Abstract
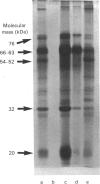
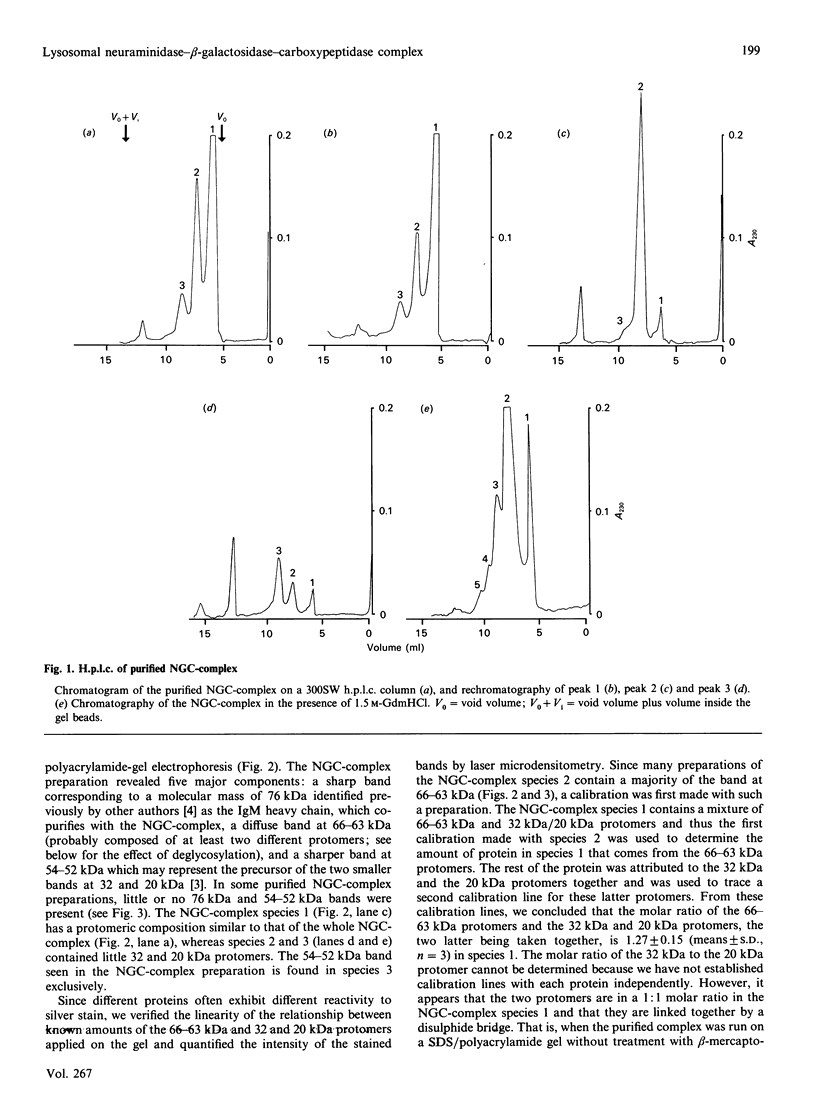
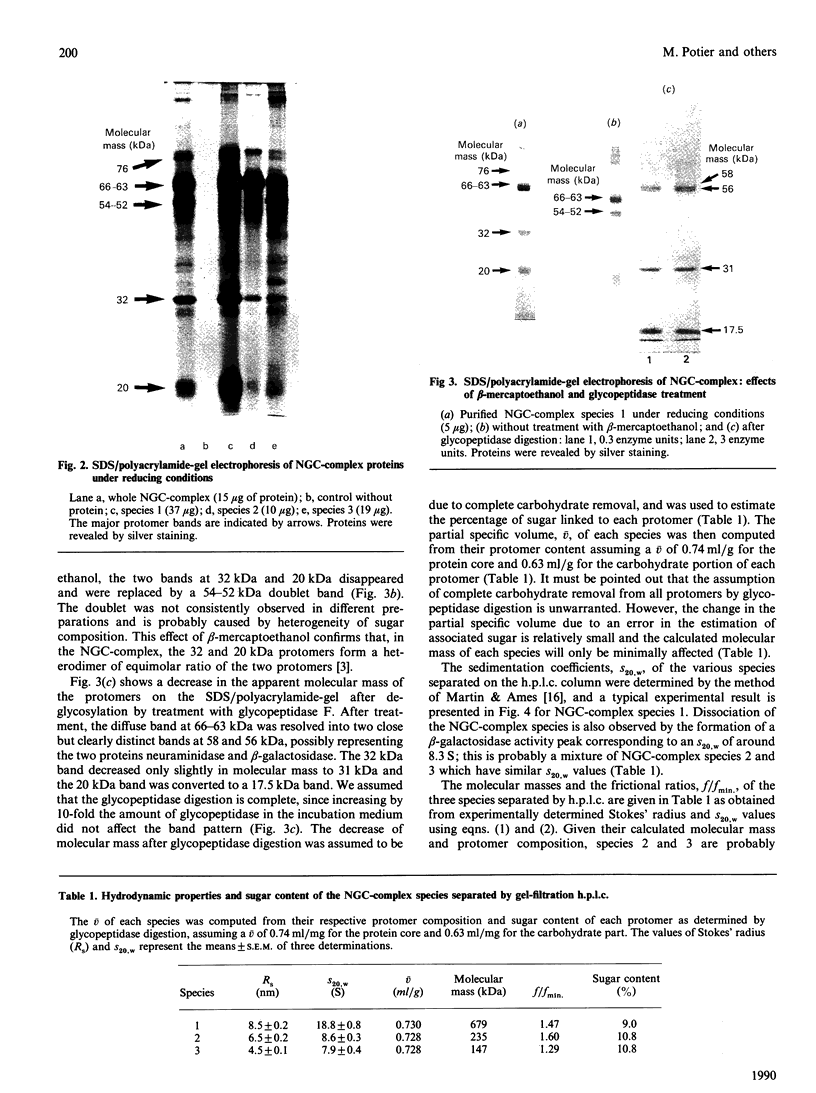
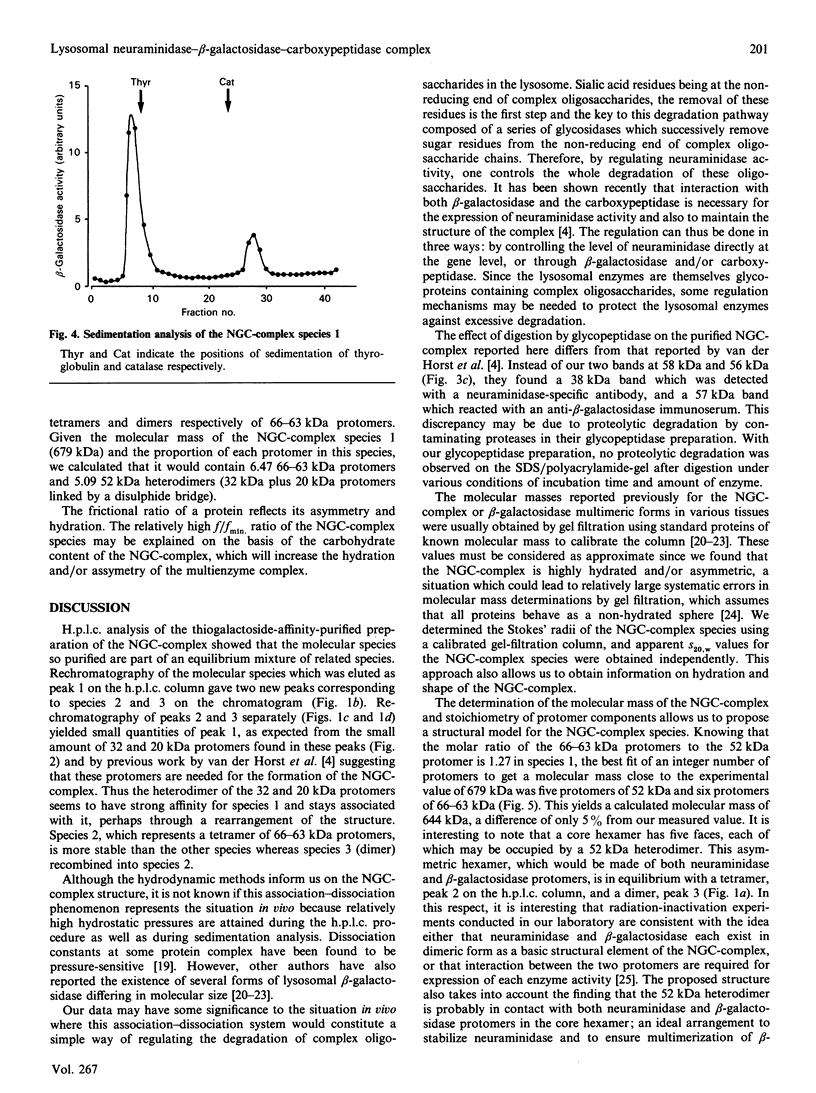
Lysosomal neuraminidase (sialidase; EC 3.2.1.18) and beta-galactosidase (EC 3.2.1.23), together with a carboxypeptidase, the so-called 'protective protein', were co-purified from the human placenta by affinity chromatography on a concanavalin A-Sepharose column followed by a thiogalactoside-agarose affinity column for beta-galactosidase. Analysis of the purified material by gel-filtration h.p.l.c. revealed three distinct molecular forms, all with high beta-galactosidase specific activity, but only the largest one expressed neuraminidase activity. Rechromatography of each individual species separately indicated that all three are in fact part of an equilibrium system (the neuraminidase-beta-galactosidase-carboxypeptidase complex or NGC-complex) and that these species undergo slow conversion into one another through dissociation and association of protomeric components. Each species was sufficiently stable for the determination of their hydrodynamic properties by gel-filtration h.p.l.c. and sedimentation velocity. The largest species had an apparent sedimentation coefficient S20.w, of 18.8 S and a Stokes' radius of 8.5 nm, giving a molecular mass of 679 kDa and a fractional ratio, f/f min, of 1.47. The latter value indicates that the macromolecule is asymmetric or highly hydrated. This large species is composed of four types of polypeptide chains of molecular mass 66 kDa (neuraminidase), 63 kDa (beta-galactosidase), 32 kDa and 20 kDa (carboxypeptidase heterodimer). The 32 kDa and 20 kDa protomers are linked together by a disulphide bridge. Glycopeptidase F digestion of the NGC-complex transformed the diffuse 66-63 kDa band on the SDS gel into