Abstract
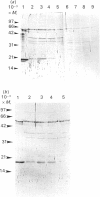
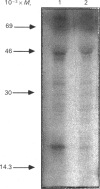
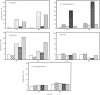
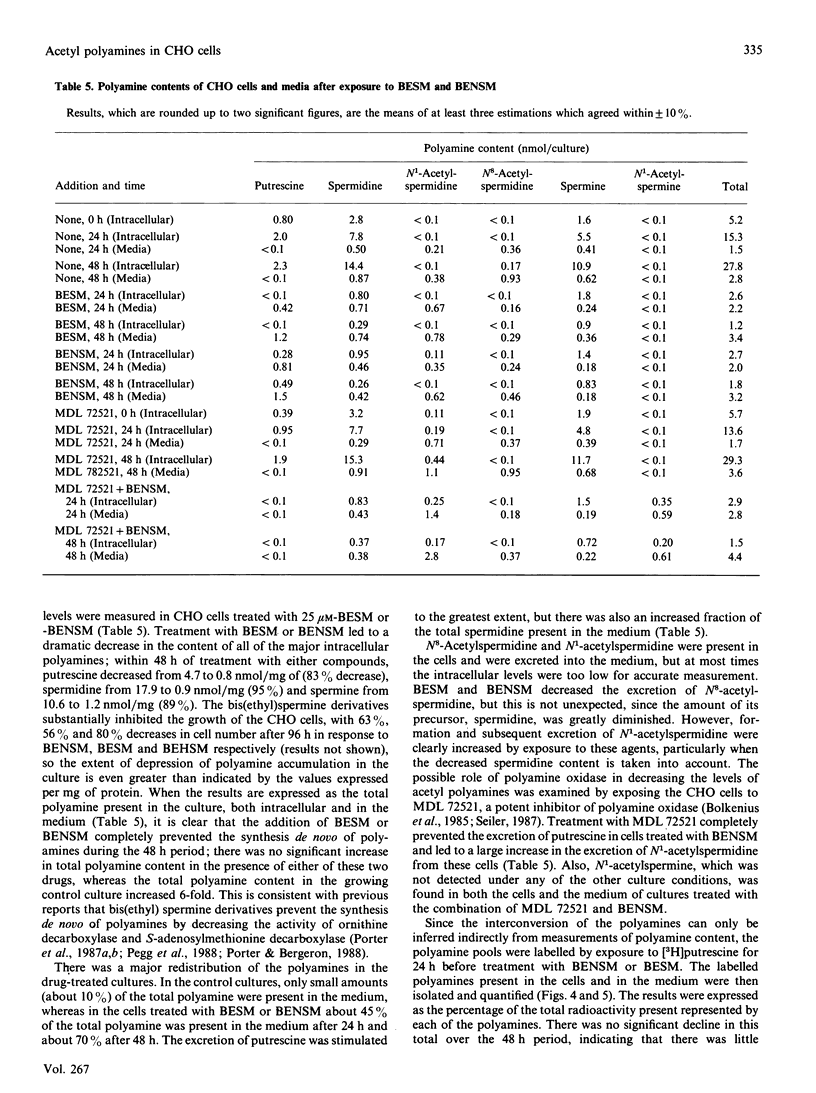
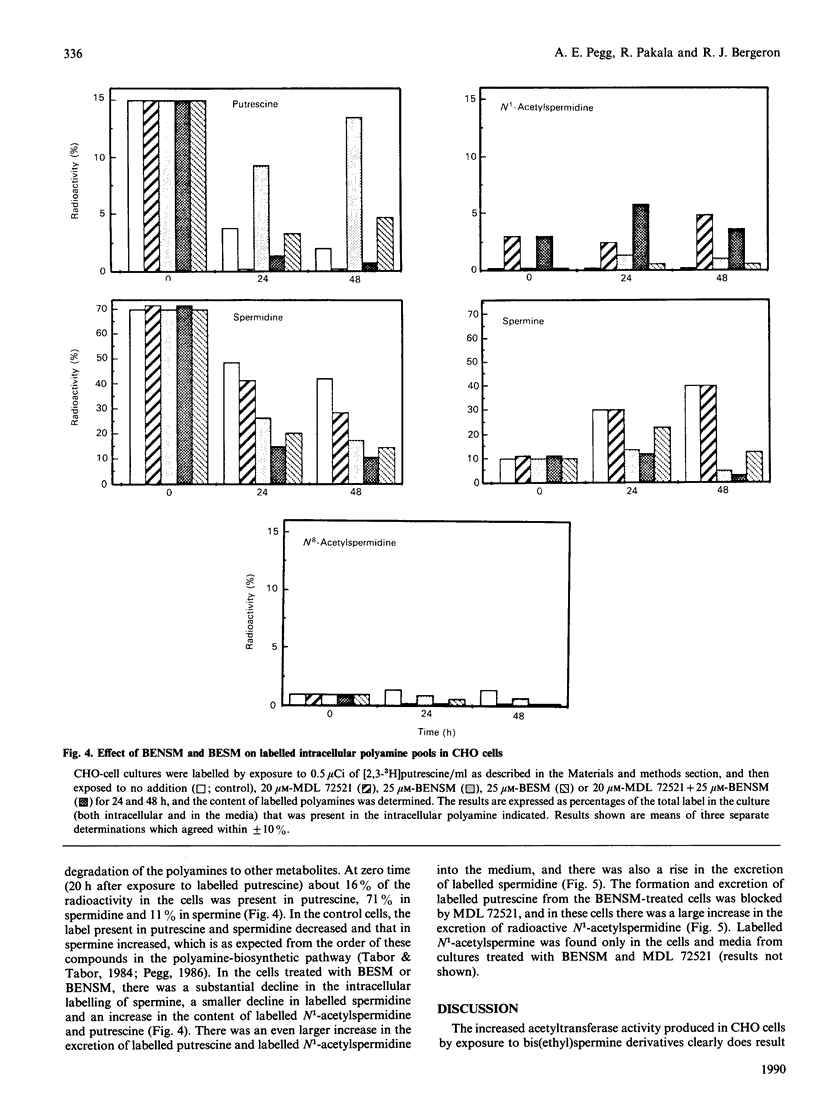
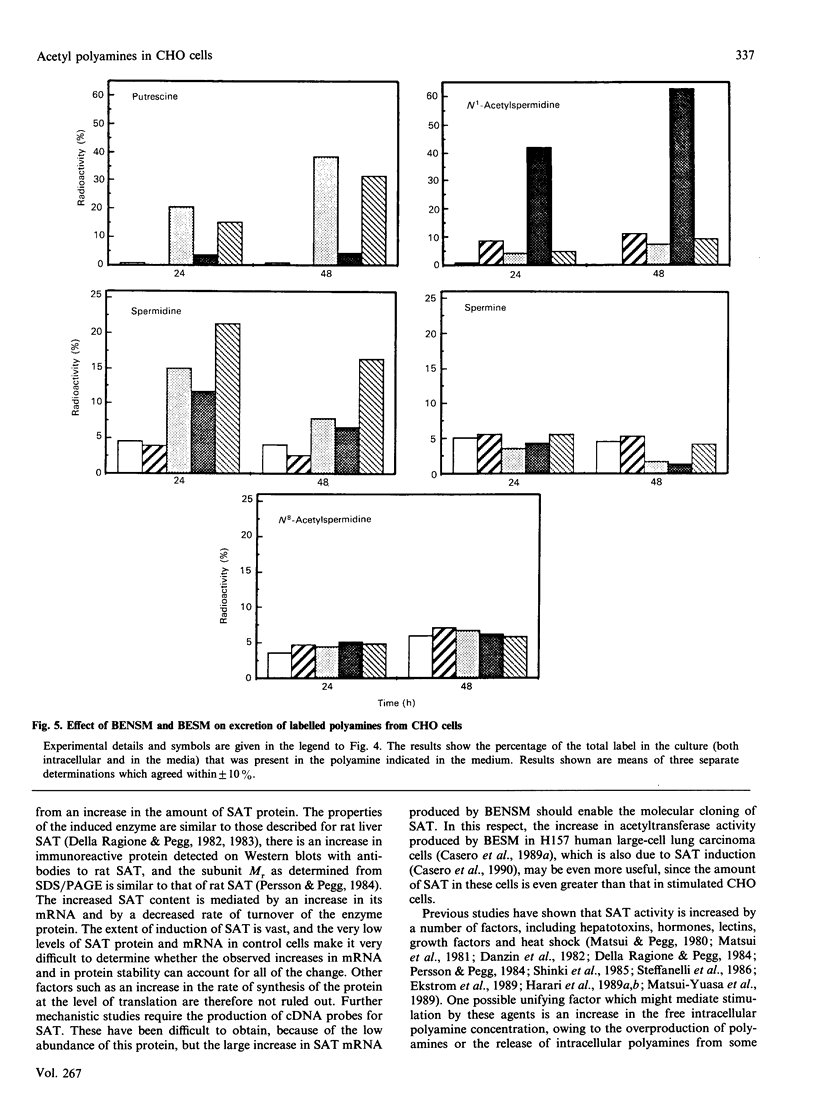
Treatment of Chinese-hamster ovary (CHO) cells with N1N11-bis(ethyl)norspermine (BENSM) led to a very large increase in the activity of spermidine/spermine N1-acetyltransferase (SAT), which rose by about 600-fold within 48 h. Smaller, but still very large increases, were also produced in decreasing order of potency by 3,7,11,15,19-penta-azaheneicosane, N1N12-bis(ethyl)spermine and by N1N14-bis(ethyl)homospermine. The rise in acetyltransferase activity was due to an increase in enzyme protein, as indicated by immunoblotting using antibodies directed against rat liver SAT. There was an increase in the content of mRNA for SAT, indicating that BENSM regulates the level of enzyme protein partly by means of a change in transcription or stability of the mRNA. There was also a decreased rate of degradation of the protein in CHO cells trated with the drug. This may be due to the binding of BENSM, which is a competitive inhibitor of the enzyme with a Ki of 120 microM. Exposure to BENSM led to an increased conversion of spermidine into N1-acetylspermidine and putrescine, a rapid fall in the content of intracellular polyamines and the excretion from the cell of putrescine, N1-acetylspermidine and spermidine. When polyamine oxidase activity in the treated cells was blocked, increases in N1-acetylspermidine and N1-acetylspermine were much greater, and the formation of putrescine was prevented. These results indicate that the induction of SAT facilities the degradation of spermine and spermidine to putrescine and the subsequent excretion of putrescine from the cell. When the degradation of the N1-acetyl derivatives by polyamine oxidase is blocked, the cells excrete N1-acetylspermidine instead of putrescine. CHO cells also contained and excreted N8-acetylspermidine, but its synthesis was not increased in cells treated with BENSM, confirming data obtained in vitro that SAT does not produce this derivative.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergeron R. J., Hawthorne T. R., Vinson J. R., Beck D. E., Jr, Ingeno M. J. Role of the methylene backbone in the antiproliferative activity of polyamine analogues on L1210 cells. Cancer Res. 1989 Jun 1;49(11):2959–2964. [PubMed] [Google Scholar]
