Abstract
Background
Women with severe primary mitral regurgitation (MR) have lower surgery rates than men and could suffer from delayed referral for mitral valve (MV) intervention, exposing them to an increased risk of postoperative adverse outcomes.
Objectives
The purpose of this study was to assess the sex-based differences in patients with primary MR.
Methods
The study sample consisted of 420 patients (median age: 62 years, 26% women) with primary MR due to valve prolapse referred for preoperative assessment who underwent transthoracic echocardiography (TTE) and cardiac magnetic resonance (CMR) imaging. Multiple endpoints (abnormally increased left ventricular size, NYHA functional class III/IV, severe left atrial [LA] dilatation, pulmonary hypertension) were studied using areas under the curves and logistic regression models.
Results
Women were older than men, had higher NYHA functional class and larger indexed LA volumes (all P ≤ 0.031), despite displaying lower MR effective regurgitant orifice area, regurgitant volumes (RegVol), and ventricular volumes than men (all P ≤ 0.002). The optimal cut-off values of RegVol associated with abnormally increased left ventricular size according to reference normal values were lower in women (TTE: 67 ml, CMR: 50 ml) than in men (TTE: 77 ml, CMR: 65 ml). MR regurgitant fraction, but not RegVol, was associated in women and men with NYHA functional class III/IV, severe LA dilatation, and pulmonary hypertension (all areas under the curves, P ≤ 0.024).
Conclusions
Despite having hallmarks of more advanced valvular heart disease, women with significant primary MR demonstrate lower mitral RegVol and ventricular volumes than men. In contrast, the systematic calculation of MR regurgitant fraction could standardize MR quantification irrespective of sex.
Key words: mitral regurgitant fraction, mitral regurgitant volume, primary mitral regurgitation, sex differences, women
Central Illustration
Chronic significant mitral regurgitation (MR) causes left ventricular (LV) volume overload and dilatation, eventually progressing to LV dysfunction.1 Currently, the grading of MR severity is based primarily on absolute mitral regurgitant volume (RegVol) and effective regurgitant orifice area (EROA).2
However, normal LV and cardiac stroke volumes depend on body size and are influenced by gender and age.3,4 Also, the clinical and morphological cardiac response to a fixed RegVol varies between patients due to comorbidities, LV and left atrial (LA) function, and the timing and duration of the volume overload.5,6 Therefore, a given amount of MR RegVol is likely to impact LV remodeling and hemodynamics differently depending on these factors. Hence, the idea of a unique RegVol cut-off value for grading MR severity in every patient is debatable.7 Likewise, previous studies suggested that sex could influence the quantification of primary MR and the degree of cardiac remodeling in response to volume overload.8,9
Degenerative mitral valve (MV) disease is more common in women, but they have lower surgery rates than men and suffer from delayed referral for MV intervention.10,11 This leads to a worse clinical presentation and an increased risk of postoperative cardiovascular morbi-mortality.12, 13, 14 Understanding why women are likely to undergo MV surgery less and later than men and how to address this situation and ensure that both sexes receive equivalent care is of utmost importance. Indeed, the current recommended echocardiographic threshold values for MR quantitative parameters (EROA, RegVol) and LV remodeling in primary MR (LV end-systolic diameter [ESD]) are not sex-specific and have been based on epidemiological studies where women were underrepresented.10,15,16
Since the evidence regarding the influence of sex on primary MR quantification and cardiac remodeling in contemporary practice remains limited, we investigated whether there are sex-based differences in the clinical and imaging phenotypes of patients with significant primary MR managed in contemporary practice. We specifically aimed at making use of the advantages of cardiac magnetic resonance (CMR) imaging to precisely evaluate LV remodeling and hemodynamics.17 We thus evaluated relations between MR severity and cardiac remodeling using echocardiography and CMR in a large cohort of patients with significant primary MR due to valve prolapse referred to tertiary centers for MV intervention.
Methods
Study sample
This study involved patients with primary chronic primary MR due to prolapse referred for MV intervention in 3 heart valve centers (Brussels, Lille, and Monaco) between January 2005 and December 2022 who had undergone a comprehensive transthoracic echocardiography (TTE) and a CMR within 3 months. Inclusion criteria were patients at least 18 years of age with significant (moderate-to-severe or severe according to American Society of Echocardiography/European Association of Cardiovascular Imaging guidelines) primary MR who underwent MV intervention within 6 months after baseline CMR (99% of patients underwent MV surgery and 1% transcatheter mitral edge-to-edge repair).15,16 Exclusion criteria were: acute MR, MR from another etiology than prolapse, prior cardiac surgery, more than mild another left valvular heart disease; prosthetic valve or intracardiac shunt; pregnancy; standard contraindication for CMR; poor TTE image quality; refusal to participate in the study; and evidence of ischemic myocardial scar.
In each center, patients were prospectively invited to undergo CMR during hospitalization for preoperative work-up of primary MR. Doppler-echocardiography and CMR data were prospectively entered into an electronic database in each center and pooled retrospectively. Clinical data were obtained by chart review. The Logistic EuroSCORE II was calculated for all patients.18 Coronary artery disease was considered >50% epicardial stenosis. The present study had been approved by local institute independent ethics committees and was conducted in accordance with institutional policies, national legal requirements, and the revised Declaration of Helsinki. Authorization for research participation was obtained for all patients.
Echocardiography
All patients had comprehensive Doppler-echocardiographic exams performed using commercially available ultrasound systems by experienced echocardiographers and analyzed locally in each center by the different investigators according to European Association of Cardiovascular Imaging/American Society of Echocardiography guidelines.4 MR severity was graded according to a multiparametric approach, as recommended by guidelines, and MR-EROA and regurgitant volume (Echo-RegVol) values were computed by the proximal isovelocity surface area technique without correction for constraint.19 Details regarding the echocardiographic assessment of the MV are provided in the Supplemental Appendix.