two close but sharp bands at 58 and 56 kDa. The two smaller species which were separated on the h.p.l.c. column correspond to tetrameric and dimeric forms of the 66-63 kDa protomers and express exclusively beta-galactosidase activity. Treatment of the NGC-complex with increasing concentrations of guanidinium hydrochloride up to 1.5 M also resulted in dissociation of the complex into the same smaller species mentioned above plus two protomers of molecular mass around 60 and 50 kDa. A model of the largest molecular species as a hexamer of the 66-63 kDa protomers associated to five carboxypeptidase heterodimers (32 kDa and 20 kDa) is proposed
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cantz M., Gehler J., Spranger J. Mucolipidosis I: increased sialic acid content and deficiency of an alpha-N-acetylneuraminidase in cultured fibroblasts. Biochem Biophys Res Commun. 1977 Jan 24;74(2):732–738. doi: 10.1016/0006-291x(77)90363-1. [DOI] [PubMed] [Google Scholar]
- Cheetham P. S., Dance N. E. The separation and characterization of the methylumbelliferyl beta-galactosidases of human liver. Biochem J. 1976 Jul 1;157(1):189–195. doi: 10.1042/bj1570189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost R. G., Holmes E. W., Norden A. G., O'Brien J. S. Characterization of purified human liver acid beta-D-galactosidases A2 and A3. Biochem J. 1978 Oct 1;175(1):181–188. doi: 10.1042/bj1750181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galjart N. J., Gillemans N., Harris A., van der Horst G. T., Verheijen F. W., Galjaard H., d'Azzo A. Expression of cDNA encoding the human "protective protein" associated with lysosomal beta-galactosidase and neuraminidase: homology to yeast proteases. Cell. 1988 Sep 9;54(6):755–764. doi: 10.1016/s0092-8674(88)90999-3. [DOI] [PubMed] [Google Scholar]
