Abstract
Background
Ventricular arrhythmias (VAs) are a common cause of death in patients with acute myocardial infarction (AMI). Studies have shown sex differences in the incidence, presentation, and outcomes of AMI. However, less is known about sex differences in patients with AMI who develop VAs.
Objectives
The authors assessed sex differences in incidence and in-hospital outcomes of patients with AMI and VAs.
Methods
Using the National Inpatient Sample 2016 to 2020, we conducted a retrospective analysis of patients admitted for AMI with a secondary diagnosis of VAs. Multivariable logistic regression was performed to estimate the sex-specific differences in the rates and in-hospital outcomes of VAs post-AMI.
Results
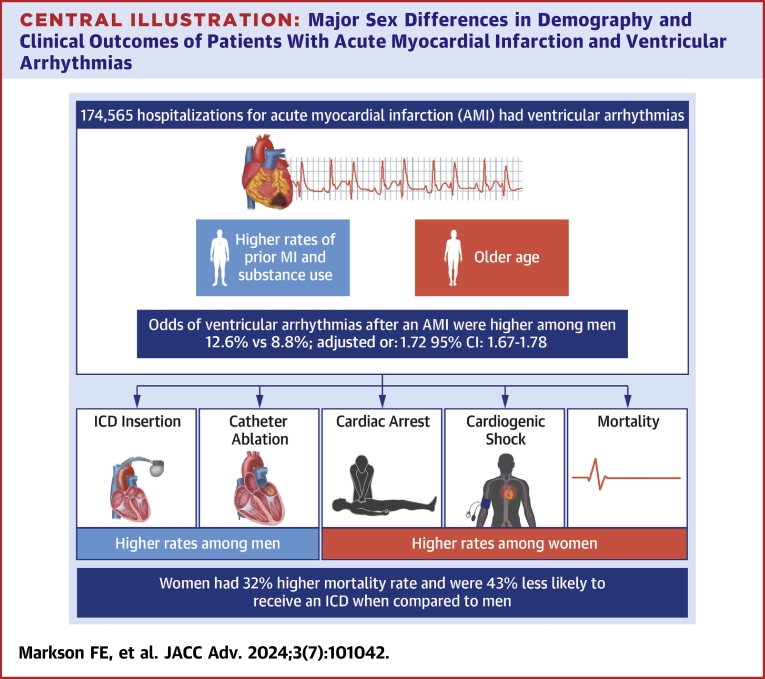
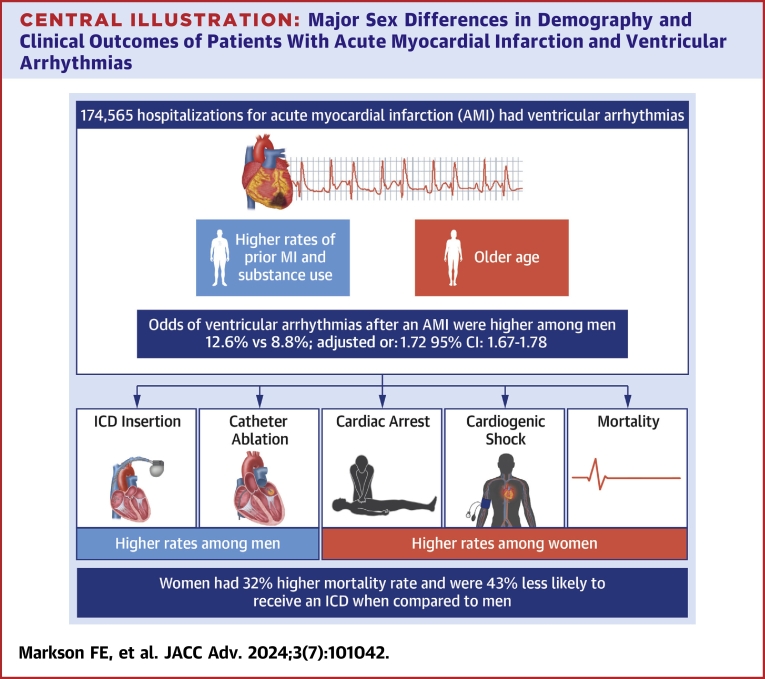
We identified 1,543,140 patients admitted with AMI. Of these, (11.3%) 174,565 patients had VAs after AMI. The odds of VAs after AMI were higher among men (12.6% vs 8.8% adjusted odds ratio [AOR]: 1.72; CI: 1.67-1.78; P < 0.001). Women had significantly higher odds of in-hospital mortality (AOR: 1.32; CI: 1.21-1.42; P < 0.001), cardiogenic shock (AOR: 1.08; CI: 1.01-1.15; P < 0.022), and cardiac arrest (AOR: 1.11; CI: 1.03-1.18; P < 0.002). Women were less likely to receive an implantable cardioverter-defibrillator (ICD) (AOR: 0.57; CI: 0.47-0.68; P < 0.001) or undergo catheter ablation (AOR: 0.51; CI: 0.27-0.98; P < 0.001) during the index admission.
