Abstract
Tetrastigma (Vitaceae) is known for its ornamental, medicinal, and ecological significance. However, the structural and variational characteristics of the Tetrastigma chloroplast genome and their impact on phylogenetic relationships remain underexplored. This study utilized bioinformatics methods to assemble and annotate the chloroplast genomes of 10 Tetrastigma species and compare them with five previously sequenced species. This study analyzed gene composition, simple sequence repeats, and codon usage patterns, revealing a high A/T content, uniquely identified pentanucleotide repeats in five species and several preferred codons. In addition, comparative analyses were conducted of the chloroplast genomes of 15 Tetrastigma species, examining their structural differences and identifying polymorphic hotspots (rps16, rps16-trnQ, trnS, trnD, psbC-trnS-psbZ, accD-psaI, psbE-petL-petG, etc.) suitable for DNA marker development. Furthermore, phylogenetic and selective pressure analyses were performed based on the chloroplast genomes of these 15 Tetrastigma species, validating and elucidating intra-genus relationships within Tetrastigma. Futhermore, several genes under positive selection, such as atpF and accD, were identified, shedding light on the adaptive evolution of Tetrastigma. Utilizing 40 Vitaceae species, the divergence time of Tetrastigma was estimated, clarifying the evolutionary relationships within Tetrastigma relative to other genera. The analysis revealed diverse divergences of Tetrastigma in the Miocene and Pliocene, with possible ancient divergence events before the Eocene. Furthermore, family-level selective pressure analysis identified key features distinguishing Tetrastigma from other genera, showing a higher degree of purifying selection. This research enriches the chloroplast genome data for Tetrastigma and offers new insights into species identification, phylogenetic analysis, and adaptive evolution, enhancing our understanding of the genetic diversity and evolutionary history of these species.
Keywords: chloroplast genome, Tetrastigma, comparative genomics, phylogenetic analysis, selective pressure analysis
1. Introduction
The genus Tetrastigma, belonging to the Vitaceae family, comprises numerous climbing plants predominantly distributed in tropical and subtropical regions, especially in Asia. These plants are favored by horticulturists for their ornamental foliage and climbing ability. Due to their unique tuberous root structures, some Tetrastigma species are rich in starch and possess edible value [1]. Additionally, Tetrastigma species hold significant medicinal value, with notable antioxidant and anti-inflammatory properties [2,3], and numerous studies have demonstrated their anti-tumor effects [4,5].
Despite their distinct morphological traits (Figure 1), the classification and identification of Tetrastigma species are challenging due to the considerable number of species, considerable morphological variation, and the occurrence of hybridization. Traditional morphological characteristics can be influenced by environmental and growth conditions, further complicating classification and identification.
Figure 1.
Four species of Tetrastigma, from left to right: (a) Tetrastigma hemsleyanum. (b) Tetrastigma leucostaphylum. (c) Tetrastigma thorsborneorum. (d) Tetrastigma voinierianum.
Common methods for phylogenetic identification of species include ITS sequences and SCoT molecular markers [6]. Simple sequence repeats (SSRs) are also used as molecular markers. SSRs, also known as microsatellites, consist of tandem repeats of nucleotide sequences, such as mononucleotides and dinucleotides. Researchers have reported the successful application of SSR markers in maize hybrid genotypes and disease-resistant tomato varieties [7,8], indicating the feasibility of SSRs as molecular markers. Single nucleotide polymorphism (SNP) markers also offer great advantages. SNPs are highly specific, as they represent variations at a single nucleotide such as nucleotide transversions with each SNP representing a nucleotide difference [9]. Boakyewaa et al. analyzed 94 early-maturing maize yellow and white varieties based on SNP markers and morphological traits, successfully identifying distinct clusters of inbred lines [10]. Exploring SSRs and SNPs in Tetrastigma species holds significant value.
Chloroplast is a crucial organelle in plant cells, with a relatively conserved genome structure and unique features such as the ycf1 DNA barcode. Research on chloroplast genomes offers several advantages: (1) The chloroplast genome is relatively small and simple in structure, making it easy to assemble and analyze without consuming extensive computational resources. Additionally, the chloroplast genome has a highly conserved quadripartite circular structure, divided into a large single-copy region (LSC), a small single-copy region (SSC), and a pair of inverted repeats (IRs) [11]. By comparing the gene boundary distances between different species’ chloroplast genomes, researchers can analyze phenomena such as rearrangements. (2) The chloroplast genome, though small, contains abundant genetic information, aiding in phylogenetic and species identification studies, for example, SSRs and SNPs mentioned earlier. (3) The evolutionary stability of the chloroplast genome makes it suitable for interspecies and intraspecies phylogenetic analysis. Researchers have reported that the evolutionary rate of synonymous substitution in the chloroplast genome is half that of the nuclear genome [12], indicating the relative stability and conservativeness of the chloroplast genome.