- Heyworth C. M., Neumann E. F., Wynn C. H. The stability and aggregation properties of human liver acid beta-D-galactosidase. Biochem J. 1981 Mar 1;193(3):773–779. doi: 10.1042/bj1930773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs R., Harrington W. F. An unusual pressure dependence for a reversibly associating protein system; sedimentation studies on myosin. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1587–1594. doi: 10.1073/pnas.58.4.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Le Maire M., Aggerbeck L. P., Monteilhet C., Andersen J. P., Møller J. V. The use of high-performance liquid chromatography for the determination of size and molecular weight of proteins: a caution and a list of membrane proteins suitable as standards. Anal Biochem. 1986 May 1;154(2):525–535. doi: 10.1016/0003-2697(86)90025-4. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- McNamara D., Beauregard G., Nguyen H. V., Yan D. L., Bélisle M., Potier M. Characterization of human placental neuraminidases. Stability, substrate specificity and molecular weight. Biochem J. 1982 Aug 1;205(2):345–351. doi: 10.1042/bj2050345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Van Keuren M. L. Gel protein stains: silver stain. Methods Enzymol. 1984;104:441–447. doi: 10.1016/s0076-6879(84)04111-2. [DOI] [PubMed] [Google Scholar]
- Okada S., O'Brien J. S. Generalized gangliosidosis: beta-galactosidase deficiency. Science. 1968 May 31;160(3831):1002–1004. doi: 10.1126/science.160.3831.1002. [DOI] [PubMed] [Google Scholar]
- Potier M., Mameli L., Bélisle M., Dallaire L., Melançon S. B. Fluorometric assay of neuraminidase with a sodium (4-methylumbelliferyl-alpha-D-N-acetylneuraminate) substrate. Anal Biochem. 1979 Apr 15;94(2):287–296. doi: 10.1016/0003-2697(79)90362-2. [DOI] [PubMed] [Google Scholar]
