Abstract
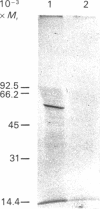
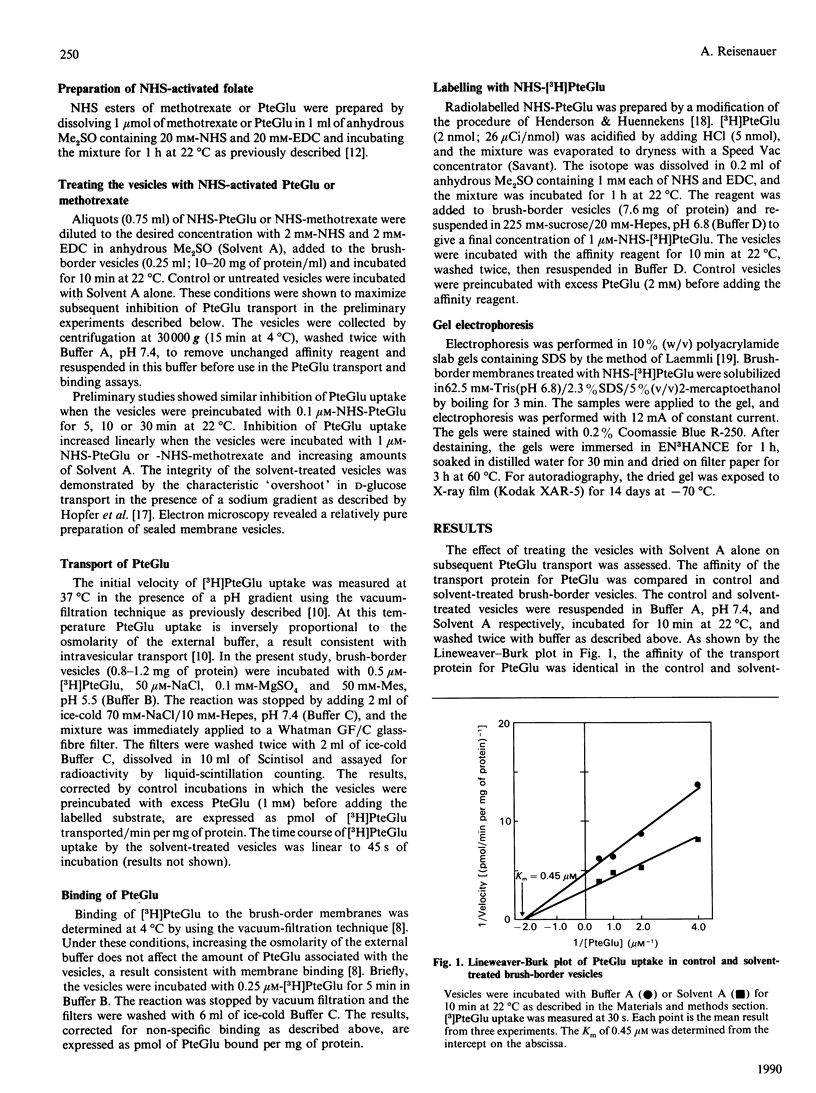
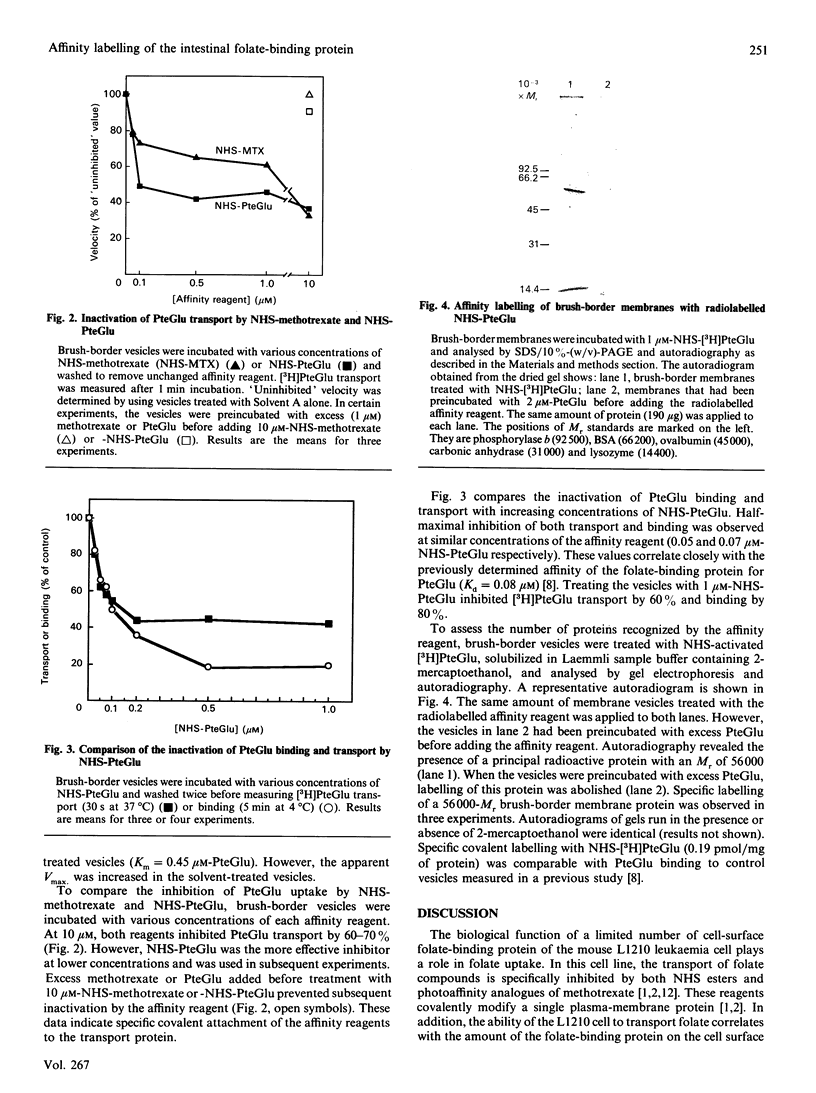
A specific transport system for folate and a high-affinity folate-binding protein have been identified in pig intestinal brush-border membranes. To determine if the binding protein plays a role in folic acid (PteGlu) uptake in to the cell, the inactivation of folate binding and transport by N-hydroxysuccinimide esters of folic acid (NHS-PteGlu) was compared. In addition, the number of brush-border proteins modified by the affinity reagent was assessed. Brush-border vesicles were incubated with various concentrations of NHS-PteGlu or NHS-methotrexate. Transport and binding of [3H]PteGlu by the vesicles were measured at 37 and 4 degrees C respectively by using the vacuum-filtration technique. NHS-methotrexate and NHS-PteGlu specifically inhibited PteGlu transport. Incubating the vesicles with 1 microM-NHS-PteGlu inactivated [3H]PteGlu transport by 60% and binding by 80%. Half-maximal inhibition of both transport and binding was observed at similar concentrations of the affinity reagent (0.05 and 0.07 microM-NHS-PteGlu respectively). Treating the vesicles with radiolabelled NHS-PteGlu followed by gel electrophoresis and autoradiography revealed a specifically labelled protein with an Mr of 56,000. These results indicate that the intestinal folate-binding and transport proteins are identical and that the function of the folate-binding protein is to transport folate into the cell.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen B. A., Newman R. A. High-performance liquid chromatographic separation of clinically important folic acid derivatives using ion-pair chromatography. J Chromatogr. 1980 Mar 21;190(1):241–245. doi: 10.1016/s0021-9673(00)85542-9. [DOI] [PubMed] [Google Scholar]
- Antony A. C., Kane M. A., Portillo R. M., Elwood P. C., Kolhouse J. F. Studies of the role of a particulate folate-binding protein in the uptake of 5-methyltetrahydrofolate by cultured human KB cells. J Biol Chem. 1985 Dec 5;260(28):14911–14917. [PubMed] [Google Scholar]
- Antony A. C., Utley C., Van Horne K. C., Kolhouse J. F. Isolation and characterization of a folate receptor from human placenta. J Biol Chem. 1981 Sep 25;256(18):9684–9692. [PubMed] [Google Scholar]