Furthermore, the simple structure and relatively small size of the chloroplast genome make more extensive horizontal research possible, such as pan-chloroplast genomes. Distinct from conventional chloroplast genome comparative analysis, research on pan-chloroplast genomes typically involves dozens to hundreds of samples, which can be different species or different varieties of the same species. The methods of pan-chloroplast genome research have been applied, for example, to help develop molecular markers for peppers and clarify genetic variation relationships [13]. In addition, research on genetic variation and temperature adaptation in cucumbers [14], as well as research on accession-specific markers in the genus Hibiscus [15], have all been supplemented by the methods of pan-chloroplast genome research. Whether it is pan-chloroplast genomes or conventional chloroplast genome comparative analysis, both can deepen people’s understanding of molecular markers, phylogenetics, and other aspects.
With the advancement of next-generation sequencing technologies and the reduction in sequencing costs, chloroplast genomes have been widely used in phylogenetic analyses in recent years [16,17,18], demonstrating the feasibility of using chloroplast genomes for phylogenetic analysis and species identification. Recent research has reported that nearly 13,000 plant chloroplast genomes have been published (NCBI, September 2023) [12]. In recent years, the complete chloroplast genomes of species such as Tetrastigma hemsleyanum and Tetrastigma planicaule have been assembled and annotated [19,20]. With ongoing sequencing studies and improvements in assembly software [21], more data resources for Tetrastigma species are being supplemented. However, data alone are not enough; corresponding analyses must follow. Compared to other genera in the Vitaceae family, evolutionary relationship studies on Tetrastigma are still limited. Although some studies have analyzed the phylogenetic relationships of Tetrastigma based on ten chloroplast DNA regions [22], they only include a portion of the Tetrastigma species. Some Tetrastigma species only have sequencing data with limited related studies, especially on chloroplast genomes.
When studying phylogenetic relationships, constructing phylogenetic trees based on methods such as Maximum Likelihood (ML) and maximum parsimony (MP) is an effective approach. Additionally, a time-calibrated tree allows for researchers to delve into species’ evolutionary relationships over long time scales, clarifying some controversies arising from constructing phylogenetic trees using conventional methods. Nie and colleagues reconstructed the phylogenetic relationships of the genus Ampelopsis within the Vitaceae family, providing detailed divergence times using fossil data combined with global geography [23]. Similarly, the evolutionary time scale of the genus Vitis has also been studied [24]. Selecting an appropriate molecular clock is crucial in molecular dating studies. Previous research has analyzed the divergence times and absolute substitution rates of six genera: Ampelocissus, Ampelopsis, Nekemias, Parthenocissus, Rhoicissus, and Vitis under different molecular clocks [24]. However, studies on the molecular clock selection and time scale for the genus Tetrastigma are still lacking, or they are merely used as reference outgroups.
Selective pressure analysis is an important method for studying the evolutionary processes of species. By analyzing selective pressure, researchers can understand the selective pressure on genes or genomes during evolution, revealing the conservation and adaptive changes in gene functions. Nonsynonymous substitution rates (Ka) and synonymous substitution rates (Ks) are important parameters for measuring gene evolutionary rates. Ka represents the rate of mutations that lead to amino acid changes in protein-coding genes, while Ks represents the rate of mutations that do not change amino acids. The Ka/Ks ratio is a crucial indicator for assessing gene selective pressure. Researchers have used the Ka/Ks ratio to explore the selective pressure on Lilium ledebourii [25]. During the evolution of Tetrastigma species, what selective pressure was applied by the key genes distinguishing this genus from others experience? How did evolution occur within the genus? These questions are worth in-depth research.
Our study aims to address the following questions: (1) What are the SSR and SNPs molecular markers for species identification in the Tetrastigma genus? (2) Based on chloroplast genome data, what are the phylogenetic relationships within the Tetrastigma genus? (3) What is the divergence time of Tetrastigma species, and how does it compare to the evolutionary timeline of other genera within the Vitaceae family? (4) What selective pressures have key genes within the Tetrastigma genus experienced during evolution, and how do these pressures compare to those in other genera within the Vitaceae family? Thus, we selected ten sequenced species: Tetrastigma thorsborneorum, Tetrastigma serrulatum, Tetrastigma pyriforme, Tetrastigma pachyphyllum, Tetrastigma nilagiricum, Tetrastigma leucostaphylum, Tetrastigma cauliflorum, Tetrastigma canarense, Tetrastigma annamense, and Tetrastigma angustifolium and assembled and annotated their respective chloroplast genomes. We then explored the characteristics of each genome and compared them with the genomes of five other Tetrastigma species. Our analysis included codon usage patterns, simple sequence repeats (SSRs), and hotspot region identification, aiming to screen for promising identification sites. Additionally, as the phylogenetic relationships within Tetrastigma are unclear, we constructed phylogenetic trees based on Maximum Likelihood and Bayesian methods for the genus Tetrastigma and the Vitaceae family, estimating divergence times to further supplement and verify the evolutionary position and phylogenetic relationships of Tetrastigma. Based on the phylogenetic trees, we also analyzed selective pressure within the genus Tetrastigma and among genera, aiming to clarify the roles of different genes in the evolution of Tetrastigma from both intra- and intergeneric perspectives.