Conclusions
We found important sex differences in the incidence and outcomes of VAs among patients with AMI. Women had lower odds of VAs but worse hospital outcomes overall. In addition, women were less likely to receive ICD. Further studies to address these sex disparities are needed.
Key words: acute myocardial infarction, sex or gender disparities, ventricular arrhythmias
Central Illustration
Ventricular arrhythmias (VAs), including ventricular tachycardia (VT) and ventricular fibrillation (VF) occur following 10 to 20% of acute myocardial infarction (AMI) cases and are associated with an increased risk of poor hospital outcomes.1, 2, 3, 4 Data from the SPACE (Saudi Project for Assessment of Coronary Events) registry have shown that patients with AMI who develop VAs are more likely to experience reinfarction, cardiogenic shock, congestive heart failure, stroke, and in-hospital mortality.5 Congruently, a study using data from the GRACE (Global Registry of Acute Coronary Events) registry showed similar outcomes in patients with AMI who develop VAs, with in-hospital mortality rates as high as 52% as compared to 1.6% in those who did not develop VAs.6
Previous studies have explored the sex-specific differences in AMI outcomes, including a higher incidence of VAs in men.5 However, data addressing sex-specific differences in the in-hospital outcomes of the subset of patients with VAs have been lacking. The FAST-MI (French Registry of Acute ST-Elevation or Non-ST elevation MI found that women with VAs had higher 1-year mortality but were less likely to receive cardiac interventions.7 Given these findings, using a national representative sample of the United States, we sought to determine whether sex differences exist in VA incidence and in-hospital outcomes in patients admitted for AMI.
Methodology
Study design and data source
This study is reported following the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) reporting guidelines.8
We analyzed hospitalizations between January 1, 2016, and December 31, 2020, in the U.S. National Inpatient Sample (NIS). The NIS is the largest publicly available all-payer inpatient database in the United States and is maintained by the Agency for Healthcare Research and Quality; it is designed as a stratified probability sample of discharges representing nonfederal acute care hospitals nationwide. Samples from these hospitals are recorded and then weighted to ensure that they are nationally representative. Further details on the content and design of the NIS database can be found on the Healthcare Cost and Utilization Project (HCUP) website.9 Institutional Review Board approval was waived since all patient data in NIS were deidentified.
Study sample
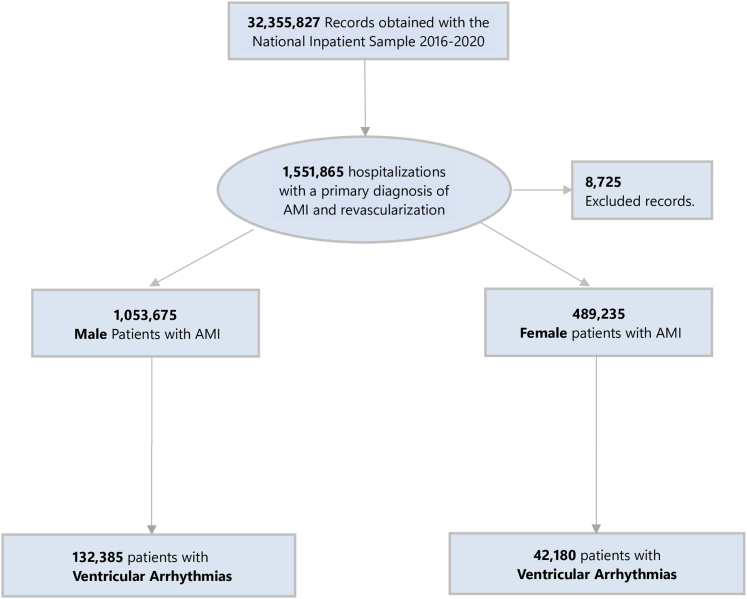
The study population consisted of all inpatient hospitalizations with a primary diagnosis of type 1 AMI who underwent revascularization (percutaneous coronary intervention or coronary artery bypass grafting) and had a secondary diagnosis of VAs (VT or VF). Diagnoses were identified using ICD-10-CM codes (type 1 AMI “I21.0,” “I21.1,” “I21.2,” “I21.3,” “I21.4,” “I21.9,” ventricular tachycardia–“I47.2” and ventricular fibrillation-“I49.0”) as recommended by the American College of Cardiology.10 We excluded patients <18 years and those who had infiltrative cardiac diseases (amyloidosis, sarcoidosis), Lyme disease, or long QT syndrome (Figure 1). Additional codes on revascularization documented are given in Supplemental Table 1.