- Bergeron R. J., Neims A. H., McManis J. S., Hawthorne T. R., Vinson J. R., Bortell R., Ingeno M. J. Synthetic polyamine analogues as antineoplastics. J Med Chem. 1988 Jun;31(6):1183–1190. doi: 10.1021/jm00401a019. [DOI] [PubMed] [Google Scholar]
- Bolkenius F. N., Bey P., Seiler N. Specific inhibition of polyamine oxidase in vivo is a method for the elucidation of its physiological role. Biochim Biophys Acta. 1985 Jan 28;838(1):69–76. doi: 10.1016/0304-4165(85)90251-x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Casero R. A., Jr, Celano P., Ervin S. J., Porter C. W., Bergeron R. J., Libby P. R. Differential induction of spermidine/spermine N1-acetyltransferase in human lung cancer cells by the bis(ethyl)polyamine analogues. Cancer Res. 1989 Jul 15;49(14):3829–3833. [PubMed] [Google Scholar]
- Casero R. A., Jr, Ervin S. J., Celano P., Baylin S. B., Bergeron R. J. Differential response to treatment with the bis(ethyl)polyamine analogues between human small cell lung carcinoma and undifferentiated large cell lung carcinoma in culture. Cancer Res. 1989 Feb 1;49(3):639–643. [PubMed] [Google Scholar]
- Danzin C., Bolkenius F. N., Claverie N., Wagner J., Grove J. Secretin-induced accumulation of N1-acetylspermidine and putrescine in the rat pancreas. Biochem Biophys Res Commun. 1982 Dec 31;109(4):1234–1239. doi: 10.1016/0006-291x(82)91909-x. [DOI] [PubMed] [Google Scholar]
- Della Ragione F., Pegg A. E. Effect of analogues of 5'-methylthioadenosine on cellular metabolism. Inactivation of S-adenosylhomocysteine hydrolase by 5'-isobutylthioadenosine. Biochem J. 1983 Feb 15;210(2):429–435. doi: 10.1042/bj2100429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dredar S. A., Blankenship J. W., Marchant P. E., Manneh V., Fries D. S. Design and synthesis of inhibitors of N8-acetylspermidine deacetylase. J Med Chem. 1989 May;32(5):984–989. doi: 10.1021/jm00125a010. [DOI] [PubMed] [Google Scholar]
