Abstract
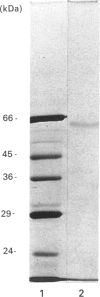
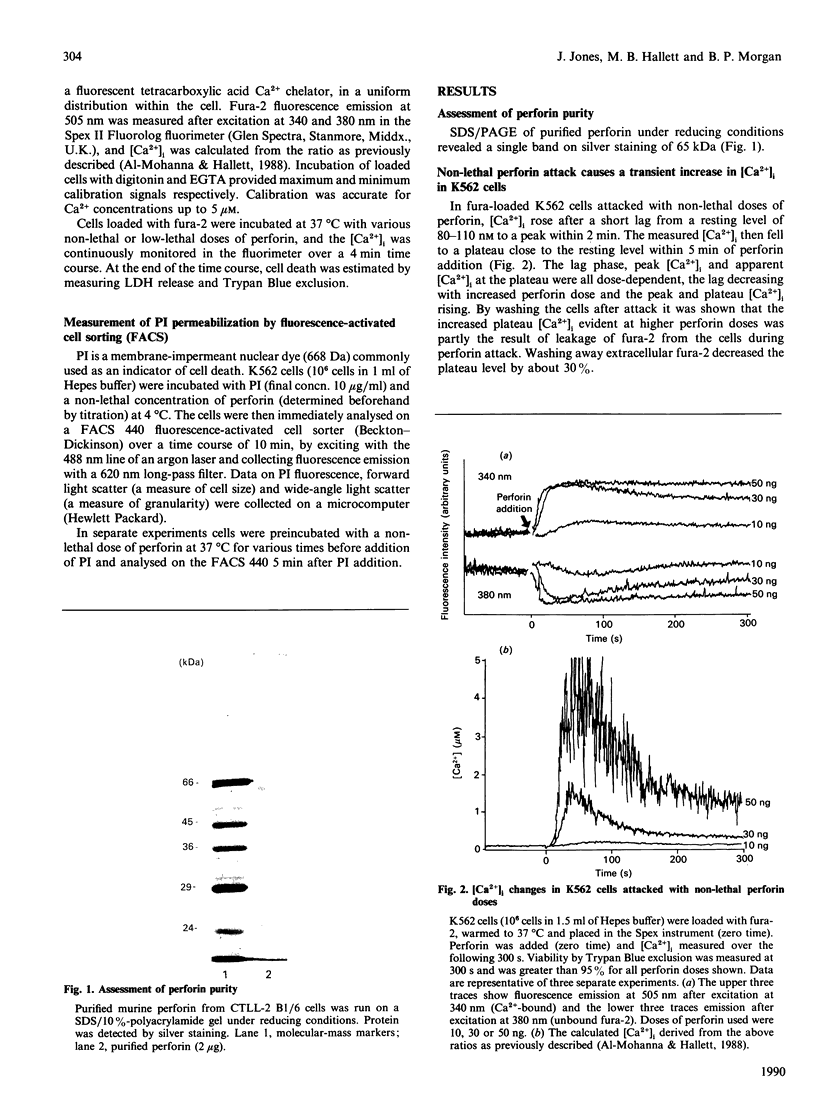
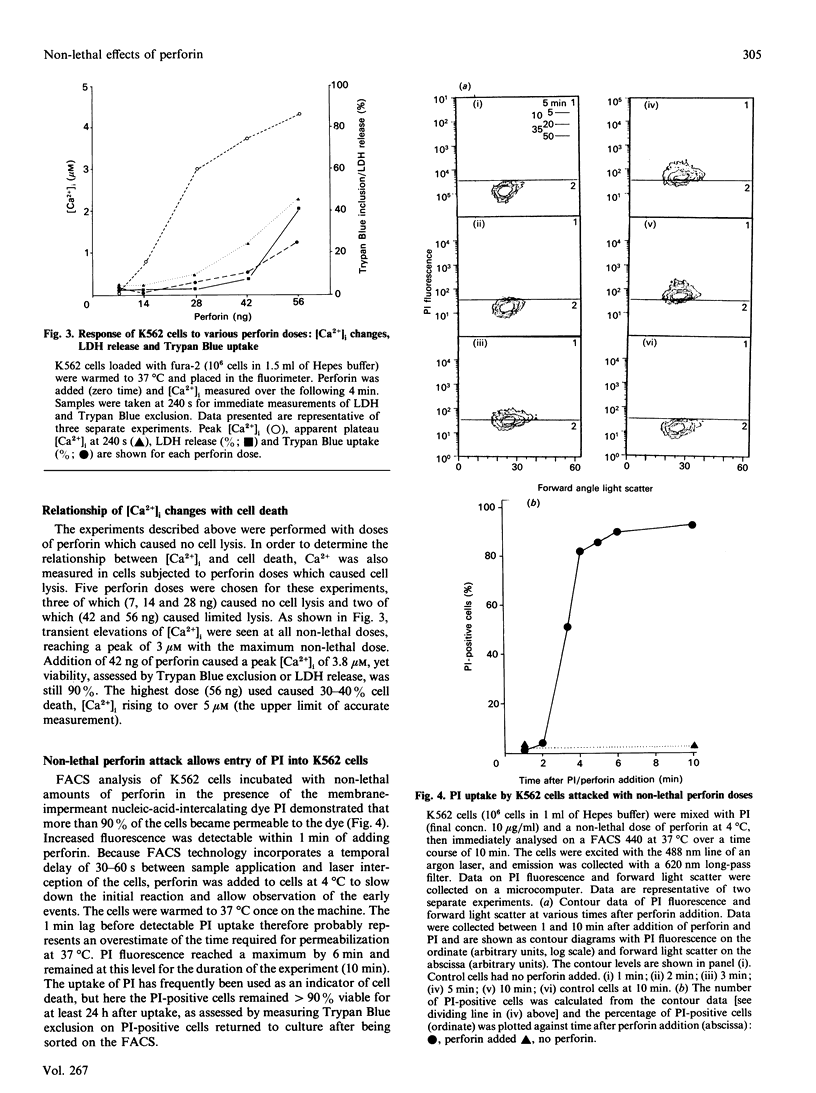
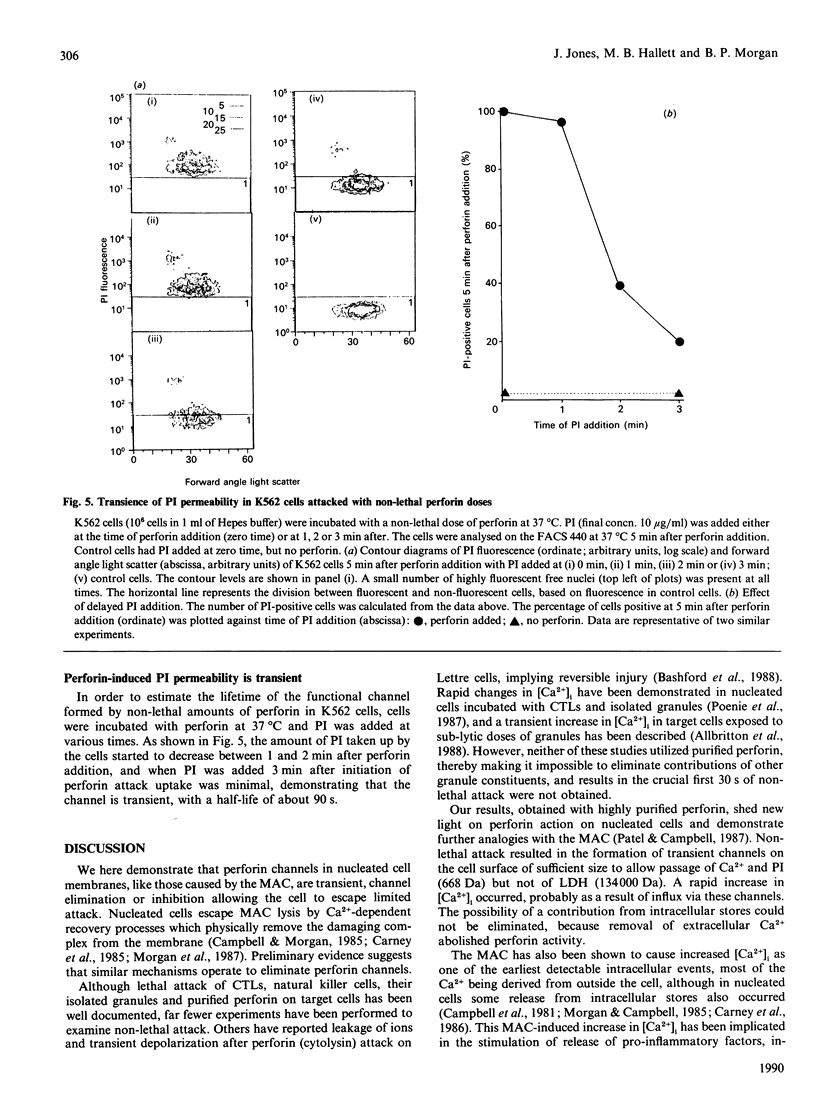
The non-lethal effects of the lymphocyte-derived pore-forming toxin perforin on the human erythroleukaemia cell line K562 were investigated. By using the fluorescent Ca2+ indicator fura-2, perforin was shown to cause intracellular Ca2+ concentration to rise transiently into the micromolar range in the absence of cell death. By fluorescence-activated cell sorting it was demonstrated that K562 cells took up the membrane-impermeant nuclear stain propidium iodide (PI) when exposed to non-lethal doses of perforin. The permeability to PI was short-lived, confirming the transience of the perforin pore. Analogies with non-lethal effects and recovery processes occurring in nucleated cells exposed to the membrane-attack complex of complement are drawn.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Mohanna F. A., Hallett M. B. The use of fura-2 to determine the relationship between cytoplasmic free Ca2+ and oxidase activation in rat neutrophils. Cell Calcium. 1988 Feb;9(1):17–26. doi: 10.1016/0143-4160(88)90034-6. [DOI] [PubMed] [Google Scholar]
- Allbritton N. L., Verret C. R., Wolley R. C., Eisen H. N. Calcium ion concentrations and DNA fragmentation in target cell destruction by murine cloned cytotoxic T lymphocytes. J Exp Med. 1988 Feb 1;167(2):514–527. doi: 10.1084/jem.167.2.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashford C. L., Menestrina G., Henkart P. A., Pasternak C. A. Cell damage by cytolysin. Spontaneous recovery and reversible inhibition by divalent cations. J Immunol. 1988 Dec 1;141(11):3965–3974. [PubMed] [Google Scholar]