Figure 1.
Flow Chart of Our Study Population
The flow chart displays the selection of patients with acute myocardial infarction and ventricular arrhythmias (ventricular tachycardia and ventricular fibrillation) from year January 1, 2016, to December 31, 2020. AMI = acute myocardial infarction.
Variables
The study population was divided into 2 subgroups based on the gender/sex. In the NIS, the sex of the patient was recorded as binary, either “female or male,” with all nonfemale and nonmale (eg, “other”) values set to missing. Hence to maintain uniformity, we consistently used “sex’’ instead of “gender” throughout this article. Baseline demographic variables including age, sex, race, hospital characteristics, and medical comorbidities (computed from Charlson index for comorbidities) were identified as variables present in the data set. Other comorbidities were obtained using ICD-10-CM and ICD-10-PCS codes (Supplemental Table 1). These codes have been used in prior nationally representative studies and recommended for use by the Centers for Medicare and Medicaid Services.11,12
Study outcomes
The primary endpoint of this study was to determine the sex differences in the rates of VAs among patients with AMI, while the secondary outcomes were sex differences in rates of in-hospital mortality, cardiogenic shock, cardiac arrest, implantable cardiac defibrillator insertion, palliative care consultation, catheter ablation, and length of hospitalization. We also performed a subanalysis to assess the sex differences in the outcomes of patients with AMI and either VT or VF, and the VA outcomes in patients with NSTEMI or STEMI.
Statistical analysis
Data analysis was performed using the HCUP STATA package, which incorporates the NIS-specific variables, including hospital identifiers, stratum, and discharge weights to account for clustering and large survey-weighted data analysis according to HCUP recommendations.13 Stratified weighted data were used in our analysis using the Svy command to obtain nationwide estimates. Baseline sociodemographic, hospital, and clinical characteristics of patients with AMI and VAs by sex were reported. In our descriptive statistics, continuous variables were presented as means ± SD and compared using a Student’s t-test while categorical variables were presented in percentages (%) and compared using the chi-square test. Multivariable logistic and linear regression was used to determine the adjusted odds ratio (AOR)/adjusted risk ratio of the categorical and continuous outcomes of interest, respectively. Confounders were obtained from a literature review of prior similar studies and included age, race, income status, hospital region, hyperlipidemia, heart failure, history of MI, chronic kidney disease, diabetes mellitus, hypertension, anemia, and obesity.14, 15, 16 A P value of <0.05 was considered statistically significant, and all tests were 2-sided with a 95% CI.
Results
Baseline characteristics
From 2016 to 2020, there were 3,191,049 admissions for AMI. After applying exclusions, our final cohort comprised of 1,542,910 patients with AMI. Of these, 174,565 (11.3%) patients had VAs. Among patients with AMI and VAs 132,385 (75.8%) were men. Baseline sociodemographic, hospital, and clinical characteristics of patients with AMI who had VAs are summarized by sex in (Table 1). Overall, women were older, more likely to have congestive heart failure, diabetes mellitus, obesity, atrial arrhythmia, and had higher index of illness severity by the All-Payer Refined Diagnostic Related Group severity of illness scale, and more likely to have do-not-resuscitate status.
Table 1.
