Abstract
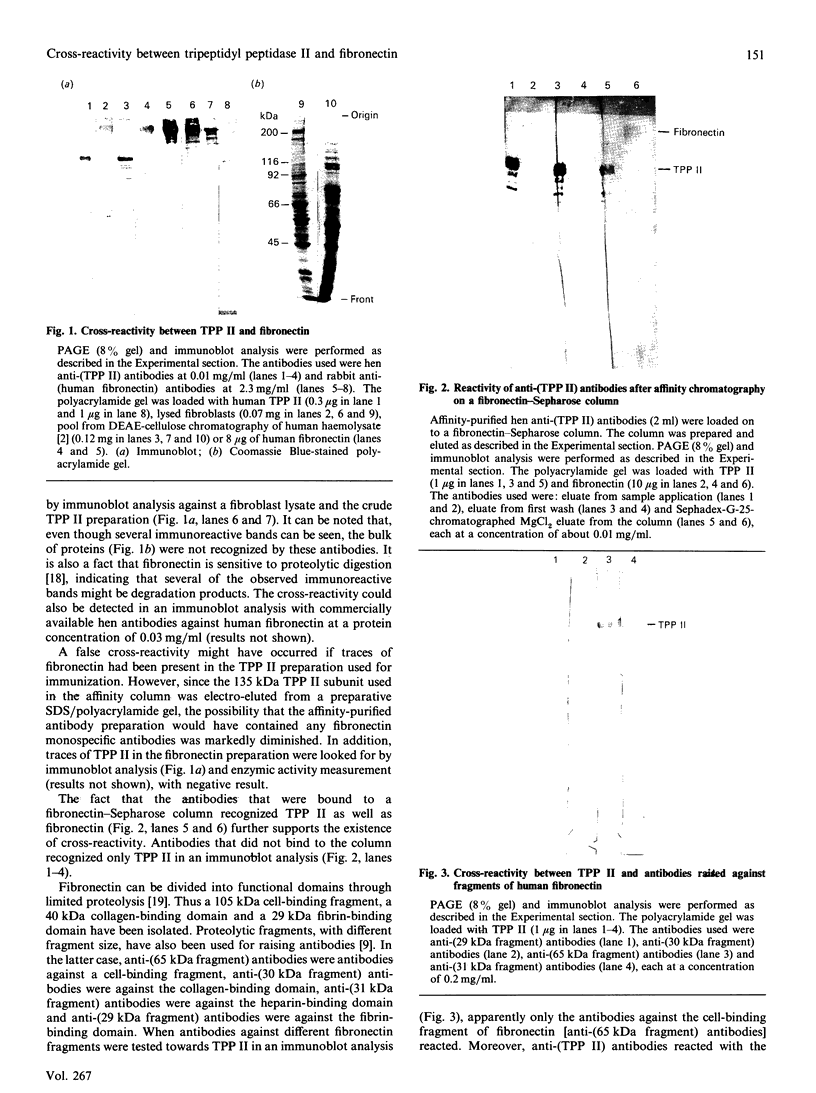
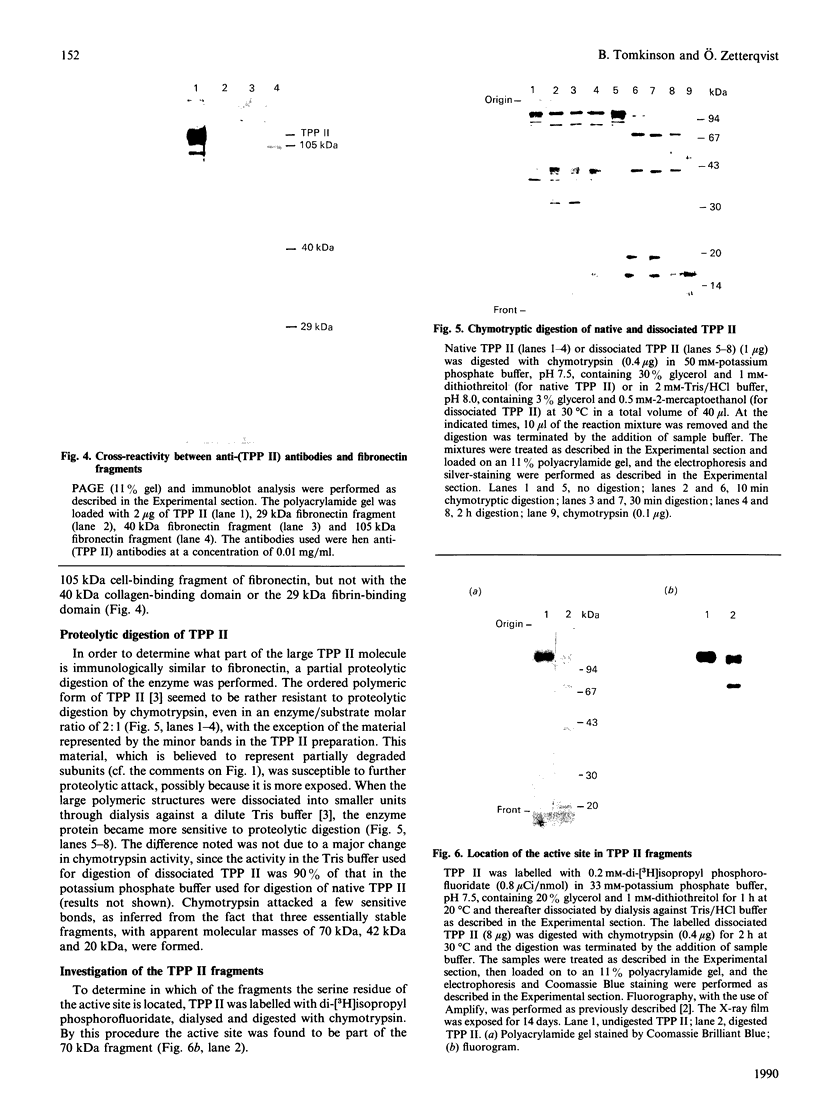
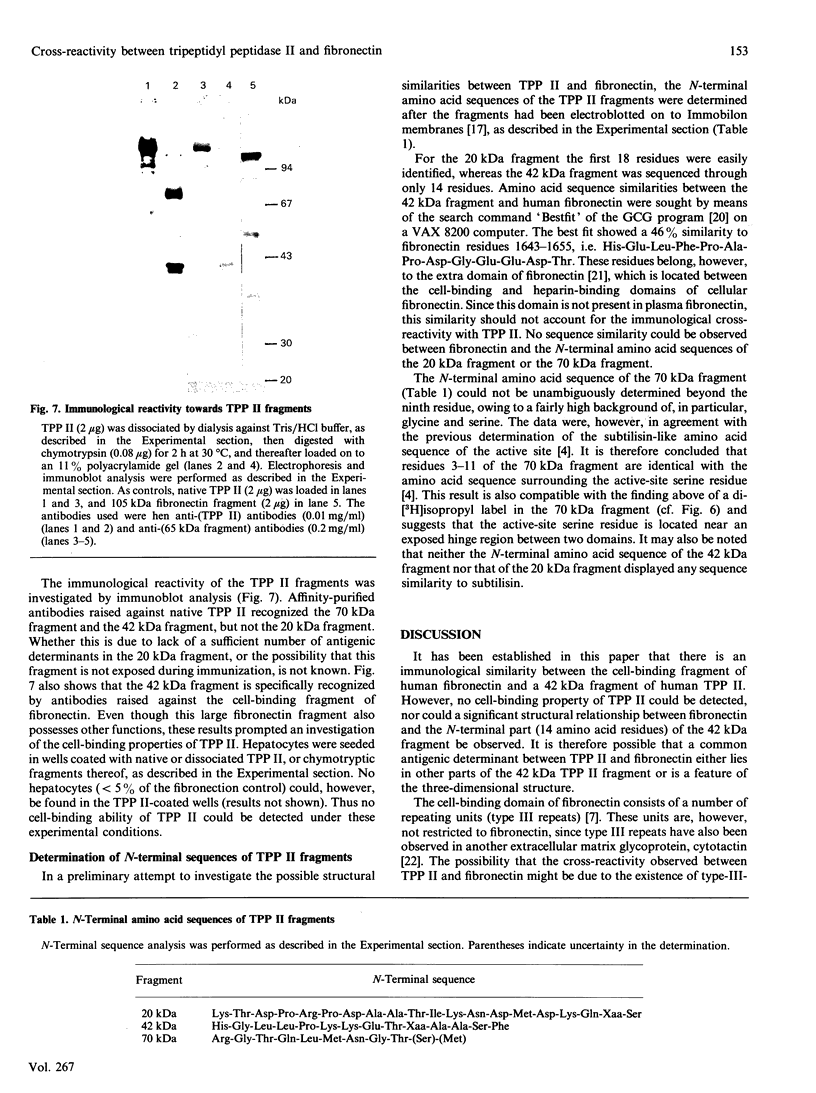
Tripeptidyl peptidase II (TPP II) is a large intracellular exopeptidase with an active site of the subtilisin type. Affinity-purified hen antibodies against human erythrocyte TPP II cross-reacted with fibronectin in an immunoblot analysis. Furthermore, antibodies against human fibronectin cross-reacted with TPP II. Antibodies against a 65 kDa cell-binding fragment of fibronectin specifically reacted with TPP II, whereas antibodies against the collagen-binding domain, the main heparin-binding domain or the N-terminal fibrin-binding domain did not react. Moreover, the affinity-purified antibodies against TPP II reacted with a 105 kDa cell-binding fragment of fibronectin but not with the fibrin-binding domain or the collagen-binding domain. When native TPP II was dissociated into smaller units through dialysis against a dilute Tris buffer, it could be digested by chymotrypsin into three stable fragments of 70 kDa, 42 kDa and 20 kDa. It could be demonstrated that the 42 kDa fragment was specifically recognized by antibodies against the 65 kDa cell-binding fragment of fibronectin. Furthermore, labelling with di-[3H]isopropyl phosphorofluoridate and N-terminal sequence determination showed that the 70 kDa fragment contained the active-site serine residue. In conclusion, our findings suggest that one domain of the TPP II molecule bears structural resemblance to a cell-binding fragment of fibronectin.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama S. K., Yamada K. M. Fibronectin. Adv Enzymol Relat Areas Mol Biol. 1987;59:1–57. doi: 10.1002/9780470123058.ch1. [DOI] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bålöw R. M., Eriksson I. Tripeptidyl peptidase II in haemolysates and liver homogenates of various species. Biochem J. 1987 Jan 1;241(1):75–80. doi: 10.1042/bj2410075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bålöw R. M., Ragnarsson U., Zetterqvist O. Tripeptidyl aminopeptidase in the extralysosomal fraction of rat liver. J Biol Chem. 1983 Oct 10;258(19):11622–11628. [PubMed] [Google Scholar]