Cardiac magnetic resonance imaging
Details regarding the CMR assessment are provided in the Supplemental Appendix. Briefly, CMR studies were performed on clinical scanners and analyzed locally by experienced operators blinded to the echocardiographic data of the patient. LV and right ventricular (RV) end-diastolic volume (EDV), end-systolic volume (ESV), and ejection fraction (EF) were assessed from consecutive short-axis cine steady-state free precession pulse images covering the entire LV from the mitral plane to the apex. The LV total stroke volume (LVTSV) was obtained by subtracting the LVESV from the LVEDV. Aortic stroke volume was derived from quantitative through-plane phase-contrast measurement performed at the level of the sino-tubular junction perpendicular to the aorta.20 Mitral regurgitant volume (CMR-RegVol) was calculated by subtracting the aortic stroke volume from the total LVTSV. Mitral regurgitant fraction (CMR-RegFrac) was defined as CMR-RegVol divided by the LVTSV, expressed as a percentage.
Statistical analysis
Multiple endpoints were used in the present study.
-
•
LV dilatation according to CMR-indLVEDV either as a continuous covariate or according to age and sex-stratified upper reference limit normal values from the CMR UK Biobank study (women <55 years: 101 ml/m2, 55-64 years: 94 ml/m2, ≥65 years: 96 ml/m2; men <55 years: 112 ml/m2, 55-64 years: 117 ml/m2, ≥65 years: 110 ml/m2).3
-
•
Severe LA dilatation is defined as Echo-indLAV ≥60 ml/m2
-
•
Pulmonary hypertension is defined as an RV peak pressure gradient of ≥50 mm Hg
-
•
Heart failure symptoms are defined as NYHA functional class III-IV.
These endpoints were used to compare between women and men with significant primary MR referred for preoperative assessment: 1/baseline differences in the clinical and imaging (Echo, CMR) characteristics 2/MR quantitative parameters (MR-EROA, Echo-RegVol, CMR-RegVol, and CMR-RegFrac).
Data were analyzed with R version 4.1.1 (R Foundation for Statistical Computing) and GraphPad Prism (GraphPad Software). Quantitative data are reported as median (25th-75th percentile), while qualitative data are presented as absolute numbers (percentages). Patients were stratified by sex. Associations between sex and baseline categorical variables were examined using either the Pearson chi-square statistic or Fisher’s exact test. Individual differences for continuous variables were compared using Mann-Whitney U tests. Multivariable logistic regression models were employed to assess the relationship between MR quantitative parameters, cardiac remodeling, and sex while controlling for age, body surface area (BSA), and NYHA functional class. Receiver operating characteristic (ROC) curves were used to evaluate the association according to sex between MR quantitative parameters and abnormally increased LV size, defined as a value of indLVEDV greater than age and sex-stratified upper reference limit normal values.3 Optimal threshold values of MR quantitative parameters associated with abnormally increased indLVEDV were determined separately for each sex by selecting the point on the ROC curve closest to the top-left corner. Linear regression analyses were performed to model the relationship between CMR-RegFrac and indLVEDV in women and men and compared by analysis of covariance. The relationship between MR-EROA, Echo-RegVol, CMR-RegVol, CMR-RegFrac, and NYHA functional class III/IV, Echo-indLAV ≥60 ml/m2, RV peak pressure gradient ≥50 mm Hg, stratified by sex, was assessed using areas under the curves and logistic regression models. All analyses considered a 2-tailed P value of <0.05 as statistically significant.
Results
Clinical and echocardiographic characteristics of patients with significant primary MR according to sex
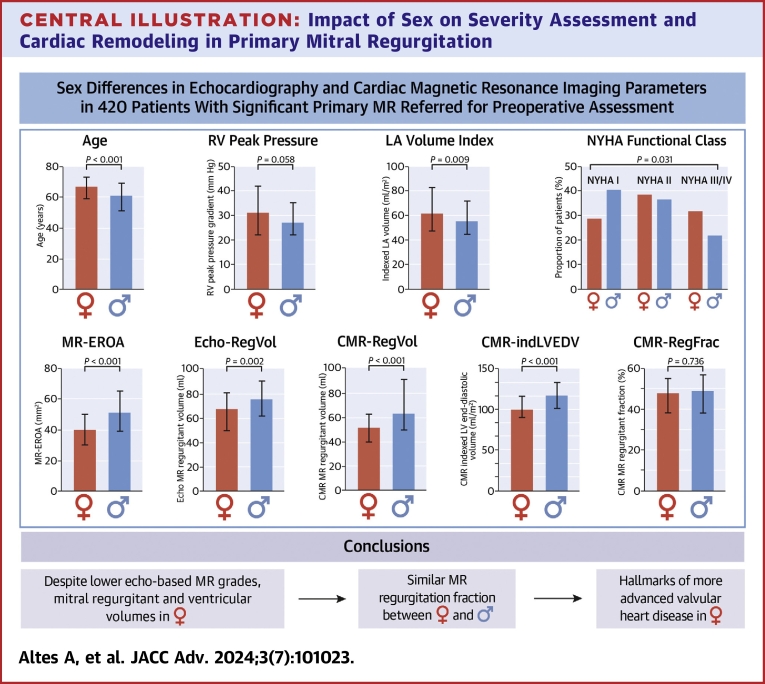
We first describe the differences in clinical and imaging characteristics between women and men with significant primary MR referred for preoperative assessment (Tables 1 and 2). Among the 420 patients included, 26% (n = 108) were women. Women were older than men (P < 0.001) and more symptomatic (NYHA functional class III-IV; 33% vs 22%, P = 0.031) (Central Illustration).
Table 1.