- Ekström J., Månsson B., Nilsson B. O., Rosengren E., Tobin G. Spermidine/spermine-N1-acetyltransferase activity in isoprenaline-stimulated rat salivary glands. Acta Physiol Scand. 1989 Mar;135(3):249–254. doi: 10.1111/j.1748-1716.1989.tb08574.x. [DOI] [PubMed] [Google Scholar]
- Erwin B. G., Pegg A. E. Regulation of spermidine/spermine N1-acetyltransferase in L6 cells by polyamines and related compounds. Biochem J. 1986 Sep 1;238(2):581–587. doi: 10.1042/bj2380581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erwin B. G., Persson L., Pegg A. E. Differential inhibition of histone and polyamine acetylases by multisubstrate analogues. Biochemistry. 1984 Aug 28;23(18):4250–4255. doi: 10.1021/bi00313a036. [DOI] [PubMed] [Google Scholar]
- Harari P. M., Fuller D. J., Gerner E. W. Heat shock stimulates polyamine oxidation by two distinct mechanisms in mammalian cell cultures. Int J Radiat Oncol Biol Phys. 1989 Feb;16(2):451–457. doi: 10.1016/0360-3016(89)90341-6. [DOI] [PubMed] [Google Scholar]
- Harari P. M., Tome M. E., Fuller D. J., Carper S. W., Gerner E. W. Effects of diethyldithiocarbamate and endogenous polyamine content on cellular responses to hydrogen peroxide cytotoxicity. Biochem J. 1989 Jun 1;260(2):487–490. doi: 10.1042/bj2600487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyvönen T. Excretion of acetylated and free polyamines by polyamine depleted Chinese hamster ovary cells. Int J Biochem. 1989;21(3):313–316. doi: 10.1016/0020-711x(89)90189-4. [DOI] [PubMed] [Google Scholar]
- Kameji T., Pegg A. E. Inhibition of translation of mRNAs for ornithine decarboxylase and S-adenosylmethionine decarboxylase by polyamines. J Biol Chem. 1987 Feb 25;262(6):2427–2430. [PubMed] [Google Scholar]
- Libby P. R., Bergeron R. J., Porter C. W. Structure-function correlations of polyamine analog-induced increases in spermidine/spermine acetyltransferase activity. Biochem Pharmacol. 1989 May 1;38(9):1435–1442. doi: 10.1016/0006-2952(89)90182-2. [DOI] [PubMed] [Google Scholar]
- Marchant P., Dredar S., Manneh V., Alshabanah O., Matthews H., Fries D., Blankenship J. A selective inhibitor of N8-acetylspermidine deacetylation in mice and HeLa cells without effects on histone deacetylation. Arch Biochem Biophys. 1989 Aug 15;273(1):128–136. doi: 10.1016/0003-9861(89)90170-7. [DOI] [PubMed] [Google Scholar]