- Cezard J. P., Conklin K. A., Das B. C., Gray G. M. Incomplete intracellular forms of intestinal surface membrane sucrase-isomaltase. J Biol Chem. 1979 Sep 25;254(18):8969–8975. [PubMed] [Google Scholar]
- Chandler C. J., Wang T. T., Halsted C. H. Pteroylpolyglutamate hydrolase from human jejunal brush borders. Purification and characterization. J Biol Chem. 1986 Jan 15;261(2):928–933. [PubMed] [Google Scholar]
- Elwood P. C., Kane M. A., Portillo R. M., Kolhouse J. F. The isolation, characterization, and comparison of the membrane-associated and soluble folate-binding proteins from human KB cells. J Biol Chem. 1986 Nov 25;261(33):15416–15423. [PubMed] [Google Scholar]
- Goldman I. D., Lichtenstein N. S., Oliverio V. T. Carrier-mediated transport of the folic acid analogue, methotrexate, in the L1210 leukemia cell. J Biol Chem. 1968 Oct 10;243(19):5007–5017. [PubMed] [Google Scholar]
- Henderson G. B., Huennekens F. M. Membrane-associated folate transport proteins. Methods Enzymol. 1986;122:260–269. doi: 10.1016/0076-6879(86)22180-1. [DOI] [PubMed] [Google Scholar]
- Henderson G. B., Montague-Wilkie B. Irreversible inhibitors of methotrexate transport in L1210 cells. Characteristics of inhibition by an N-hydroxysuccinimide ester of methotrexate. Biochim Biophys Acta. 1983 Oct 26;735(1):123–130. doi: 10.1016/0005-2736(83)90267-5. [DOI] [PubMed] [Google Scholar]
- Henderson G. B., Potuznik S. Irreversible inhibition of folate transport in Lactobacillus casei by covalent modification of the binding protein with carbodiimide-activated folate. Arch Biochem Biophys. 1982 Jun;216(1):27–33. doi: 10.1016/0003-9861(82)90184-9. [DOI] [PubMed] [Google Scholar]
- Henderson G. B., Zevely E. M. Affinity labeling of the 5-methyltetrahydrofolate/methotrexate transport protein of L1210 cells by treatment with an N-hydroxysuccinimide ester of [3H]methotrexate. J Biol Chem. 1984 Apr 10;259(7):4558–4562. [PubMed] [Google Scholar]
- Hopfer U., Nelson K., Perrotto J., Isselbacher K. J. Glucose transport in isolated brush border membrane from rat small intestine. J Biol Chem. 1973 Jan 10;248(1):25–32. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leslie G. I., Rowe P. B. Folate binding by the brush border membrane proteins of small intestinal epithelial cells. Biochemistry. 1972 Apr 25;11(9):1696–1703. doi: 10.1021/bi00759a026. [DOI] [PubMed] [Google Scholar]