- Tarentino A. L., Gómez C. M., Plummer T. H., Jr Deglycosylation of asparagine-linked glycans by peptide:N-glycosidase F. Biochemistry. 1985 Aug 13;24(17):4665–4671. doi: 10.1021/bi00338a028. [DOI] [PubMed] [Google Scholar]
- Verheijen F. W., Palmeri S., Hoogeveen A. T., Galjaard H. Human placental neuraminidase. Activation, stabilization and association with beta-galactosidase and its protective protein. Eur J Biochem. 1985 Jun 3;149(2):315–321. doi: 10.1111/j.1432-1033.1985.tb08928.x. [DOI] [PubMed] [Google Scholar]
- Verheijen F., Brossmer R., Galjaard H. Purification of acid beta-galactosidase and acid neuraminidase from bovine testis: evidence for an enzyme complex. Biochem Biophys Res Commun. 1982 Sep 30;108(2):868–875. doi: 10.1016/0006-291x(82)90911-1. [DOI] [PubMed] [Google Scholar]
- Wenger D. A., Tarby T. J., Wharton C. Macular cherry-red spots and myoclonus with dementia: coexistent neuraminidase and beta-galactosidase deficiencies. Biochem Biophys Res Commun. 1978 May 30;82(2):589–595. doi: 10.1016/0006-291x(78)90915-4. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y., Nishimura K. Aggregation-dissociation and stability of acid beta-galactosidase purified from porcine spleen. Int J Biochem. 1986;18(4):327–335. doi: 10.1016/0020-711x(86)90038-8. [DOI] [PubMed] [Google Scholar]
- le Maire M., Ghazi A., Møller J. V., Aggerbeck L. P. The use of gel chromatography for the determination of sizes and relative molecular masses of proteins. Interpretation of calibration curves in terms of gel-pore-size distribution. Biochem J. 1987 Apr 15;243(2):399–404. doi: 10.1042/bj2430399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Horst G. T., Galjart N. J., d'Azzo A., Galjaard H., Verheijen F. W. Identification and in vitro reconstitution of lysosomal neuraminidase from human placenta. J Biol Chem. 1989 Jan 15;264(2):1317–1322. [PubMed] [Google Scholar]