- Campbell A. K., Daw R. A., Hallett M. B., Luzio J. P. Direct measurement of the increase in intracellular free calcium ion concentration in response to the action of complement. Biochem J. 1981 Feb 15;194(2):551–560. doi: 10.1042/bj1940551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell A. K., Morgan B. P. Monoclonal antibodies demonstrate protection of polymorphonuclear leukocytes against complement attack. Nature. 1985 Sep 12;317(6033):164–166. doi: 10.1038/317164a0. [DOI] [PubMed] [Google Scholar]
- Carney D. F., Hammer C. H., Shin M. L. Elimination of terminal complement complexes in the plasma membrane of nucleated cells: influence of extracellular Ca2+ and association with cellular Ca2+. J Immunol. 1986 Jul 1;137(1):263–270. [PubMed] [Google Scholar]
- Carney D. F., Koski C. L., Shin M. L. Elimination of terminal complement intermediates from the plasma membrane of nucleated cells: the rate of disappearance differs for cells carrying C5b-7 or C5b-8 or a mixture of C5b-8 with a limited number of C5b-9. J Immunol. 1985 Mar;134(3):1804–1809. [PubMed] [Google Scholar]
- Hallett M. B., Luzio J. P., Campbell A. K. Stimulation of Ca2+-dependent chemiluminescence in rat polymorphonuclear leucocytes by polystyrene beads and the non-lytic action of complement. Immunology. 1981 Nov;44(3):569–576. [PMC free article] [PubMed] [Google Scholar]
- Morgan B. P., Campbell A. K. The recovery of human polymorphonuclear leucocytes from sublytic complement attack is mediated by changes in intracellular free calcium. Biochem J. 1985 Oct 1;231(1):205–208. doi: 10.1042/bj2310205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan B. P. Complement membrane attack on nucleated cells: resistance, recovery and non-lethal effects. Biochem J. 1989 Nov 15;264(1):1–14. doi: 10.1042/bj2640001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan B. P., Dankert J. R., Esser A. F. Recovery of human neutrophils from complement attack: removal of the membrane attack complex by endocytosis and exocytosis. J Immunol. 1987 Jan 1;138(1):246–253. [PubMed] [Google Scholar]