2. Results
2.1. Genome Organization and Features
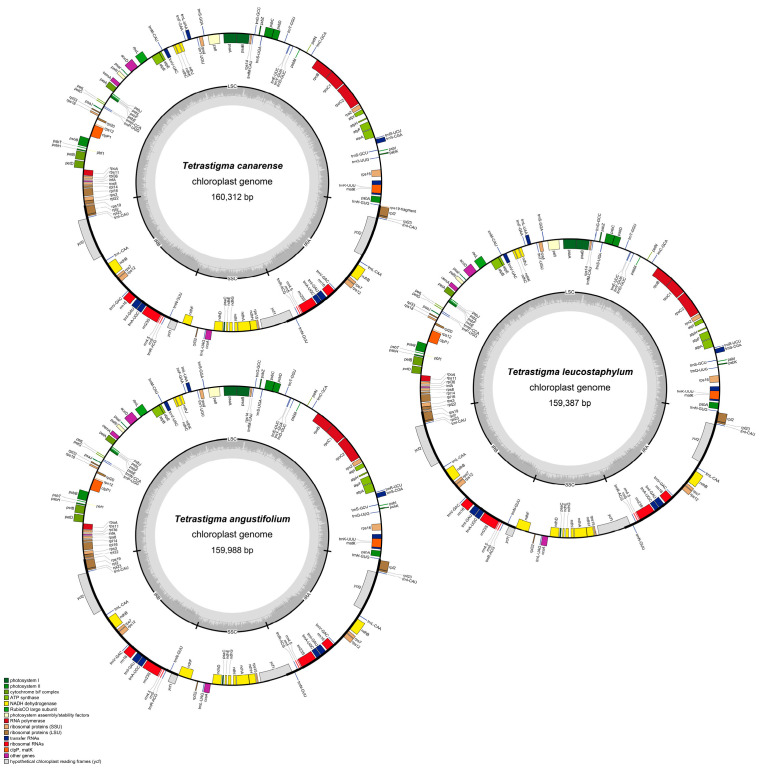
The chloroplast genomes of ten Tetrastigma species were successfully assembled, resulting in gap-free, circularly structured genomes (Figure 2). Additionally, chloroplast genomes of five other Tetrastigma species were downloaded from NCBI, allowing for the analysis of a total of fifteen species. The sizes of these 15 Tetrastigma chloroplast genomes (Table 1) range from 159,387 base pairs (bp) in T. nilagiricum to 160,736 bp in T. annamense, with total GC content varying between 37.35% in T. voinierianum to 37.62% in T. cauliflorum. All genomes exhibit the typical quadripartite structure, consisting of a large single-copy (LSC) region, a pair of inverted repeats (IRs), and a small single-copy (SSC) region. The LSC regions range in length from 87,499 bp in T. nilagiricum to 88,821 bp in T. annamense, the IR regions range from 26,288 bp in T. annamense to 26,934 bp in T. thorsborneorum, and the SSC regions range from 18,649 bp in T. thorsborneorum to 19,339 bp in T. annamense. The corresponding GC contents for these regions are 35.34–35.63%, 42.73–42.99%, and 31.41–31.79%, respectively.
Figure 2.
Chloroplast genome maps of Tetrastigma, taking T. angustifolium, T. canarense and T. leucostaphylum as examples.
Table 1.