- Bålöw R. M., Tomkinson B., Ragnarsson U., Zetterqvist O. Purification, substrate specificity, and classification of tripeptidyl peptidase II. J Biol Chem. 1986 Feb 15;261(5):2409–2417. [PubMed] [Google Scholar]
- DULBECCO R., FREEMAN G. Plaque production by the polyoma virus. Virology. 1959 Jul;8(3):396–397. doi: 10.1016/0042-6822(59)90043-1. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman G. M. CAMs and Igs: cell adhesion and the evolutionary origins of immunity. Immunol Rev. 1987 Dec;100:11–45. doi: 10.1111/j.1600-065x.1987.tb00526.x. [DOI] [PubMed] [Google Scholar]
- Jones F. S., Burgoon M. P., Hoffman S., Crossin K. L., Cunningham B. A., Edelman G. M. A cDNA clone for cytotactin contains sequences similar to epidermal growth factor-like repeats and segments of fibronectin and fibrinogen. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2186–2190. doi: 10.1073/pnas.85.7.2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornblihtt A. R., Umezawa K., Vibe-Pedersen K., Baralle F. E. Primary structure of human fibronectin: differential splicing may generate at least 10 polypeptides from a single gene. EMBO J. 1985 Jul;4(7):1755–1759. doi: 10.1002/j.1460-2075.1985.tb03847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landegren U. Measurement of cell numbers by means of the endogenous enzyme hexosaminidase. Applications to detection of lymphokines and cell surface antigens. J Immunol Methods. 1984 Mar 16;67(2):379–388. doi: 10.1016/0022-1759(84)90477-0. [DOI] [PubMed] [Google Scholar]