- Matsui-Yuasa I., Otani S., Yukioka K., Goto H., Morisawa S. Two mechanisms of spermidine/spermine N1-acetyltransferase-induction. Arch Biochem Biophys. 1989 Jan;268(1):209–214. doi: 10.1016/0003-9861(89)90581-x. [DOI] [PubMed] [Google Scholar]
- Matsui I., Pegg A. E. Effect of thioacetamide, growth hormone or partial hepatectomy on spermidine acetylase activity of rat liver cytosol. Biochim Biophys Acta. 1980 Nov 17;633(1):87–94. doi: 10.1016/0304-4165(80)90040-9. [DOI] [PubMed] [Google Scholar]
- Matsui I., Wiegand L., Pegg A. E. Properties of spermidine N-acetyltransferase from livers of rats treated with carbon tetrachloride and its role in the conversion of spermidine into putrescine. J Biol Chem. 1981 Mar 10;256(5):2454–2459. [PubMed] [Google Scholar]
- Okayama H., Kawaichi M., Brownstein M., Lee F., Yokota T., Arai K. High-efficiency cloning of full-length cDNA; construction and screening of cDNA expression libraries for mammalian cells. Methods Enzymol. 1987;154:3–28. doi: 10.1016/0076-6879(87)54067-8. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., Erwin B. G. Induction of spermidine/spermine N1-acetyltransferase in rat tissues by polyamines. Biochem J. 1985 Oct 15;231(2):285–289. doi: 10.1042/bj2310285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E., Erwin B. G., Persson L. Induction of spermidine/spermine N1-acetyltransferase by methylglyoxal bis(guanylhydrazone). Biochim Biophys Acta. 1985 Oct 17;842(2-3):111–118. doi: 10.1016/0304-4165(85)90192-8. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., Madhubala R., Kameji T., Bergeron R. J. Control of ornithine decarboxylase activity in alpha-difluoromethylornithine-resistant L1210 cells by polyamines and synthetic analogues. J Biol Chem. 1988 Aug 5;263(22):11008–11014. [PubMed] [Google Scholar]
- Pegg A. E. Polyamine metabolism and its importance in neoplastic growth and a target for chemotherapy. Cancer Res. 1988 Feb 15;48(4):759–774. [PubMed] [Google Scholar]
- Pegg A. E. Recent advances in the biochemistry of polyamines in eukaryotes. Biochem J. 1986 Mar 1;234(2):249–262. doi: 10.1042/bj2340249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E., Wechter R., Pakala R., Bergeron R. J. Effect of N1,N12-bis(ethyl)spermine and related compounds on growth and polyamine acetylation, content, and excretion in human colon tumor cells. J Biol Chem. 1989 Jul 15;264(20):11744–11749. [PubMed] [Google Scholar]