Gender Differences in the Sociodemographic and Comorbid Characteristics of AMI Patients With Ventricular Arrhythmias
| All Ventricular Arrhythmias (N = 174,565, 11.3%) |
P Value | ||
|---|---|---|---|
| Male (n = 132,385, 75.8%) |
Female (n = 42,180, 24.2%) |
||
| Age (y) | 62 ± 0.2 | 66 ± 0.3 | <0.001 |
| Race | <0.0001 | ||
| White | 78.4 | 77.3 | |
| Blacks | 7.76 | 11.7 | |
| Hispanics | 6.99 | 6.2 | |
| Asians | 2.63 | 1.6 | |
| Native Americans | 0.6 | 0.5 | |
| Others | 3.7 | 2.7 | |
| Median household income | <0.0001 | ||
| 1 | 25.8 | 29.1 | |
| 2 | 26.4 | 29.0 | |
| 3 | 25.4 | 23.8 | |
| 4 | 22.3 | 18.1 | |
| Primary insurance payer | <0.0001 | ||
| Medicaid | 40.84 | 56.5 | |
| Medicare | 10.03 | 10.86 | |
| Private pay | 37.78 | 25.98 | |
| Self-pay | 6.86 | 4.83 | |
| No-charge | 0.54 | 0.3 | |
| Others | 3.95 | 1.53 | |
| Hospital bed size | 0.623 | ||
| Small | 15.49 | 15.85 | |
| Medium | 29.78 | 29.34 | |
| Large | 54.73 | 54.81 | |
| Hospital location/teaching status | 0.093 | ||
| Rural | 5.08 | 5.69 | |
| Urban nonteaching | 20.57 | 20.41 | |
| Urban teaching | 74.36 | 73.9 | |
| Hospital region | <0.0001 | ||
| Northeast | 16.62 | 16.39 | |
| Midwest | 24.53 | 26.67 | |
| South | 37.93 | 38.8 | |
| West | 20.91 | 18.14 | |
| Old MI | 14.3 | 11.2 | <0.0001 |
| Old PCI | 1.1 | 0.9 | 0.056 |
| Old CABG | 5.6 | 3.8 | <0.0001 |
| Old pacemaker | 1.4 | 1.6 | 0.145 |
| Old catheter ablation | 2.7 | 1.6 | <0.0001 |
| Hypertension | 39.9 | 39.4 | 0.379 |
| Diabetes mellitus | 11.2 | 12.2 | 0.013 |
| Peripheral vascular disease | 3.6 | 5.1 | <0.0001 |
| Smoking | 25.9 | 21.2 | <0.0001 |
| Obesity | 16.6 | 20.5 | <0.0001 |
| Cocaine use | 1.0 | 0.6 | 0.0005 |
| CHF | 34.6 | 39.5 | <0.0001 |
| Elixhauser comorbidity categories | <0.0001 | ||
| 1 | 5.67 | 3.57 | |
| 2 | 13.96 | 8.88 | |
| ≥3 | 80.37 | 87.55 | |
| Dyslipidemia | 63.3 | 61.9 | 0.025 |
| Prior catheter ablation | 7.3 | 2.7 | <0.0001 |
| Atrial fibrillation | 19.5 | 21.5 | 0.0001 |
| Palliative care | 4.5 | 5.9 | <0.0001 |
| DNR | 6.8 | 10.0 | <0.0001 |
| ADPRG severity scale | |||
| 1 | 1.5 | 2.1 | |
| 2 | 34.4 | 26.9 | |
| 3 | 24.7 | 25.6 | |
| 4 | 39.4 | 45.4 | |
Values are percentage unless otherwise indicated. Estimated median household incomes are zip code-specific, updated annually and are classified into 4 quartiles indicating the poorest to wealthiest populations.
ADPRG = All-Payer Refined Diagnostic Related Group; AMI = acute myocardial infarction; CABG = coronary artery bypass grafting; CHF = congestive heart failure; DNR = do-not-resuscitate; MI = myocardial infarction; PCI = percutaneous coronary intervention.