- Macpherson E., Tomkinson B., Bålöw R. M., Höglund S., Zetterqvist O. Supramolecular structure of tripeptidyl peptidase II from human erythrocytes as studied by electron microscopy, and its correlation to enzyme activity. Biochem J. 1987 Nov 15;248(1):259–263. doi: 10.1042/bj2480259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Neurath H. Evolution of proteolytic enzymes. Science. 1984 Apr 27;224(4647):350–357. doi: 10.1126/science.6369538. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Patthy L. Evolution of the proteases of blood coagulation and fibrinolysis by assembly from modules. Cell. 1985 Jul;41(3):657–663. doi: 10.1016/s0092-8674(85)80046-5. [DOI] [PubMed] [Google Scholar]
- Perris R., Johansson S. Amphibian neural crest cell migration on purified extracellular matrix components: a chondroitin sulfate proteoglycan inhibits locomotion on fibronectin substrates. J Cell Biol. 1987 Dec;105(6 Pt 1):2511–2521. doi: 10.1083/jcb.105.6.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin K., Kjellén L., Obrink B. Intercellular adhesion between juvenile liver cells. A method to measure the formation of stable lateral contacts between cells attached to a collagen gel. Exp Cell Res. 1977 Oct 15;109(2):413–422. doi: 10.1016/0014-4827(77)90021-0. [DOI] [PubMed] [Google Scholar]
- Tomkinson B., Wernstedt C., Hellman U., Zetterqvist O. Active site of tripeptidyl peptidase II from human erythrocytes is of the subtilisin type. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7508–7512. doi: 10.1073/pnas.84.21.7508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vartio T., Seppä H., Vaheri A. Susceptibility of soluble and matrix fibronectins to degradation by tissue proteinases, mast cell chymase and cathepsin G. J Biol Chem. 1981 Jan 10;256(1):471–477. [PubMed] [Google Scholar]
- Vuento M., Vaheri A. Purification of fibronectin from human plasma by affinity chromatography under non-denaturing conditions. Biochem J. 1979 Nov 1;183(2):331–337. doi: 10.1042/bj1830331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods A., Johansson S., Hök M. Fibronectin fibril formation involves cell interactions with two fibronectin domains. Exp Cell Res. 1988 Aug;177(2):272–283. doi: 10.1016/0014-4827(88)90461-2. [DOI] [PubMed] [Google Scholar]