- Persson L., Pegg A. E. Studies of the induction of spermidine/spermine N1-acetyltransferase using a specific antiserum. J Biol Chem. 1984 Oct 25;259(20):12364–12367. [PubMed] [Google Scholar]
- Porter C. W., Berger F. G., Pegg A. E., Ganis B., Bergeron R. J. Regulation of ornithine decarboxylase activity by spermidine and the spermidine analogue N1N8-bis(ethyl)spermidine. Biochem J. 1987 Mar 1;242(2):433–440. doi: 10.1042/bj2420433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter C. W., Bergeron R. J. Enzyme regulation as an approach to interference with polyamine biosynthesis--an alternative to enzyme inhibition. Adv Enzyme Regul. 1988;27:57–79. doi: 10.1016/0065-2571(88)90009-x. [DOI] [PubMed] [Google Scholar]
- Porter C. W., McManis J., Casero R. A., Bergeron R. J. Relative abilities of bis(ethyl) derivatives of putrescine, spermidine, and spermine to regulate polyamine biosynthesis and inhibit L1210 leukemia cell growth. Cancer Res. 1987 Jun 1;47(11):2821–2825. [PubMed] [Google Scholar]
- Ragione F. D., Pegg A. E. Purification and characterization of spermidine/spermine N1-acetyltransferase from rat liver. Biochemistry. 1982 Nov 23;21(24):6152–6158. doi: 10.1021/bi00267a020. [DOI] [PubMed] [Google Scholar]
- Seiler N. Functions of polyamine acetylation. Can J Physiol Pharmacol. 1987 Oct;65(10):2024–2035. doi: 10.1139/y87-317. [DOI] [PubMed] [Google Scholar]
- Shinki T., Takahashi N., Kadofuku T., Sato T., Suda T. Induction of spermidine N1-acetyltransferase by 1 alpha,25-dihydroxyvitamin D3 as an early common event in the target tissues of vitamin D. J Biol Chem. 1985 Feb 25;260(4):2185–2190. [PubMed] [Google Scholar]
- Stefanelli C., Carati D., Rossoni C., Flamigni F., Caldarera C. M. Accumulation of N1-acetylspermidine in heart and spleen of isoprenaline-treated rats. Biochem J. 1986 Aug 1;237(3):931–934. doi: 10.1042/bj2370931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Polyamines. Annu Rev Biochem. 1984;53:749–790. doi: 10.1146/annurev.bi.53.070184.003533. [DOI] [PubMed] [Google Scholar]