Baseline Clinical and Echocardiographic Characteristics of the Study Sample According to Sex
| All Patients (N = 420) | Women (n = 108, 26%) | Men (n = 312, 74%) | Overall P Value | |
|---|---|---|---|---|
| Clinical and biological parameters | ||||
| Age (y) | 62 (53-71) | 67 (59-73) | 61 (51-69) | <0.001 |
| Height (cm) | 175 (167-180) | 164 (158-168) | 178 (172-183) | <0.001 |
| Weight (kg) | 75 (66-84) | 62.5 (55-70) | 78 (72-86) | <0.001 |
| BSA (m2) | 1.91 (1.77-23) | 1.68 (1.58-1.78) | 1.96 (1.86-2.07) | <0.001 |
| BMI (kg/m2) | 24.6 (22.6-26.9) | 23.5 (20.7-27) | 24.8 (23.1-26.9) | 0.001 |
| Hypertension | 148 (35%) | 36 (33%) | 112 (36%) | 0.716 |
| Dyslipidemia | 103 (24%) | 25 (23%) | 78 (25%) | 0.798 |
| Coronary artery disease | 58 (14%) | 6 (6%) | 52 (17%) | 0.006 |
| EuroSCORE II (%) | 1.04 (0.70-1.86) | 1.52 (0.99-2.48) | 0.96 (0.67-1.56) | <0.001 |
| History of atrial fibrillation | 87 (21%) | 21 (19%) | 66 (21%) | 0.810 |
| NYHA functional class | 0.031 | |||
| I | 159 (38%) | 31 (29%) | 128 (41%) | |
| II | 158 (38%) | 42 (39%) | 116 (37%) | |
| III-IV | 103 (24%) | 35 (32%) | 68 (22%) | |
| Serum creatinine level (mg/dl) | 0.92 (0.83-1.06) | 0.81 (0.72-0.91) | 0.96 (0.87-1.10) | <0.001 |
| eGFR (ml/m2) | 83 (63-105) | 63 (52-85) | 88 (69-109) | <0.001 |
| Echocardiographic parameters | ||||
| LVEDD (mm) | 59 (55-62) | 55 (51-60) | 59 (56-63) | <0.001 |
| LVESD (mm) | 35 (31-40) | 33 (29-38) | 36 (32-40) | <0.001 |
| indLVEDD (mm/m2) | 31 (29-33) | 33 (29.5-36) | 30 (28-33) | <0.001 |
| indLVESD (mm/m2) | 19 (16-21) | 19 (17-22) | 18 (16-21) | 0.006 |
| Echo-LVEDV (ml) | 190 (160-219) | 146 (122-172) | 200 (179-226) | <0.001 |
| Echo-LVESV (ml) | 64 (49-81) | 46.0 (37-59) | 71.5 (57-85) | <0.001 |
| Echo-indLVEDV (ml/m2) | 99 (86-110) | 88 (76-100) | 103 (90-114) | <0.001 |
| Echo-indLVESV (ml/m2) | 34 (27-41) | 28 (22-34) | 36 (29-42) | <0.001 |
| Echo-LVEF (%) | 65 (61-71) | 68 (63-72) | 64 (60-70) | 0.001 |
| indLAV (ml/m2) | 56 (45-73) | 61 (47-82) | 55 (44-71) | 0.009 |
| RV peak pressure gradient (mm Hg) (n = 360) | 27 (22-37) | 31 (22-42) | 27 (22-35) | 0.058 |
| MR EROA (mm2) | 47 (35-62) | 40 (30-50) | 51 (39-65) | <0.001 |
| Echo-RegVol (ml) | 74 (58-89) | 68 (50-81) | 76 (62-91) | 0.002 |
| MR prolapse localization | 0.041 | |||
| Anterior | 30 (7%) | 8 (7%) | 22 (7%) | |
| Posterior | 294 (70%) | 66 (61%) | 228 (73%) | |
| Bi-leaflet | 96 (23%) | 34 (32%) | 62 (20%) | |
| Flail leaflet | 173 (41%) | 39 (36%) | 134 (43%) | 0.258 |
| Mitral annulus disjunction | 102 (24%) | 30 (27%) | 73 (23%) | 0.554 |
Values are median (IQR) or n (%). P values in bold are below the 0.05 threshold.
BMI = body mass index; BSA = body surface area; EDD = end-diastolic diameter; EDV = end-diastolic volume; EF = ejection fraction; eGFR = estimated glomerular filtration rate; EROA = effective regurgitant orifice area; ESD = end-systolic diameter; ESV = end-systolic volume; LAV = left atrial volume; LV = left ventricular; LVEF = left ventricular ejection fraction; MR = mitral regurgitant; RegVol = regurgitant volume; RV = right ventricular.
Table 2.
Baseline CMR Characteristics of the Study Sample According to Sex
| All Patients (N = 420) | Women (n = 108, 26%) | Men (n = 312, 74%) | Overall P Value | |
|---|---|---|---|---|
| CMR-LVEDV (ml) | 214 (180-255) | 173 (143-198) | 229 (196-264) | <0.001 |
| CMR-LVESV (ml) | 77 (59-99) | 57 (42-73) | 84 (64-103) | <0.001 |
| CMR-indLVEDV (ml/m2) | 113 (96-130) | 100 (90-116) | 118 (101-133) | <0.001 |
| CMR-indLVESV (ml/m2) | 40 (32-51) | 35 (25-43) | 42 (33-52) | <0.001 |
| LV mass (g) | 148 (125-172) | 114 (99-131) | 159 (140-181) | <0.001 |
| indLV mass (g/m2) | 78 (67-87) | 67 (58-77) | 81 (71-92) | <0.001 |
| CMR-LVEF (%) | 64 (58-70) | 67 (61-72) | 63 (57-68) | <0.001 |
| LV sphericity index | 0.61 (0.56-0.66) | 0.64 (0.59-0.71) | 0.60 (0.55-0.64) | <0.001 |
| LV fibrosis, (n = 358) | 61 (17%) | 16 (17%) | 45 (17%) | 1.00 |
| Aortic forward volume (ml) | 70 (57-84) | 57 (46-73) | 74 (62-87) | <0.001 |
| Ind-Aortic forward volume (ml/m2) | 37.5 (30-44) | 35 (27-42) | 38 (32-44) | 0.010 |
| CMR-RegVol (ml) | 60 (46-85) | 52 (40-63) | 64 (50-92) | <0.001 |
| indCMR-RegVol (ml/m2) | 32 (24-45) | 30 (23-39) | 33 (25-46) | 0.030 |
| CMR-RegFrac (%) | 48 (38-57) | 48 (38-55) | 49 (38-57) | 0.736 |
| RVEDV (ml) | 142 (117-170) | 114 (92-139) | 152 (130-177) | <0.001 |
| RVESV (ml) | 66 (52-88) | 52 (35-66) | 73 (58-94) | <0.001 |
| indRVEDV (ml/m2) | 76 (64-89) | 69 (57-81) | 78 (66-90) | <0.001 |
| indRVESV (ml/m2) | 35 (28-45) | 31 (22-40) | 37 (29-46) | <0.001 |
| RVEF (%) | 52 (47-58) | 54 (47-62) | 52 (46-57) | 0.011 |
Values are median (IQR) or n (%). P values in bold are below the 0.05 threshold.
