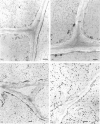
Abstract
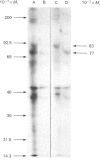
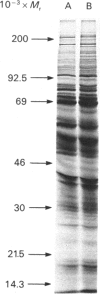
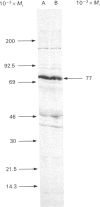
1. Excised discs of potato (Solanum tuberosum) tuber were incubated with [3H]fucose and extracts were prepared and incubated with an antibody to phenylalanine ammonia-lyase. Analysis of the resulting immunoprecipitated proteins by SDS/PAGE showed [3H]mannose- and [3H]fucose-labelled bands with Mr values corresponding to those of phenylalanine ammonia-lyase subunits. 2. When potato discs were incubated with [3H]sugars in the presence of tunicamycin, an inhibitor of N-linked protein glycosylation, incorporation of radioactivity from [3H]mannose into the immunoprecipitated enzyme subunits was virtually eliminated, whereas that from [3H]fucose was only marginally inhibited. 3. Tunicamycin reduced the level of extractable phenylalanine ammonia-lyase activity induced in excised potato tuber discs. Kinetic analysis revealed that the Vmax value of the enzyme in crude extracts from tunicamycin-treated tissue was reduced, whereas the apparent Km values were unaffected. 4. Immunoprecipitation of the enzyme labelled in vivo with [35S]methionine showed that tunicamycin did not inhibit the synthesis of the enzyme protein per se, nor did it increase the degradation of the enzyme protein. 5. Immunoprecipitation of the enzyme labelled in vitro with [14C]nitromethane showed that tunicamycin did not affect the introduction of the dehydroalanine residue into the active site. 6. These results are consistent with the following hypothesis: tunicamycin inhibits the N-linked glycosylation of phenylalanine ammonia-lyase which, in turn, results in imperfect folding of the enzyme protein. The orientation of the active site is changed in such a way that the affinity of the enzyme for its substrate is unaffected, whereas the catalytic activity of the enzyme is reduced. 7. Both optical- and electron-microscopic immunolocalization studies with antibody to phenylalanine ammonia-lyase showed increased deposition of silver granules in cells in sections of potato discs in which induction of the enzyme was allowed to occur compared with cells from newly wounded tissue. The enzyme was located in the cytoplasm, and was possibly membrane-associated.