- Patel A. K., Campbell A. K. The membrane attack complex of complement induces permeability changes via thresholds in individual cells. Immunology. 1987 Jan;60(1):135–140. [PMC free article] [PubMed] [Google Scholar]
- Podack E. R., Dennert G. Assembly of two types of tubules with putative cytolytic function by cloned natural killer cells. 1983 Mar 31-Apr 6Nature. 302(5907):442–445. doi: 10.1038/302442a0. [DOI] [PubMed] [Google Scholar]
- Podack E. R., Konigsberg P. J. Cytolytic T cell granules. Isolation, structural, biochemical, and functional characterization. J Exp Med. 1984 Sep 1;160(3):695–710. doi: 10.1084/jem.160.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podack E. R., Young J. D., Cohn Z. A. Isolation and biochemical and functional characterization of perforin 1 from cytolytic T-cell granules. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8629–8633. doi: 10.1073/pnas.82.24.8629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poenie M., Tsien R. Y., Schmitt-Verhulst A. M. Sequential activation and lethal hit measured by [Ca2+]i in individual cytolytic T cells and targets. EMBO J. 1987 Aug;6(8):2223–2232. doi: 10.1002/j.1460-2075.1987.tb02494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J. D., Hengartner H., Podack E. R., Cohn Z. A. Purification and characterization of a cytolytic pore-forming protein from granules of cloned lymphocytes with natural killer activity. Cell. 1986 Mar 28;44(6):849–859. doi: 10.1016/0092-8674(86)90007-3. [DOI] [PubMed] [Google Scholar]
- Zalman L. S., Brothers M. A., Chiu F. J., Müller-Eberhard H. J. Mechanism of cytotoxicity of human large granular lymphocytes: relationship of the cytotoxic lymphocyte protein to the ninth component (C9) of human complement. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5262–5266. doi: 10.1073/pnas.83.14.5262. [DOI] [PMC free article] [PubMed] [Google Scholar]