Sex differences in in-hospital outcomes
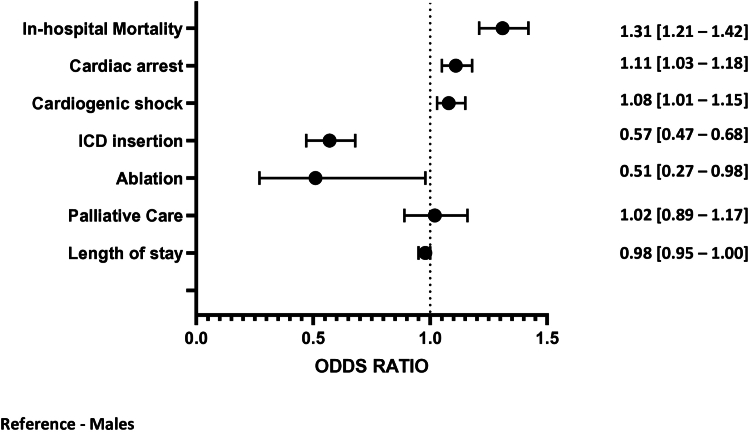
Compared with men, women were found to have a lower incidence of VAs following AMI (8.6% for women vs 12.6% for men; AOR: 0.67 [0.65-0.69]; P < 0.001). However, women had worse secondary outcomes with higher rates in hospital mortality (AOR: -1.31 [1.21-1.42] P < 0.0001), cardiogenic shock (AOR: 1.08 [1.01-1.15]; P = 0.022), and cardiac arrest (AOR: 1.11 [1.03-1.18]; P < 0.002) (Figure 2).
Figure 2.
Sex Differences in the In-Hospital Outcomes of Patients With AMI Complicated by Ventricular Arrhythmias
The Forest plot shows the in-hospital outcomes of ventricular arrhythmias in women compared to men, represented with adjusted ORs and their 95% CIs. AMI = acute myocardial infarction; ICD = implantable cardioverter-defibrillator.
Procedural outcomes
Women were also found to have lower rates of ICD insertion (AOR: 0.57 [0.47-0.68] P < 0.0001) and less likely to receive ablative therapies (AOR: 0.51 [0.27-0.98] P < 0.0001) following VAs, as compared to men (Figure 2). A subanalysis considering VT and VF patients separately found the disparity to apply to both types of VAs: (2.0% vs 2.9%), AOR 0.60 [0.49-0.74] P < 0.001 for VT and (1.7% vs 2.7%), AOR 0.56 [0.47-0.68] P < 0.001 for VF. Similarly, women were less likely to undergo catheter ablation during the index hospitalization as compared to men (AOR: 0.51; CI: 0.27-0.98; P < 0.001).
Subanalysis of outcomes in NSTEMI vs STEMI patients
Subgroup analysis on the sex differences in outcomes of STEMI patients revealed that women were less likely to experience VAs post-STEMI (AOR: 0.63 [0.60-0.66] P < 0.001), but they had worse in-hospital mortality and were less likely to receive an ICD (Table 2). Similar findings were also observed in the NSTEMI subgroup of patients as shown in (Table 3).
Table 2.
Sex Differences in the Outcomes of Patients With STEMI and Ventricular Arrhythmias
| Malesa | Females | OR (95% CI) | P Value | |
|---|---|---|---|---|
| In-hospital mortality | 12.5 | 17.9 | 1.24 (1.13-1.35) | <0.001 |
| Cardiogenic shock | 26.4 | 30.8 | 1.03 (0.95-1.11) | 0.435 |
| Cardiac arrest | 21.8 | 23.4 | 1.05 (0.97-1.13) | 0.209 |
| ICD insertion | 2.0 | 1.30 | 0.57 (0.43-0.72) | <0.001 |
| Ablation | 0.1 | 0.01 | 0.49 (0.18-1.33) | 0.165 |
In males represent “reference variable”.
Table 3.
Sex Differences in the Outcomes of Patients With NSTEMI and Ventricular Arrhythmias
| Malesa | Females | OR (95% CI) | P Value | |
|---|---|---|---|---|
| In-hospital mortality | 8.5 | 13.4 | 1.48 (1.27-1.74) | <0.001 |
| Cardiogenic shock | 14.7 | 17.7 | 1.15 (1.01-1.32) | 0.04 |
| Cardiac arrest | 13.3 | 17.3 | 1.28 (1.12-1.46) | <0.001 |
| ICD insertion | 4.9 | 3.1 | 0.61 (0.47-0.78) | <0.001 |
| Ablation | 0.5 | 0.3 | 0.57 (0.26-1.25) | 0.163 |
In males represent “reference variable”.