Full text
PDF

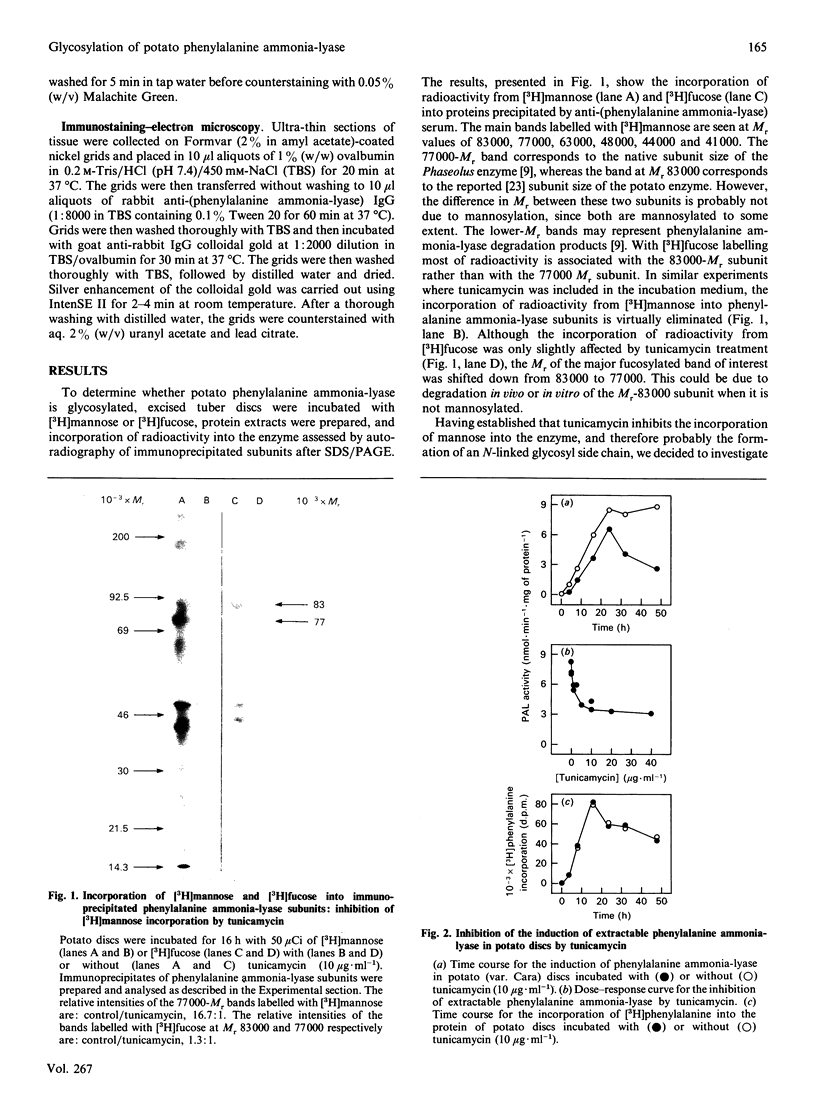
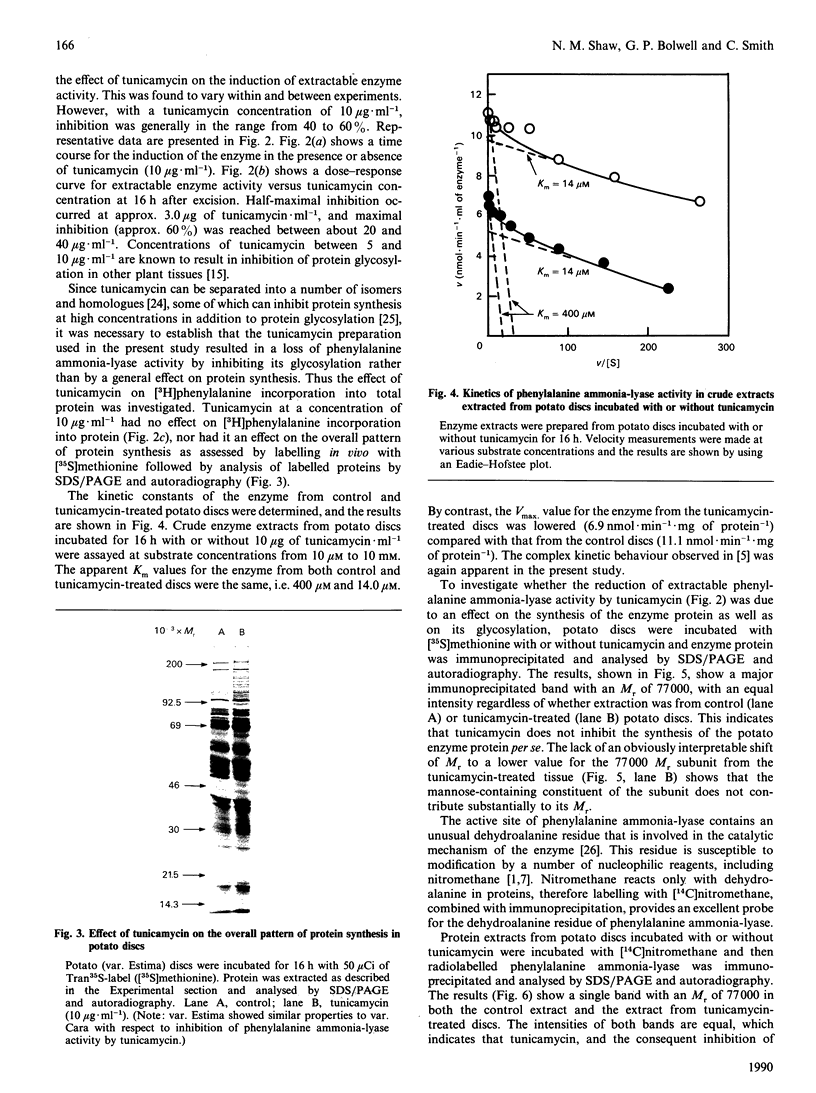


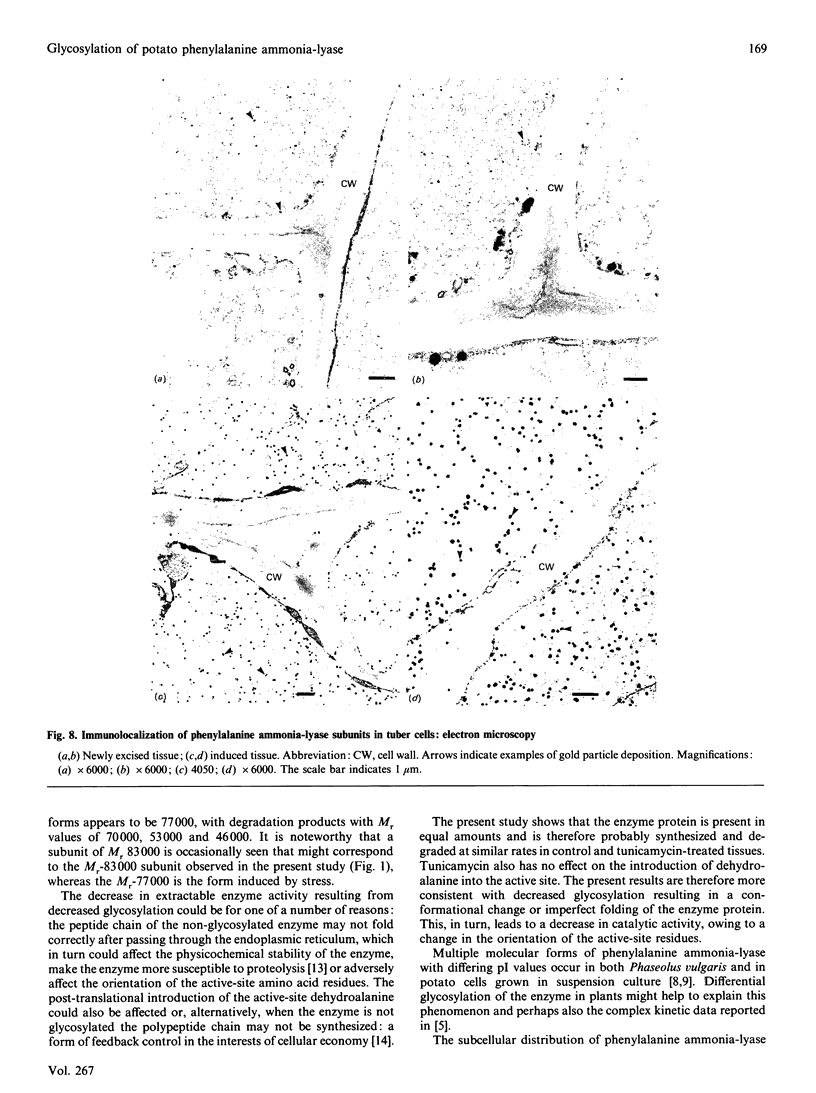

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
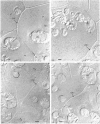
- Acton G. J., Schopfer P. Control over activation or synthesis of phenylalanine ammonia-lyase by phytochrome in mustard (Sinapis alba L.)? A contribution to eliminate some misconceptions. Biochim Biophys Acta. 1975 Oct 9;404(2):231–242. doi: 10.1016/0304-4165(75)90329-3. [DOI] [PubMed] [Google Scholar]
- Bevan M., Shufflebottom D., Edwards K., Jefferson R., Schuch W. Tissue- and cell-specific activity of a phenylalanine ammonia-lyase promoter in transgenic plants. EMBO J. 1989 Jul;8(7):1899–1906. doi: 10.1002/j.1460-2075.1989.tb03592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolwell G. P., Bell J. N., Cramer C. L., Schuch W., Lamb C. J., Dixon R. A. L-Phenylalanine ammonia-lyase from Phaseolus vulgaris. Characterisation and differential induction of multiple forms from elicitor-treated cell suspension cultures. Eur J Biochem. 1985 Jun 3;149(2):411–419. doi: 10.1111/j.1432-1033.1985.tb08941.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Danscher G. Localization of gold in biological tissue. A photochemical method for light and electronmicroscopy. Histochemistry. 1981;71(1):81–88. doi: 10.1007/BF00592572. [DOI] [PubMed] [Google Scholar]
- Duksin D., Mahoney W. C. Relationship of the structure and biological activity of the natural homologues of tunicamycin. J Biol Chem. 1982 Mar 25;257(6):3105–3109. [PubMed] [Google Scholar]