Summary of 15 Tetrastigma chloroplast genome characteristics.
| Species | Genome Size | LSC Length | IR Length | SSC Length | Total GC Content | GC Content of LSC | GC Content of IR | GC Content of SSC | Number of Total Genes | Number of CDS | Number of rRNA Genes | Number of tRNA Genes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T. angustifolium (NC_029339) | 159,988 | 88,107 | 26,510 | 18,861 | 37.55% | 35.54% | 42.89% | 31.77% | 131 | 86 | 8 | 37 |
| T. annamense | 160,736 | 88,821 | 26,288 | 19,339 | 37.56% | 35.35% | 42.98% | 31.55% | 132 | 87 | 8 | 37 |
| T. canarense | 160,312 | 88,010 | 26,627 | 19,048 | 37.57% | 35.53% | 42.82% | 31.77% | 132 | 87 | 8 | 37 |
| T. cauliflorum | 160,382 | 88,238 | 26,513 | 19,118 | 37.62% | 35.50% | 42.87% | 31.65% | 131 | 86 | 8 | 37 |
| T. hemsleyanum | 159,889 | 87,901 | 26,511 | 18,966 | 37.53% | 35.58% | 42.89% | 31.77% | 132 | 87 | 8 | 37 |
| T. lawsonii (NC_061673) | 160,360 | 88,117 | 26,469 | 19,306 | 37.53% | 35.50% | 42.93% | 31.57% | 131 | 86 | 8 | 37 |
| T. leucostaphylum | 159,387 | 87,499 | 26,506 | 18,876 | 37.43% | 35.59% | 42.88% | 31.71% | 131 | 86 | 8 | 37 |
| T. nilagiricum | 160,116 | 88,021 | 26,492 | 19,111 | 37.58% | 35.63% | 42.90% | 31.69% | 132 | 87 | 8 | 37 |
| T. pachyphyllum | 159,317 | 87,384 | 26,529 | 188,875 | 37.49% | 35.62% | 42.86% | 31.79% | 131 | 86 | 8 | 37 |
| T. planicaule (NC_057118) | 160,323 | 88,181 | 26,523 | 19,096 | 37.39% | 35.52% | 42.87% | 31.66% | 131 | 86 | 8 | 37 |
| T. pyriforme | 160,893 | 88,979 | 26,319 | 19,276 | 37.48% | 35.35% | 42.97% | 31.51% | 131 | 86 | 8 | 37 |
| T. rafflesiae (NC_061671) | 159,805 | 87,920 | 26,306 | 19,273 | 37.55% | 35.63% | 42.99% | 31.62% | 131 | 86 | 8 | 37 |
| T. serrulatum | 160,113 | 88,084 | 26,441 | 19,147 | 37.50% | 35.34% | 42.85% | 31.41% | 132 | 87 | 8 | 37 |
| T. thorsborneorum | 160,676 | 88,159 | 26,934 | 18,649 | 37.48% | 35.43% | 42.73% | 31.62% | 131 | 86 | 8 | 37 |
| T.voinierianum (NC_061711) | 160,005 | 87,874 | 26,510 | 19,111 | 37.35% | 35.59% | 42.87% | 31.65% | 131 | 86 | 8 | 37 |
Excluding the pseudogenes ψycf1 and ψrps19-fragment, the Tetrastigma chloroplast genomes encode a total of 130 genes, comprising 85 CDS, 8 rRNA genes, and 37 tRNA genes. These genes are broadly categorized into four types (Table 2): self-replication genes, photosynthesis-related genes, other genes, and genes of unknown function, with the majority located in the LSC region.
Table 2.
Gene types of Tetrastigma chloroplast genome.
| Category | Gene Group | Gene Name |
|---|---|---|
| Photosynthesis | Subunits of photosystem I | psaA, psaB, psaC, psaI, psaJ |
| Subunits of photosystem II | psbA, psbB, psbC, psbD, psbE, psbF, psbH, psbI, psbJ, psbK, psbL, psbM, psbT, psbZ | |
| Subunits of NADH dehydrogenase | ndhA *, ndhB *(2), ndhC, ndhD, ndhE, ndhF, ndhG, ndhH, ndhI, ndhJ, ndhK | |
| Subunits of cytochrome b/f complex | petA, petB *, petD *, petG, petL, petN | |
| Subunits of ATP synthase | atpA, atpB, atpE, atpF *, atpH, atpI | |
| Large subunit of rubisco | rbcL | |
| Self-replication | Proteins of large ribosomal subunit | rpl14, rpl16 *, rpl2 *(2), rpl20, rpl22, rpl23(2), rpl32, rpl33, rpl36 |
| Proteins of small ribosomal subunit | rps11, rps12 **(2), rps14, rps15, rps16 *, rps18, rps19, rps2, rps3, rps4, rps7(2), rps8, ψrps19-fragment | |
| Subunits of RNA polymerase | rpoA, rpoB, rpoC1 *, rpoC2 | |
| Ribosomal RNAs | rrn16(2), rrn23S(2), rrn4.5(2), rrn5(2) | |
| Transfer RNAs | trnAUGC *(2), trnCGCA, trnDGUC, trnEUUC, trnFGAA, trnGGCC, trnHGUG, trnICAU(2), trnIGAU *(2), trnKUUU *, trnLCAA(2), trnLUAA *, trnLUAG, trnMCAU, trnNGUU(2), trnPUGG, trnQUUG, trnRACG(2), trnRUCU, trnSCGA *, trnSGCU, trnSGGA, trnSUGA, trnTGGU, trnTUGU, trnVGAC(2), trnVUAC *, trnWCCA, trnYGUA, trnfMCAU | |
| Other genes | Maturase | matK |
| Protease | clpP1 ** | |
| Envelope membrane protein | cemA | |
| Acetyl-CoA carboxylase | accD | |
| c-type cytochrome synthesis gene | ccsA | |
| Translation initiation factor | infA | |
| Other | pafI **, pafII, pbf1 | |
| Genes of unknown function | Conserved hypothetical chloroplast ORF | ycf1, ψycf1, ycf2(2) |
Notes: Gene *: Gene with one intron; Gene **: Gene with two introns; ψGene: Pseudo gene; Gene(2): Number of copies of multi-copy genes.