CMR = cardiac magnetic resonance imaging; ind = indexed to body surface area; RegFrac = regurgitant fraction; other abbreviations as in Table 1.
Central Illustration.
Impact of Sex on Severity Assessment and Cardiac Remodeling in Primary Mitral Regurgitation
For continuous variables, IQRs are displayed in bar plots. Differences for age, RV peak pressure, indLAV, and NYHA class are displayed above, while those for MR EROA, Echo-RegVol, CMR-RegVol, CMR-indLVEDV, and CMR-RegFrac are displayed below. BSA = body surface area; EDV = end-diastolic volume; EROA = effective regurgitant orifice area; ind = indexed to BSA; LAV = left atrial volume; LV = left ventricular; RegFrac = regurgitant fraction; RegVol = regurgitant volume; RV = right ventricular.
Women had more bileaflet prolapse than men (32% vs 20%, P = 0.041). The proportion of flail leaflet was similar between women and men (P = 0.258). MR-EROA (40 [30-50.5] mm2 vs 51 [39-65] mm2, P < 0.001) and Echo-RegVol (68 [50-81] mL vs 76 [62-91] mL, P = 0.002) were lower in women than in men (Central Illustration). Accordingly, women had more often moderate-to-severe (defined as MR-EROA <40 mm2 and Echo-RegVol <60 mL) rather than severe MR (25% vs 13%, P = 0.007) (Figure 1). Yet women had a larger indLAV than men (61 [47-84] mL/m2 vs 55 [43-70] mL/m2, P = 0.009).
Figure 1.
Relationship Between MR Severity, LV Dysfunction, and LA Dilatation Stratified by Sex in Primary MR
Bar graphs show the proportion of women and men with primary MR regarding MR severity grading (moderate-to-severe or severe), LV dysfunction (LVEF <60%, LVESD ≥40 mm) and severe LA dilatation (indLAV ≥60 ml/m2). EF = ejection fraction; ESD = end-systolic diameter; LA = left atrial; LAV = left atrial volume; LV = left ventricular; MR = mitral regurgitant/regurgitation.
Absolute LV diameters were smaller, while indexed LV diameters were larger in women than in men (Table 1). However, both absolute and indexed LV volumes were lower in women (P < 0.001 for all). Median LVEF values were higher for women compared to men (P = 0.001), but the proportion of patients with LVEF <60% was similar in women and men (16% vs 21%, P = 0.332). Women less commonly presented with absolute LVESD ≥40 mm (18% vs 31%, P = 0.012) but more frequently had severe LA dilatation than men (55% vs 40%, P = 0.013) (Figure 1). After adjusting for age, BSA, and symptoms, the differences in MR-EROA, Echo-RegVol, and indexed LV volumes persisted between women and men (Figure 2A).
Figure 2.
Relationship Between MR Quantitative Parameters, Cardiac Remodeling, and Sex
Relationship between (A) echocardiographic and (B) CMR MR quantitative parameters (MR-EROA, Echo-RegVol, CMR-RegVol) or cardiac remodeling (LVESD, indLVESD, indLVEDV, indLVESV, indRVEDV, LVSI) or function (LVEF, RVEF) characteristics and sex, while controlling for age, BSA, and NYHA functional class. Multivariable logistic regression models were used for each imaging characteristic tested, adjusting for age, BSA, and NYHA functional class. In each analysis conducted, the dependent variable was being male or being female, while the independent covariables included age, BSA, NYHA functional class, and each of the imaging characteristics sequentially. Adjusted ORs were calculated for a 1-SD increase in each imaging characteristic. The forest plots depict the adjusted OR (represented by circles) along with their corresponding 95% CIs (horizontal line). The vertical dashed line at an OR of 1.00 indicates no relationship between the analyzed imaging characteristic and sex after adjusting for age, BSA, and NYHA functional class. Adjusted OR and their 95% CI on the left side of the vertical line suggest imaging characteristics are more likely to be associated with primary MR male patients with increased values. Conversely, adjusted OR and their 95% CI on the right side of the vertical line suggest imaging characteristics are more likely to be associated with primary MR female patients with increased values. BSA = body surface area; CMR = cardiac magnetic resonance imaging; EDV = end-diastolic volume; EF = ejection fraction; EROA = effective regurgitant orifice area; ESD = end-systolic diameter; ESV = end-systolic volume; ind = indexed to BSA; LAV = left atrial volume; LV = left ventricular; MR = mitral regurgitant; RegFrac = regurgitant fraction; RegVol = regurgitant volume; RV = right ventricular; SI = sphericity index.
CMR characteristics of patients with significant primary MR according to sex
CMR-assessed LV volumes were lower in women than in men (all P < 0.001) (Table 2). Women had a lower median CMR-RegVol than men (52 [40-64] mL vs 64 [50-92] mL, P < 0.001), and this difference persisted when indexing for BSA. In contrast, CMR-RegFrac values were similar between sexes (P = 0.736) (Central Illustration). The proportion of patients with CMR-RegFrac ≥40% was similar between sexes (71% vs 70%, P = 1.00). LV shape was more spherical in women than in men (LV sphericity index: 0.64 [0.59-0.71] vs 0.60 [0.55-0.64], P < 0.001). After adjusting for age, BSA, and symptoms, the differences in CMR-RegVol, indexed LV, and RV volumes persisted between women and men (Figure 2B).
After excluding patients with coronary artery disease, similar differences in the phenotypic presentation of women and men with primary MR were found (Supplemental Table 1). Excluding patients with atrial fibrillation at the time of echocardiography and CMR (n = 27, 6%) yielded similar results (Supplemental Table 2).
Relationship between MR quantitative parameters and abnormal LV remodeling according to sex
We examined the relationship between MR quantitative parameters (MR-EROA, Echo-RegVol, and CMR-RegVol) and LV dilatation based on indLVEDV. Figure 3 shows ROC curves evaluating the relationship between MR-EROA, Echo-RegVol, and CMR-RegVol and abnormal LV remodeling (indLVEDV greater than age and sex-stratified upper reference limit normal values). Optimal thresholds of MR-EROA (women: 40 mm2, men: 53 mm2), Echo-RegVol (women: 67 ml, men: 77 ml), and CMR-RegVol (women: 50 ml, men: 65 ml) associated with abnormally increased indLVEDV were consistently lower in women than in men.