- Faye L., Johnson K. D., Chrispeels M. J. Oligosaccharide Side Chains of Glycoproteins that Remain in the High-Mannose Form Are Not Accessible to Glycosidases. Plant Physiol. 1986 May;81(1):206–211. doi: 10.1104/pp.81.1.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira R. B., Davies D. D. Protein degradation in lemna with particular reference to ribulose bisphosphate carboxylase: I. The effect of light and dark. Plant Physiol. 1987 Apr;83(4):869–877. doi: 10.1104/pp.83.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson K. R., Havir E. A. L-phenylalanine ammonia-lyase. IV. Evidence that the prosthetic group contains a dehydroalanyl residue and mechanism of action. Arch Biochem Biophys. 1970 Nov;141(1):1–17. doi: 10.1016/0003-9861(70)90100-1. [DOI] [PubMed] [Google Scholar]
- Hashim O. H., Cushley W. Minor modifications to the structure of tunicamycin lead to loss of the biological activity of the antibiotic. Biochim Biophys Acta. 1987 Mar 19;923(3):362–370. doi: 10.1016/0304-4165(87)90044-4. [DOI] [PubMed] [Google Scholar]
- Havir E. A., Hanson K. R. L-Phenylalanine ammonia-lyase. I. Purification and molecular size of the enzyme from potato tubers. Biochemistry. 1968 May;7(5):1896–1903. doi: 10.1021/bi00845a038. [DOI] [PubMed] [Google Scholar]
- Havir E. A., Hanson K. R. L-phenylalanine ammonia-lyase (maize and potato). Evidence that the enzyme is composed of four subunits. Biochemistry. 1973 Apr 10;12(8):1583–1591. doi: 10.1021/bi00732a019. [DOI] [PubMed] [Google Scholar]
- Havir E. A., Hanson K. R. L-phenylalanine ammonia-lyase (maize, potato, and Rhodotorula glutinis). Studies of the prosthetic group with nitromethane. Biochemistry. 1975 Apr 22;14(8):1620–1626. doi: 10.1021/bi00679a012. [DOI] [PubMed] [Google Scholar]
- Havir E. A., Hanson K. R. L-phenylalanine ammonia-lyase. II. Mechanism and kinetic properties of the enzyme from potato tubers. Biochemistry. 1968 May;7(5):1904–1914. doi: 10.1021/bi00845a039. [DOI] [PubMed] [Google Scholar]
- Havir E. A. Phenylalanine ammonia-lyase: purification and characterization from soybean cell suspension cultures. Arch Biochem Biophys. 1981 Oct 15;211(2):556–563. doi: 10.1016/0003-9861(81)90490-2. [DOI] [PubMed] [Google Scholar]