2.2. SSRs Analysis
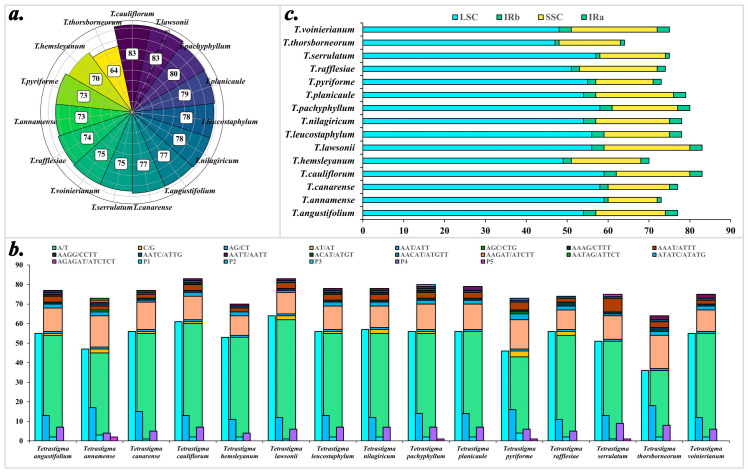
Overall, 1139 SSRs were detected. T. lawsonii and T. cauliflorum have the highest number of SSRs, totaling eighty-three, while T. thorsborneorum has the fewest, with only sixty-four. Mapping the identified simple sequence repeats to the chloroplast genome reveals the distribution regions of SSRs. The results indicate that SSRs in the fifteen species are primarily distributed in the LSC region, followed by the SSC region, with the least in the IR regions, where the numbers of SSRs detected in IRa and IRb are equal (Table 3).
Table 3.
SSRs detected in the Tetrastigma chloroplast genome P1-mononucleotide, P2-dinucleotide, P3-trinucleotide, P4-tetranucleotide, P5-pentanucleotide.
| Species | P1 | P2 | P3 | P4 | P5 | Total | LSC | IRb | SSC | IRa |
|---|---|---|---|---|---|---|---|---|---|---|
| T. angustifolium | 55 | 13 | 2 | 7 | 0 | 77 | 54 | 3 | 17 | 3 |
| T. annamense | 47 | 17 | 3 | 4 | 2 | 73 | 59 | 1 | 12 | 1 |
| T. canarense | 56 | 15 | 1 | 5 | 0 | 77 | 58 | 2 | 15 | 2 |
| T. cauliflorum | 61 | 13 | 2 | 7 | 0 | 83 | 59 | 3 | 18 | 3 |
| T. hemsleyanum | 53 | 11 | 2 | 4 | 0 | 70 | 49 | 2 | 17 | 2 |
| T. lawsonii | 64 | 12 | 1 | 6 | 0 | 83 | 56 | 3 | 21 | 3 |
| T. leucostaphylum | 56 | 13 | 2 | 7 | 0 | 78 | 56 | 3 | 16 | 3 |
| T. nilagiricum | 57 | 12 | 2 | 7 | 0 | 78 | 54 | 3 | 18 | 3 |
| T. pachyphyllum | 56 | 14 | 2 | 7 | 1 | 80 | 58 | 3 | 16 | 3 |
| T. planicaule | 56 | 14 | 2 | 7 | 0 | 79 | 54 | 3 | 19 | 3 |
| T. pyriforme | 46 | 16 | 4 | 6 | 1 | 73 | 55 | 2 | 14 | 2 |
| T. rafflesiae | 56 | 11 | 2 | 5 | 0 | 74 | 51 | 2 | 19 | 2 |
| T. serrulatum | 51 | 13 | 1 | 9 | 1 | 75 | 57 | 1 | 16 | 1 |
| T. thorsborneorum | 36 | 18 | 2 | 8 | 0 | 64 | 47 | 1 | 15 | 1 |
| T. voinierianum | 55 | 12 | 2 | 6 | 0 | 75 | 48 | 3 | 21 | 3 |
From the perspective of nucleotide repeat count (Figure 3), the SSRs in the 15 Tetrastigma species mainly consist of mononucleotide repeats (70.68%), followed by dinucleotide repeats (17.91%), trinucleotide repeats (2.63%), tetranucleotide repeats (8.34%), and pentanucleotide repeats (0.44%). Among them, pentanucleotide repeats are very rare. In terms of nucleotide composition types (Table S2), considering sequence complementarity, a total of seventeen repeat types were detected, mainly A/T and AT/AT. A detailed analysis of different nucleotide composition types, considering only mononucleotide and dinucleotide repeats, shows that repeats containing C and G bases are exceedingly rare, with A/T and AT/AT accounting for 69.27% and 16.59%, respectively, while C/G and AG/CT account for only 1.40% and 1.32%. This trend is also observed in tetranucleotide repeats, where the number of SSRs without C/G is greater than those with C/G, except for the pairs AAAG/CTTT and AATC/ATTG. However, the total number of these repeats is too small to reliably analyze frequencies solely based on count.