Figure 3.
Relationship Between MR Quantitative Parameters and Abnormally Increased Indexed LV End-Diastolic Volume Stratified by Sex
ROC curves display the relationship stratified by sex (A, women; B, men) between MR-EROA, Echo-RegVol, CMR-RegVol, and abnormally increased LV size (defined as values of CMR-assessed indLVEDV >95% upper limit age- and sex-stratified normal reference values). CMR = cardiac magnetic resonance imaging; EROA = effective regurgitant orifice area; LV = left ventricular; MR = mitral regurgitant; RegVol = regurgitant volume; ROC = receiver operating characteristic.
Figure 4 shows the relationship between CMR-RegFrac and indLVEDV stratified by sex. The slope of the regression lines for women and men were similar (P for interaction = 0.613), but the intercept was smaller in women (P for intercept <0.001), showing that for a similar RegFrac value, women had smaller indLVEDV than men.
Figure 4.
Relationship Between MR Regurgitant Fraction and Indexed LV End-Diastolic Volume (indLVEDV) Stratified by Sex
Dashed red and blue lines display the upper limit reference values of indLVEDV in women (101 mL/m2) and men (117 mL/m2), respectively. LV = left ventricular; MR = mitral regurgitant.
Relationship between MR quantitative parameters, symptoms, LA remodeling, and pulmonary pressures according to sex
We assessed the relationship between MR quantitative parameters (MR-EROA, Echo-RegVol, CMR-RegVol, and CMR-RegFrac) and known features of postoperative adverse outcome in primary MR: NYHA functional class III-IV, severe LA dilatation, and pulmonary hypertension. Table 3 summarizes the ROC analysis of MR quantitative parameters associated with NYHA functional class III/IV, indLAV ≥60 ml/m2, and RV peak pressure gradient ≥50 mm Hg. Unlike MR-EROA, Echo-RegVol, or CMR-RegVol, CMR-RegFrac was consistently associated with NYHA functional class III/IV, indLAV ≥60 ml/m2, and RV peak pressure gradient ≥50 mm Hg in both sexes. The optimal thresholds of CMR-RegVol associated with indLAV ≥60 ml/m2 and RV peak pressure gradient ≥50 mm Hg were consistently lower in women than in men. By opposition, those of CMR-RegFrac were similar between women and men.
Table 3.
Comparison of Area Under the Curve and Receiver Operator Curves, Optimal Cutoff, and Diagnostic Accuracies of Different MR Quantitative Parameters Associated With NYHA Functional Class III-IV, LA Dilatation, or Pulmonary Hypertension
| Echo-EROA (mm2) |
Echo-RegVol (ml) |
CMR-RegVol (ml) |
CMR-RegFrac (%) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Women | Men | Women | Men | Women | Men | Women | Men | ||
| NYHA functionalclass III-IV | AUC(95% CI)P value | 0.63 (0.51-0.76) P = 0.016 | 0.58 (0.50-0.66) P = 0.032 | 0.56 (0.44-0.68) P = 0.169 | 0.53 (0.45-0.62) P = 0.205 | 0.60 (0.48-0.71) P = 0.051 | 0.54 (0.46-0.62) P = 0.136 | 0.65 (0.54-0.76) P = 0.006 | 0.58 (0.50-0.66) P = 0.024 |
| Optimal cutoff value | 39 | 49 | NA | NA | NA | NA | 49 | 50 | |
| Sensitivity/specificity | 72/62 | 63/50 | NA | NA | NA | NA | 66/68 | 62/63 | |
| indLAV ≥60 ml/m2 | AUC(95% CI)P value | 0.52 (0.40-0.64) P = 0.355 | 0.61 (0.54-0.68) P < 0.001 | 0.57 (0.46-0.69) P = 0.110 | 0.64 (0.58-0.71) P < 0.001 | 0.66 (0.55-0.77, P = 0.003 | 0.67 (0.60-0.73) P < 0.001 | 0.65 (0.54-0.76) P = 0.005 | 0.64 (0.57-0.70) P < 0.001 |
| Optimal cutoff value | NA | 50 | NA | 74 | 50 | 67 | 47 | 50 | |
| Sensitivity/specificity | NA | 63/59 | NA | 67/57 | 73/61 | 63/66 | 64/61 | 60/63 | |
| RV peak pressure gradient ≥50 mm Hg | AUC(95% CI)P value | 0.61 (0.44-0.78) P = 0.138 | 0.57 (0.43-0.71) P = 0.151 | 0.56 (0.41-0.70) P = 0.274 | 0.60 (0.48-0.72) P = 0.063 | 0.64 (0.51-0.77) P = 0.062 | 0.63 (0.50-0.76) P = 0.023 | 0.75 (0.62-0.89) P = 0.003 | 0.69 (0.57-0.80) P = 0.002 |
| Optimal cutoff value | NA | NA | NA | NA | NA | 70 | 54 | 53 | |
| Sensitivity/specificity | NA | NA | NA | NA | NA | 64/59 | 73/76 | 68/68 | |
Table 4 shows the age-adjusted OR for MR-EROA, Echo-RegVol, CMR-RegVol, and CMR-RegFrac associated with NYHA functional class III/IV, indLAV ≥60 ml/m2, and RV peak pressure gradient ≥50 mm Hg, stratified by sex. Only CMR-RegFrac was consistently associated with each of these hallmarks of adverse outcomes in primary MR in both sexes.
Table 4.