Discussion
Our study set out to explore the sex differences in the incidence and in-hospital outcomes of VAs following an AMI using a nationally representative sample of patients admitted for AMI in the United States. The salient findings of this study are that while women were less likely to have VAs following AMI, they suffered worse in-hospital outcomes in terms of in-hospital cardiogenic shock, cardiac arrest, and in-hospital mortality, and were less likely to receive procedural care such as ICD implantation or ablation therapy (Figure 2, Central Illustration).
Central Illustration.
Major Sex Differences in Demography and Clinical Outcomes of Patients With Acute Myocardial Infarction and VentricularArrhythmias
ICD = implantable cardioverter-defibrillator; MI = myocardial infarction.
VAs are known complications of AMI. However, to the best of our knowledge, this study represents the largest study to date to examine sex differences in the incidence and in-hospital outcomes of VAs in patients admitted with AMI. Consistent with the findings of other studies, our work confirms observations that women are less likely to experience VAs after AMI.17,18 While the reasons for sex differences in the risk of VAs post-AMI is not entirely understood, our study showed a higher rate of prior MI and ischemic heart disease (IHD) among men, which might result in a greater myocardial scar burden as a substrate for potential re-entrant circuits. Our findings are supported by reports from the landmark MADIT-CRT (Multicenter Automatic Defibrillator Implantation with Cardiac Resynchronization Therapy Trial), which also reported higher rates of IHD in men. They also noted that the probability of ICD shocks due to VT/VF among women was half that observed in men (HR: 0.51; P = 0.003).19 In addition to a greater burden of proarrhythmic substrate, other sex-dependent characteristics due to hormonal influences on ion channel expression and action potential may also help explain our findings.20, 21, 22, 23
Although women had a lower likelihood of VAs following AMI, women had higher odds of in-hospital mortality, cardiogenic shock, and cardiac arrest among those with VAs. These findings can be partly explained by the general comorbid profile of women with AMI, as they were older, had higher rates of congestive heart failure, diabetes mellitus and worse Charlson comorbidity index scores. However, this excess risk was observed even after accounting for these differences. In a similar study by Shih et al,24 as compared to men, women had higher mortality rates despite equivalent rates of VAs with primary drivers of these higher rates being older age, post-MI Heart Failure, and less frequent utilization of guideline-directed therapies. We noted persistence of these worse outcomes in women with VAs even after subdivision into AMI groups (STEMI and non-STEMI) (Tables 2 and 3).
In addition, women were less likely to receive ICDs or catheter ablation therapies during hospital admission. While ICD is recommended as a secondary prevention therapy for women as it is for men with ventricular tachyarrhythmias in the setting of AMI, there continue to be sex differences in the recruitment to ICD trials and implantation in women as previously demonstrated.18,25 Whether the difference in implantation is related to suggestions of higher complications of ICD implantation in women or a greater benefit in men, as suggested in some trials. The available data indicate an underrepresentation of women in these studies, with no definite sex differences in mortality and an equivocal benefit of ICD therapy postimplantation.26, 27, 28 Therefore, future efforts should be directed toward minimizing the sex disparities in ICD implantation in AMI patients with VAs.
This study should be interpreted in the context of some limitations. First, this is an observational and nonrandomized study, utilizing an administrative data set. Although we used a robust statistical model in the analysis, the effect of residual confounders cannot be fully excluded. Secondly, our clinical and procedural variables were defined by ICD-10-CM and PCS codes which are vulnerable to coding errors. Third, the data available to us lack the granularity necessary to determine the clinical severity on presentation, laboratory, and echocardiographic parameters. Similarly, lack of data on medications limited our assessment of sex disparities in treatment with guideline medical therapy. Finally, due to the lack of longitudinal data on individual patients, we cannot extrapolate our findings to the posthospitalization period.
Conclusions
In this large nationwide analysis of patients with AMI, we found a lower incidence of complicating VAs in women as compared to men. While the lower prevalence of previous IHD might partially explain this, further studies are needed to understand the potential contributions of other factors, such as endocrine and genetic factors to explain this sex difference in VAs following AMI. Despite having less VAs, women were found to have worse in-hospital outcomes (mortality, cardiac arrest, and cardiogenic shock) in the setting of AMI and VAs but were less likely to receive ICD implantation or ablation therapy. These findings highlight the importance of assessing for sex differences in cardiovascular disease to improve the care of both women and men. Further investigation is needed to determine what treatments can improve the outcomes in women.