Figure 3.
Analysis of chloroplast genome SSRs in 15 Tetrastigma species. (a) The total number of SSRs in the 15 species; (b) The total number of repeat types classified by repeat unit type and repeat unit count. P1—mono, P2—di, P3—tri, P4—tetra, P5—penta; (c) Statistics of SSR distribution region.
Notably, pentanucleotide repeats were detected in 4 of the 15 Tetrastigma species: T. annamense, T. pachyphyllum, T. pyriforme, and T. serrulatum.
2.3. Codon Usage Bias Analysis
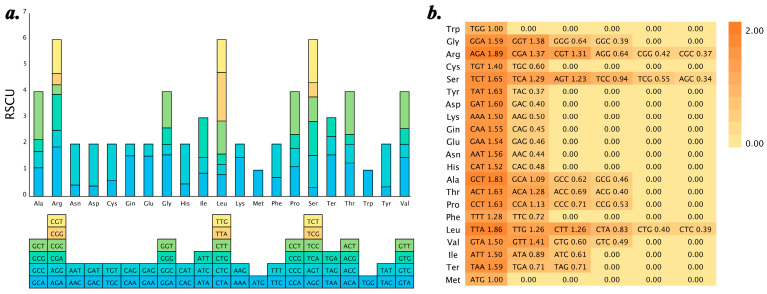
Codon usage and amino acid frequency were analyzed for the fifteen species, excluding stop codons. The number of codons used across the fifteen species showed insignificant variation, ranging from 49,986 in T. leucostaphylum to 50,614 in T. pyriforme with an average of 50,300 codons. The twenty amino acids are encoded by sixty-one codons, with Leu, Arg, Ser, and Phe being the most common, and Met and Trp being the least common. Among the sixty-one codons, TTT and AAA are the most frequent, while CGC and GCG are the least frequent.
RSCU values were calculated, excluding RNA editing and only considering genes with the start codon ATG (Figure 4). The usage frequency of codons was determined by an RSCU value of one, with RSCU > 1 indicating higher usage frequency. For the sixteen species analyzed, RSCU results were presented in the form of stacked bar charts and heatmaps, where the color intensity in the heatmaps represented the magnitude of the RSCU values. The results showed that 30 codons had RSCU > 1, while Trp and Met both had RSCU values equal to one. Most codons tend to end with A/T, with a few exceptions such as Leu (TTG) and Ser (TCG), which end with G.
Figure 4.
Relative synonymous codon usage (RSCU) in Tetrastigma plants, taking T. canarense as an example. (a) Stacked bar chart of the relative synonymous codon usage (RSCU) in Tetrastigma plants; (b) Heatmap displaying RSCU values.