- Price E. M., Freisheim J. H. Photoaffinity analogues of methotrexate as folate antagonist binding probes. 2. Transport studies, photoaffinity labeling, and identification of the membrane carrier protein for methotrexate from murine L1210 cells. Biochemistry. 1987 Jul 28;26(15):4757–4763. doi: 10.1021/bi00389a024. [DOI] [PubMed] [Google Scholar]
- Reisenauer A. M., Chandler C. J., Halsted C. H. Folate binding and hydrolysis by pig intestinal brush-border membranes. Am J Physiol. 1986 Oct;251(4 Pt 1):G481–G486. doi: 10.1152/ajpgi.1986.251.4.G481. [DOI] [PubMed] [Google Scholar]
- Selhub J., Dhar G. J., Rosenberg I. H. Gastrointestinal absorption of folates and antifolates. Pharmacol Ther. 1983;20(3):397–418. doi: 10.1016/0163-7258(83)90034-7. [DOI] [PubMed] [Google Scholar]
- Selhub J., Emmanouel D., Stavropoulos T., Arnold R. Renal folate absorption and the kidney folate binding protein. I. Urinary clearance studies. Am J Physiol. 1987 Apr;252(4 Pt 2):F750–F756. doi: 10.1152/ajprenal.1987.252.4.F750. [DOI] [PubMed] [Google Scholar]
- Selhub J., Franklin W. A. The folate-binding protein of rat kidney. Purification, properties, and cellular distribution. J Biol Chem. 1984 May 25;259(10):6601–6606. [PubMed] [Google Scholar]
- Selhub J., Nakamura S., Carone F. A. Renal folate absorption and the kidney folate binding protein. II. Microinfusion studies. Am J Physiol. 1987 Apr;252(4 Pt 2):F757–F760. doi: 10.1152/ajprenal.1987.252.4.F757. [DOI] [PubMed] [Google Scholar]
- Selhub J., Rosenberg I. H. Folate transport in isolated brush border membrane vesicles from rat intestine. J Biol Chem. 1981 May 10;256(9):4489–4493. [PubMed] [Google Scholar]
- Sirotnak F. M., Moccio D. M., Yang C. H. A novel class of genetic variants of the L1210 cell up-regulated for folate analogue transport inward. Isolation, characterization, and degree of metabolic instability of the system. J Biol Chem. 1984 Nov 10;259(21):13139–13144. [PubMed] [Google Scholar]
- Suleiman S. A., Spector R. Purification and characterization of a folate binding protein from porcine choroid plexus. Arch Biochem Biophys. 1981 Apr 15;208(1):87–94. doi: 10.1016/0003-9861(81)90126-0. [DOI] [PubMed] [Google Scholar]
- Wang T. T., Reisenauer A. M., Halsted C. H. Comparison of folate conjugase activities in human, pig, rat and monkey intestine. J Nutr. 1985 Jun;115(6):814–819. doi: 10.1093/jn/115.6.814. [DOI] [PubMed] [Google Scholar]