Age-Adjusted OR (Per 1-SD Increase) Associated With NYHA Functional Class III-IV, Severe Left Atrial Dilatation, or Pulmonary Hypertension
| NYHA Functional Class III-IV |
LA Volume ≥60 ml/m2 |
RV Peak Pressure Gradient ≥50 mm Hg |
||||
|---|---|---|---|---|---|---|
| Women | Men | Women | Men | Women | Men | |
| MR-EROA | 1.68 (1.05-2.70) | 1.45 (1.09-1.92) | 1.20 (0.77-1.87) | 1.59 (1.22-2.05) | 1.40 (0.60-3.25) | 1.60 (0.96-2.66) |
| Echo-RegVol | 1.17 (0.77-1.79) | 1.23 (0.94-1.60) | 1.28 (0.84-1.94) | 1.70 (1.30-2.22) | 1.31 (0.59-2.88) | 1.60 (1.01-2.53) |
| CMR-RegVol | 1.35 (0.89-2.05) | 1.31 (1.00-1.71) | 1.91 (1.20-3.02) | 2.00 (1.53-2.61) | 1.78 (0.87-3.65) | 2.45 (1.52-3.97) |
| CMR-RegFrac | 1.61 (1.05-2.50) | 1.31 (1.00-1.72) | 1.73 (1.13-2.68) | 1.66 (1.29-2.14) | 2.66 (1.15-6.18) | 2.10 (1.28-3.40) |
Discussion
In this large contemporary cohort of patients with significant primary MR due to valve prolapse referred to tertiary centers for preoperative assessment, we demonstrated significant sex-related differences in the clinical and imaging phenotypes of MR using echocardiography and CMR. The salient findings of our study were:
1) Despite having hallmarks of more advanced valve disease including more severe symptoms and larger indLAV, women had lower mitral RegVol and ventricular volumes than men, even after adjusting for confounders such as age or BSA (Central Illustration).
2) The optimal cut-off values of EROA and RegVol associated with abnormally increased LV size according to age and sex-stratified normal reference values were consistently lower in women than in men. This suggests that using a single EROA or RegVol cut-off value for grading MR severity in all patients, regardless of sex, could underestimate the impact of the valvular load on LV remodeling in women.
3) In contrast to CMR-RegVol, similar values of CMR-RegFrac were found between women and men. Furthermore, CMR-RegFrac, but not MR-EROA, Echo-RegVol, or CMR-RegVol, was consistently associated with NYHA functional class III/IV, severe LA dilatation, or pulmonary hypertension in women and men, even accounting for age and with similar optimal threshold values. These findings support the routine use of CMR-RegFrac to normalize RegVol to LV total stroke volume and reconcile, at least in part, the differences in MR quantification and its consequences on adverse cardiac remodeling between women and men.
Clinical implications of the differences in MR quantification between women and men
Our research extends upon prior studies that have suggested that women with significant primary MR experience delays in diagnosis and initial specialist assessment, resulting in a worse postoperative outcome compared to men.13,14 Indeed, despite being older at presentation, reporting more symptoms, and having larger indexed left atrial volumes, women in our study had lower mitral EROA, RegVol, and ventricular volumes than men, resulting in MR being classified more often as “moderate-to-severe” rather than severe, consistent with the previous study by Enriquez-Sarano et al,21 where only 18% of patients with an MR EROA >40 mm2 were women. Likewise, another study from the same group demonstrated that long-term excess mortality could appear since the “moderate” range of MR grading severity.22 Similar to previous findings, women in our cohort presented with more bi-leaflet prolapse.13 Previous studies showed that the severity of MR could be underestimated by MR-EROA or Echo-RegVol compared with CMR in bi-leaflet prolapse.23,24 Interestingly, we found that the CMR-RegVol assessed by the volumetric method, valid in patients with bi-leaflet prolapse, was still lower in women. Uretsky et al showed that patients with CMR-RegVol values within the “intermediate” range (30-60 mL) could exhibit true features of severe MV disease.25 In practice, CMR-RegVol values below the 60 ml threshold retained by guidelines are frequently found for women despite clear resolution of symptoms after MV surgery. Therefore, it is likely that many women with MR initially graded as “moderate-to-severe” actually have true severe MR, since their EROA and RegVol values can be lower than those of men despite similar or even higher degrees of symptoms or adverse cardiac consequences.
Clinical implications of the differences in LV remodeling between women and men in primary MR
Both American and European guidelines indicate that patients with asymptomatic, significant primary MR and LV dysfunction should be promptly referred for MV surgery.15,16 However, LV dimensions are influenced by body size and sex.3,4 In our study, women had a lower prevalence of LVESD ≥40 mm compared to men, consistent with the original Mitral Regurgitation International Database study.26 Indexing LV diameters to BSA has been suggested to correct for the discrepancies in LV remodeling according to sex in primary MR.8 However, in our cohort, even after indexing for BSA, women exhibited lower smaller LV volumes than men, while this finding was not captured by the sole assessment of indexed LV diameters. The differences in LV size between women and men with primary MR extend beyond body size and reflect physiological variations. Women could present with more restrictive physiology, particularly at older ages, resulting in a milder degree of RegVol and LV dilatation before symptom onset. On the other hand, the LA volume is a strong predictor of outcome in primary MR, which should be particularly considered in women who are likely to present with markedly enlarged LA despite normal or mildly enlarged LV.27
The importance of assessing MR regurgitant fraction
We observed that the optimal cut-off values of EROA and RegVol associated with abnormally increased age- and sex-stratified indLVEDV were consistently lower in women than in men. This underscores the risk of underestimating MR severity in women when relying solely on RegVol, as they tend to have smaller LV cardiac volumes. It is not surprising that the strength of this association was better with CMR-RegVol than MR-EROA or Echo-RegVol, as both of the latter measures are poorly related to LV size.24 Also, our data indicate that merely correcting for body size could not sufficiently account for the differences between women and men when quantifying MR. Indeed, women demonstrated a slight but significant lower indexed CMR-RegVol compared to men, while no difference was observed for CMR-RegFrac. From a hemodynamic perspective, it is more accurate to relate the RegVol to LV rather than body size. Indeed, a fixed mitral RegVol for a given LV volume in 1 patient could not have the same clinical implications compared with another one with the same RegVol but a smaller or larger LV, irrespective of body size. Consequently, the mitral RegFrac, which accounts for LV size rather than BSA, could be more reliable to grade MR severity.9 In our study, CMR-RegFrac was the sole MR quantitative parameter consistently associated with NYHA functional class III/IV, significant LA dilatation, and pulmonary hypertension, even accounting for age, and with similar optimal threshold values between women and men. These findings underscore the importance of assessing RegFrac to standardize MR quantification, irrespective of sex.