2.4. Comparative Chloroplast Genome Analysis
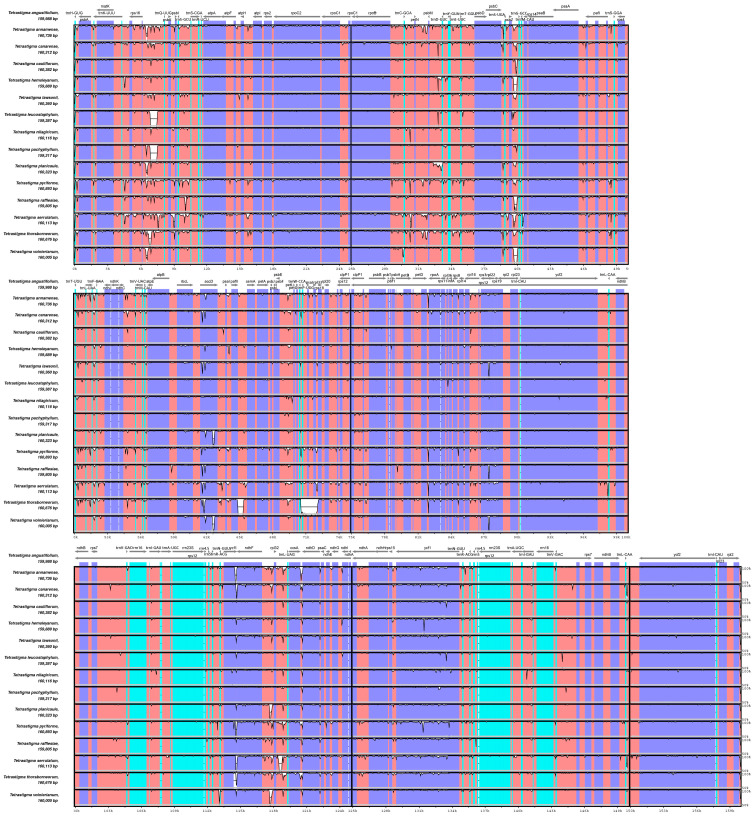
Using the chloroplast genome sequence of T. angustifolium as a reference, the mVISTA program was employed to conduct a comparative analysis of the chloroplast genomes of the remaining 14 Tetrastigma species (Figure 5). The results indicated that the 15 chloroplast genomes exhibit minor differences, with coding region sequences showing less variation than non-coding regions, except for genes such as ycf1 and accD. Significant differences were found in regions such as rps16-trnRUCU, trnCGCA-trnTGGU, trnSUGA-trnGGCC, and ndhF-trnLUAG.
Figure 5.
Visualization of chloroplast genome alignment of 14 Tetrastigma species, using T. angustifolium as a reference. The vertical scale represents the percentage of identity, ranging from 50% to 100%. The horizontal axis denotes the coordinates within the chloroplast genome. Colors are used to indicate distinctive features.
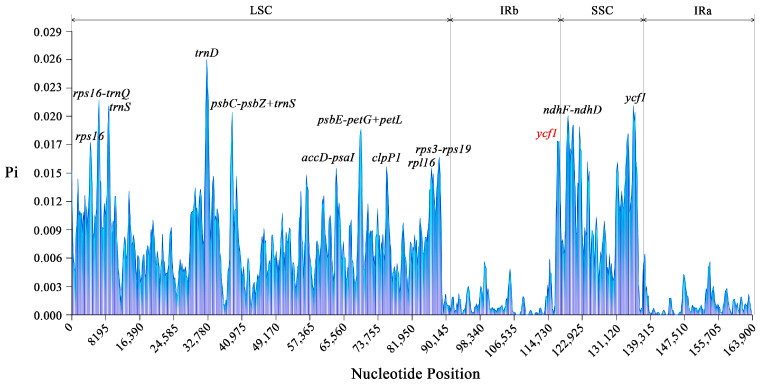
Nucleotide polymorphism (Pi) analysis of the chloroplast genomes of the 15 Tetrastigma species was performed using DnaSP V6. The results (Figure 6) showed that the Pi values of the 15 chloroplast genomes ranged from 0 to 0.02614, with an average Pi value of 0.006013. A total of 13 nucleotide polymorphism hotspots with Pi > 0.015 were detected, with 10 found in the LSC region: rps16, the intergenic region between rps16 and trnQ, trnS, trnD, the intergenic region between psbC-trnS-psbZ, the intergenic region between accD-psaI, psbE-petL-petG, clpPI, rpl16, and rps3-rps19. Two hotspots were detected in the SSC region: ndhF-ndhD and ycfI. One hotspot was detected in the IR region, which is a pseudogene fragment of ycf1. The number of polymorphic sites in the LSC and SSC regions was greater than in the IR region.
Figure 6.
Nucleotide diversity analysis based on sliding windows and the Tetrastigma chloroplast genome sequences. The X-axis represents the length coordinates of the multiple sequence alignment of the fifteen species. The Y-axis represents the nucleotide diversity at each position corresponding to each window. Red represents pseudogenes.