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE: Women are less likely to develop VAs following an acute ischemic event. However, among persons who sustained VAs post-MI, women are more likely to have worse in-hospital outcomes and less likely to receive implantable cardiac defibrillator therapy.
TRANSLATION OUTLOOK: Additional real-world studies are needed to replicate the findings from this study. In addition, further research geared toward targeted interventions to address sex-specific risk factors associated with outcomes following MI and improve postarrhythmia outcomes among women are important next steps.
Funding support and author disclosures
Dr Kwaku has received consulting services to AltaThera, Anthos Therapeutics, Biosense Webster, and Janssen Scientific Affairs; travel support from Medtronic; and contracted research support from Boston Scientific. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors wish to thank the HCUP data partners List of HCUP Data Partners for Reference in Publications(ahrq.gov) who are able to create a national information resource of encounter level health care data. HCUP would not be possible without their contributions.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For a supplemental table, please see the online version of this paper.
Supplementary data
References
- 1.Bhar-Amato J. Ventricular arrhythmia after acute myocardial infarction: ‘the Perfect Storm’. 2017. https://www.aerjournal.com/articles/ventricular-arrhythmia-after-acute-myocardial-infarction-perfect-storm [DOI] [PMC free article] [PubMed]
- 2.Piccini J.P., Berger J.S., Brown D.L. Early sustained ventricular arrhythmias complicating acute myocardial infarction. Am J Med. 2008;121(9):797–804. doi: 10.1016/j.amjmed.2008.04.024. [DOI] [PubMed] [Google Scholar]
- 3.Masuda M., Nakatani D., Hikoso S., et al. Clinical impact of ventricular tachycardia and/or fibrillation during the acute phase of acute myocardial infarction on in-hospital and 5-year mortality rates in the percutaneous coronary intervention era. Circ J. 2016;80(7):1539–1547. doi: 10.1253/circj.CJ-16-0183. [DOI] [PubMed] [Google Scholar]
- 4.Thomas D.E., Jex N., Thornley A.R. Ventricular arrhythmias in acute coronary syndromes—mechanisms and management. Contin Cardiol Educ. 2017;3(1):22–29. doi: 10.1002/cce2.51. [DOI] [Google Scholar]
- 5.Hersi A.S., Alhabib K.F., AlFaleh H.F., et al. Incidence of ventricular arrhythmia and associated patient outcomes in hospitalized acute coronary syndrome patients in Saudi Arabia: findings from the Registry of the Saudi Project for Assessment of Acute Coronary Syndrome (SPACE) Ann Saudi Med. 2012;32(4):372–377. doi: 10.5144/0256-4947.2012.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Avezum Á., Piegas L.S., Goldberg R.J., et al. Magnitude and prognosis associated with ventricular arrhythmias in patients hospitalized with acute coronary syndromes (from the GRACE Registry) Am J Cardiol. 2008;102(12):1577–1582. doi: 10.1016/j.amjcard.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 7.Weizman O., Marijon E., Narayanan K., et al. Incidence, characteristics, and outcomes of ventricular fibrillation complicating acute myocardial infarction in women admitted alive in the hospital. J Am Heart Assoc. 2022;11(17) doi: 10.1161/JAHA.122.025959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.von Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–349. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 9.NIS database documentation. https://hcup-us.ahrq.gov/db/nation/nis/nisdbdocumentation.jsp
- 10.ICD-10. American College of Cardiology. https://www.acc.org/http%3a%2f%2fwww.acc.org%2ficd10
- 11.2023 ICD-10-CM | CMS. https://www.cms.gov/medicare/icd-10/2023-icd-10-cm
- 12.Markson F.E., Akuna E., Lim C.Y., Khemani L., Amanullah A. The impact of COVID-19 on hospitalization outcomes of patients with acute myocardial infarction in the USA. Am Heart J Plus. 2023;32 doi: 10.1016/j.ahjo.2023.100305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.HCUP-US NIS overview. https://hcup-us.ahrq.gov/nisoverview.jsp