- Hermes J. D., Weiss P. M., Cleland W. W. Use of nitrogen-15 and deuterium isotope effects to determine the chemical mechanism of phenylalanine ammonia-lyase. Biochemistry. 1985 Jun 4;24(12):2959–2967. doi: 10.1021/bi00333a023. [DOI] [PubMed] [Google Scholar]
- Hori H., Elbein A. D. Tunicamycin inhibits protein glycosylation in suspension cultured soybean cells. Plant Physiol. 1981 May;67(5):882–886. doi: 10.1104/pp.67.5.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori H., Kaushal G. P., Elbein A. D. Fucosylation of membrane proteins in soybean cultured cells : effects of tunicamycin and swainsonine. Plant Physiol. 1985 Mar;77(3):687–694. doi: 10.1104/pp.77.3.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrazdina G., Wagner G. J. Metabolic pathways as enzyme complexes: evidence for the synthesis of phenylpropanoids and flavonoids on membrane associated enzyme complexes. Arch Biochem Biophys. 1985 Feb 15;237(1):88–100. doi: 10.1016/0003-9861(85)90257-7. [DOI] [PubMed] [Google Scholar]
- Lamb C. J., Merritt T. K., Butt V. S. Synthesis and removal of phenylalanine ammonia-lyase activity in illuminated discs of potato tuber parenchyme. Biochim Biophys Acta. 1979 Jan 18;582(2):196–212. doi: 10.1016/0304-4165(79)90384-2. [DOI] [PubMed] [Google Scholar]
- Lois R., Dietrich A., Hahlbrock K., Schulz W. A phenylalanine ammonia-lyase gene from parsley: structure, regulation and identification of elicitor and light responsive cis-acting elements. EMBO J. 1989 Jun;8(6):1641–1648. doi: 10.1002/j.1460-2075.1989.tb03554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwaiger H., Tanner W. Effects of gibberellic acid and of tunicamycin on glycosyl-transferase activities and on alpha-amylase secretion in barley. Eur J Biochem. 1979 Dec 17;102(2):375–381. doi: 10.1111/j.1432-1033.1979.tb04252.x. [DOI] [PubMed] [Google Scholar]
- Vayda M. E., Schaeffer H. J. Hypoxic stress inhibits the appearance of wound-response proteins in potato tubers. Plant Physiol. 1988 Nov;88(3):805–809. doi: 10.1104/pp.88.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]