Study limitations
Although the clinical, echocardiographic, and CMR data were prospectively collected in each of the centers, the present analysis is of a retrospective nature and is thus subject to inherent limitations related to such design. We focused on a homogeneous sample of patients with chronic primary MR due to prolapse; therefore, we could not assess the differences between women and men in other MR etiologies. We included patients with at least moderate-to-severe primary MR undergoing TTE and clinically indicated CMR; therefore, we could not assess the potential differences between women and men presenting with milder grades of MR. Yang et al recently showed that women with less than moderate primary MR may exhibit early LA and LV remodeling.28 This finding reinforces the hypothesis that current thresholds for MR quantitative parameters underestimate the severity of MR in women. CMR and TTE studies were not performed simultaneously for the whole study sample. However, we believe that this point did not impact our findings, since our objective was not to compare the diagnostic value of TTE vs CMR stratified by sex but rather to examine the differences between women and men with primary MR using complementary imaging modalities. Late gadolinium enhancement analysis by CMR was not performed in all patients. Myocardial T1 mapping and extracellular volume quantification emerged in last years as promising risk markers in primary MR but were not available for the vast majority of this study sample.29 This study involves a Caucasian sample of patients with primary MR. Further research is required to specifically assess the differences in phenotypes between women and men with primary MR from other ethnicities such as Afro-Caribbean or Asian. Unlike CMR-RegFrac, RegFrac assessed by 2D-echocardiography was not prospectively and systematically assessed in all patients. The vast majority of patients (94%) were in sinus rhythm at the time of assessment; hence, our findings cannot apply to those with atrial fibrillation during examination since the volumetric quantification of MR in patients with arrythmia can be cumbersome and require averaging multiple beats. Finally, due to the study’s design, we were unable to conduct survival analyses upstream of the intervention. We believe that our findings would pave the way for investigating whether absolute MR quantitative parameters (MR-EROA, Echo-RegVol, or CMR-RegVol) in women might necessitate lower threshold values than those in men for prognostic considerations. Additionally, we acknowledge the importance of investigating whether using a uniform threshold value for CMR-RegFrac irrespective of sex would yield similar clinical outcomes between women and men both before and after MV intervention.
Conclusions
Despite the clear hallmarks of more advanced valvular heart disease, women with significant primary MR due to valve prolapse demonstrate lower MR volumes and ventricular volumes compared to men. Our results support the finding that women face a risk of delayed referral for MV intervention due to the underestimation of both MR severity and its impact on cardiac remodeling when relying solely on LV size assessment. Accounting for LV size and calculating the MR fraction could help address these sex-related differences and improve MR assessment accuracy in women. These findings provide valuable insights for further research to establish sex-specific criteria for quantitative MR assessment and optimal timing for intervention.
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE: In primary MR patients, women face a risk of delayed referral for MV intervention due to the underestimation of both MR severity and its impact on cardiac remodeling when relying solely on LV size assessment. Herein, we describe the sex-based differences in the clinical and imaging phenotypes assessed by echocardiography and CMR of patients with significant primary MR. Despite the clear hallmarks of more advanced valvular heart disease, women with primary MR demonstrate lower mitral regurgitant (RegVol) and ventricular volumes compared to men. Hence, the use of a unique cut-off of MR RegVol to grade MR severity in every patient with primary MR is not warranted, especially in women. In contrast, our findings highlight the importance of standardizing mitral RegVol to LV size by RegFrac calculation in daily practice.
TRANSLATIONAL OUTLOOK: Further studies are warranted to establish sex-specific criteria for quantitative MR assessment and optimal timing for intervention to ensure that both sexes receive equivalent care. Also, further studies should focus on the potential differences according to sex in other risk markers in primary MR, such as blood brain natriuretic peptide, LV and LA longitudinal strain, or T1-mapping.
Funding support and author disclosures
This study was funded by FRSM CDR-35275164 of the Belgian Fondation de la Recherche Scientifique. Dr Gerber has received a consulting fee from BMS. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental methods and tables, please see the online version of this paper.
Supplementary data
References
- 1.Gaasch W.H., Meyer T.E. Left ventricular response to mitral regurgitation: implications for management. Circulation. 2008;118:2298–2303. doi: 10.1161/CIRCULATIONAHA.107.755942. [DOI] [PubMed] [Google Scholar]
- 2.Altes A., Vermes E., Levy F., et al. Quantification of primary mitral regurgitation by echocardiography: a practical appraisal. Front Cardiovasc Med. 2023;10 doi: 10.3389/fcvm.2023.1107724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petersen S.E., Aung N., Sanghvi M.M., et al. Reference ranges for cardiac structure and function using cardiovascular magnetic resonance (CMR) in Caucasians from the UK Biobank population cohort. J Cardiovasc Magn Reson. 2017;19:18. doi: 10.1186/s12968-017-0327-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lang R.M., Badano L.P., Mor-Avi V., et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Carabello B.A. The current therapy for mitral regurgitation. J Am Coll Cardiol. 2008;52:319–326. doi: 10.1016/j.jacc.2008.02.084. [DOI] [PubMed] [Google Scholar]
- 6.Uretsky S., Biederman R.W.W., Han Y., et al. Symptoms, outcomes, and regurgitant severity in Guideline-Directed mitral valve surgery: a multicenter prospective study. JACC Cardiovasc Imaging. 2023;16(11):1491–1493. doi: 10.1016/j.jcmg.2023.05.006. [DOI] [PubMed] [Google Scholar]