- 14.Saxena S., Goldenberg I., McNitt S., et al. Sex differences in the risk of first and recurrent ventricular tachyarrhythmias among patients receiving an implantable cardioverter-defibrillator for primary prevention. JAMA Netw Open. 2022;5(6) doi: 10.1001/jamanetworkopen.2022.17153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tarantino N., Rocca D.G.D., Zou F., Lin A., Natale A., Biase L.D. Prevalence, outcomes, and management of ventricular arrhythmias in COVID-19 patients. Card Electrophysiol Clin. 2022;14(1):11–20. doi: 10.1016/j.ccep.2021.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matetic A., Shamkhani W., Rashid M., et al. Trends of sex differences in clinical outcomes after myocardial infarction in the United States. CJC Open. 2021;3(12, Supplement):S19–S27. doi: 10.1016/j.cjco.2021.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buxton A.E., Hafley G.E., Lehmann M.H., et al. Prediction of sustained ventricular tachycardia inducible by programmed stimulation in patients with coronary artery disease. Circulation. 1999;99(14):1843–1850. doi: 10.1161/01.CIR.99.14.1843. [DOI] [PubMed] [Google Scholar]
- 18.Zaman S., Deshmukh T., Aslam A., Martin C., Kovoor P. Sex differences in electrophysiology, ventricular tachyarrhythmia, cardiac arrest and sudden cardiac death following acute myocardial infarction. Heart Lung Circ. 2020;29(7):1025–1031. doi: 10.1016/j.hlc.2019.07.017. [DOI] [PubMed] [Google Scholar]
- 19.Tompkins C.M., Kutyifa V., Arshad A., et al. Sex differences in device therapies for ventricular arrhythmias or death in the multicenter automatic defibrillator implantation trial with cardiac resynchronization therapy (MADIT-CRT) trial. J Cardiovasc Electrophysiol. 2015;26(8):862–871. doi: 10.1111/jce.12701. [DOI] [PubMed] [Google Scholar]
- 20.Dogan M., Yiginer O., Uz O., et al. The effects of female sex hormones on ventricular premature beats and repolarization parameters in physiological menstrual cycle. Pacing Clin Electrophysiol. 2016;39(5):418–426. doi: 10.1111/pace.12821. [DOI] [PubMed] [Google Scholar]
- 21.Gillis A.M. Atrial fibrillation and ventricular arrhythmias. Circulation. 2017;135(6):593–608. doi: 10.1161/CIRCULATIONAHA.116.025312. [DOI] [PubMed] [Google Scholar]
- 22.Linde C., Bongiorni M.G., Birgersdotter-Green U., et al. Sex differences in cardiac arrhythmia: a consensus document of the European Heart Rhythm Association, endorsed by the Heart Rhythm Society and Asia Pacific Heart Rhythm Society. EP Europace. 2018;20(10) doi: 10.1093/europace/euy067. 1565-1565ao. [DOI] [PubMed] [Google Scholar]
- 23.Tadros R., Ton A.T., Fiset C., Nattel S. Sex differences in cardiac electrophysiology and clinical arrhythmias: epidemiology, therapeutics, and mechanisms. Can J Cardiol. 2014;30(7):783–792. doi: 10.1016/j.cjca.2014.03.032. [DOI] [PubMed] [Google Scholar]
- 24.Shih J.Y., Chen Z.C., Chang H.Y., Liu Y.W., Ho C.H., Chang W.T. Risks of age and sex on clinical outcomes post myocardial infarction. Int J Cardiol Heart Vasc. 2019;23 doi: 10.1016/j.ijcha.2019.100350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barra S., Providência R., Boveda S., et al. Device complications with addition of defibrillation to cardiac resynchronisation therapy for primary prevention. Heart. 2018;104(18):1529–1535. doi: 10.1136/heartjnl-2017-312546. [DOI] [PubMed] [Google Scholar]
- 26.Yoder M., Dils A., Chakrabarti A., et al. Gender and race-related disparities in the management of ventricular arrhythmias. Trends Cardiovasc Med. 2023 doi: 10.1016/j.tcm.2023.10.001. [DOI] [PubMed] [Google Scholar]
- 27.Dêbski M., Ulman M., Z¥Bek A., Haberka K., Lelakowski J., Ma£ecka B. Gender differences in dual-chamber pacemaker implantation indications and long-term outcomes. Acta Cardiol. 2016;71(1):41–45. doi: 10.1080/AC.71.1.3132096. [DOI] [PubMed] [Google Scholar]
- 28.Elango K., Curtis A.B. Cardiac implantable electrical devices in women. Clin Cardiol. 2018;41(2):232–238. doi: 10.1002/clc.22903. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.