- 7.Delling F.N., Noseworthy P.A., Adams D.H., et al. Research Opportunities in the Treatment of mitral valve prolapse: JACC Expert Panel. J Am Coll Cardiol. 2022;80:2331–2347. doi: 10.1016/j.jacc.2022.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mantovani F., Clavel M.A., Michelena H.I., Suri R.M., Schaff H.V., Enriquez-Sarano M. Comprehensive imaging in women with Organic mitral regurgitation: implications for clinical outcome. JACC Cardiovasc Imaging. 2016;9:388–396. doi: 10.1016/j.jcmg.2016.02.017. [DOI] [PubMed] [Google Scholar]
- 9.House C.M., Xi M., Moriarty K.A., Nelson W.B. Gender differences in primary mitral regurgitant volumes at specific regurgitant fractions as assessed by magnetic resonance imaging. Int J Cardiovasc Imaging. 2022;38(3):663–671. doi: 10.1007/s10554-021-02449-z. [DOI] [PubMed] [Google Scholar]
- 10.DesJardin J.T., Chikwe J., Hahn R.T., Hung J.W., Delling F.N. Sex differences and Similarities in valvular heart disease. Circ Res. 2022;130:455–473. doi: 10.1161/CIRCRESAHA.121.319914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coffey S., Roberts-Thomson R., Brown A., et al. Global epidemiology of valvular heart disease. Nat Rev Cardiol. 2021;18:853–864. doi: 10.1038/s41569-021-00570-z. [DOI] [PubMed] [Google Scholar]
- 12.Hahn R.T., Clavel M.A., Mascherbauer J., Mick S.L., Asgar A.W., Douglas P.S. Sex-related factors in valvular heart disease: JACC focus Seminar 5/7. J Am Coll Cardiol. 2022;79:1506–1518. doi: 10.1016/j.jacc.2021.08.081. [DOI] [PubMed] [Google Scholar]
- 13.Avierinos J.F., Inamo J., Grigioni F., Gersh B., Shub C., Enriquez-Sarano M. Sex differences in morphology and outcomes of mitral valve prolapse. Ann Intern Med. 2008;149:787–795. doi: 10.7326/0003-4819-149-11-200812020-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu K., Ye Q., Zhao Y., Zhao C., Song L., Wang J. Sex differences in the outcomes of Degenerative mitral valve Repair. Ann Thorac Cardiovasc Surg. 2023;29(4):192–199. doi: 10.5761/atcs.oa.22-00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Otto C.M., Nishimura R.A., Bonow R.O., et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: Executive summary: a Report of the American College of Cardiology/American heart association Joint Committee on clinical practice guidelines. J Am Coll Cardiol. 2021;77:450–500. doi: 10.1016/j.jacc.2020.11.035. [DOI] [PubMed] [Google Scholar]
- 16.Vahanian A., Beyersdorf F., Praz F., et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2022;43:561–632. doi: 10.1093/eurheartj/ehab395. [DOI] [PubMed] [Google Scholar]
- 17.Lancellotti P., Pibarot P., Chambers J., et al. Multi-modality imaging assessment of native valvular regurgitation: an EACVI and ESC council of valvular heart disease position paper. Eur Heart J Cardiovasc Imaging. 2022;23:e171–e232. doi: 10.1093/ehjci/jeab253. [DOI] [PubMed] [Google Scholar]
- 18.Nashef S.A., Roques F., Sharples L.D., et al. EuroSCORE II. Eur J Cardio Thorac Surg. 2012;41:734–744. doi: 10.1093/ejcts/ezs043. discussion 744-5. [DOI] [PubMed] [Google Scholar]
- 19.Zoghbi W.A., Adams D., Bonow R.O., et al. Recommendations for Noninvasive evaluation of native valvular regurgitation: a Report from the American Society of echocardiography Developed in Collaboration with the Society for cardiovascular magnetic resonance. J Am Soc Echocardiogr. 2017;30:303–371. doi: 10.1016/j.echo.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 20.Garg P., Swift A.J., Zhong L., et al. Assessment of mitral valve regurgitation by cardiovascular magnetic resonance imaging. Nat Rev Cardiol. 2020;17:298–312. doi: 10.1038/s41569-019-0305-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Enriquez-Sarano M., Avierinos J.F., Messika-Zeitoun D., et al. Quantitative determinants of the outcome of asymptomatic mitral regurgitation. N Engl J Med. 2005;352:875–883. doi: 10.1056/NEJMoa041451. [DOI] [PubMed] [Google Scholar]
- 22.Antoine C., Benfari G., Michelena H.I., et al. Clinical outcome of Degenerative mitral regurgitation: Critical importance of echocardiographic quantitative assessment in routine practice. Circulation. 2018;138:1317–1326. doi: 10.1161/CIRCULATIONAHA.117.033173. [DOI] [PubMed] [Google Scholar]
- 23.Penicka M., Vecera J., Mirica D.C., Kotrc M., Kockova R., Van Camp G. Prognostic implications of magnetic resonance-derived quantification in asymptomatic patients with Organic mitral regurgitation: Comparison with Doppler echocardiography-derived Integrative approach. Circulation. 2018;137:1349–1360. doi: 10.1161/CIRCULATIONAHA.117.029332. [DOI] [PubMed] [Google Scholar]
- 24.Altes A., Levy F., Iacuzio L., et al. Comparison of mitral regurgitant volume assessment between Proximal Flow Convergence and volumetric methods in patients with significant primary mitral regurgitation: an echocardiographic and cardiac magnetic resonance imaging study. J Am Soc Echocardiogr. 2022;35:671–681. doi: 10.1016/j.echo.2022.03.005. [DOI] [PubMed] [Google Scholar]
- 25.Uretsky S., Argulian E., Supariwala A., et al. A Comparative assessment of echocardiographic parameters for determining primary mitral regurgitation severity using magnetic resonance imaging as a reference standard. J Am Soc Echocardiogr. 2018;31:992–999. doi: 10.1016/j.echo.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 26.Tribouilloy C., Grigioni F., Avierinos J.F., et al. Survival implication of left ventricular end-systolic diameter in mitral regurgitation due to flail leaflets a long-term follow-up multicenter study. J Am Coll Cardiol. 2009;54:1961–1968. doi: 10.1016/j.jacc.2009.06.047. [DOI] [PubMed] [Google Scholar]
- 27.Essayagh B., Antoine C., Benfari G., et al. Prognostic implications of left atrial Enlargement in Degenerative mitral regurgitation. J Am Coll Cardiol. 2019;74:858–870. doi: 10.1016/j.jacc.2019.06.032. [DOI] [PubMed] [Google Scholar]
- 28.Yang L.T., Ahn S.W., Li Z., et al. Mitral valve prolapse patients with less than moderate mitral regurgitation exhibit early cardiac chamber remodeling. J Am Soc Echocardiogr. 2020;33:815–825.e2. doi: 10.1016/j.echo.2020.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kitkungvan D., Yang E.Y., El Tallawi K.C., et al. Extracellular volume in primary mitral regurgitation. JACC Cardiovasc Imaging. 2021;14:1146–1160. doi: 10.1016/j.jcmg.2020.10.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.