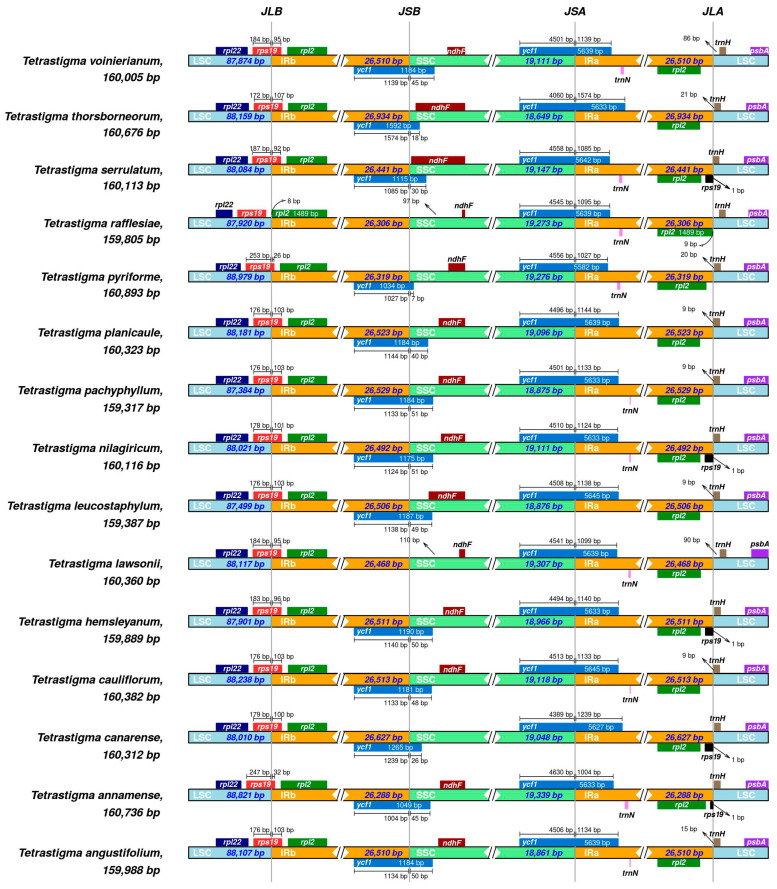
2.5. IR/SC Boundaries Analysis
IRScope was used to compare the IR/SC boundary regions of the 15 Tetrastigma species. (Figure 7). The results indicate that psbA was consistently located in the LSC region, at the IRa/LSC boundary. The distance from trnH to the boundary varied from 1 to 94 bp, all within the LSC region, specifically the distances for T. planicaule, T. pachyphyllum, T. leucostaphylum, and T. cauliflorum were all 9 bp, whereas the distances for T. lawsonii and T. voinierianum were 90 bp and 86 bp, which is significantly different from the other species. The rpl22 gene was consistently located in the LSC region, while the rps19 gene, except for T. rafflesiae, spanned the LSC/IRb boundary in the other fourteen species, with the portion in IRb ranging from 26 to 107 bp from the LSC/IRb boundary. The ndhF gene was in the SSC region, with distances from the IRb/SSC boundary ranging from 4 to 110 bp. The ycf1 gene (5582–5645 bp) spanned the SSC/IRa boundary, and genes partially spanning IR and SC regions were often annotated as pseudogenes, such as the ycf1 pseudogene at the IRb and SSC boundary. Similarly, in the 15 species, certain species such as T. serrulatum had fragments of the rps19 gene annotated in the IRa region, which were also considered pseudogene fragments without the original gene function.
Figure 7.
Comparison of LSC, SSC, and IR boundaries in the chloroplast genomes of 15 Tetrastigma species. Genes are annotated in assorted colors and are labeled with their distances from the boundaries and the lengths of these distances. The lengths of these color blocks are not indicative of the gene lengths but are compressed or expanded based on the differing distances. LSC, SSC, and IR regions are represented in assorted colors. JLB, JSB, JSA, and JLA, respectively, denote the boundaries of the four regions.
2.6. Phylogenetic Analysis
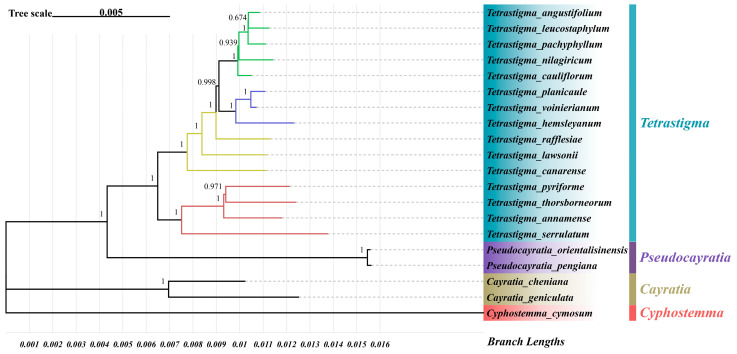
A phylogenetic tree was constructed based on the complete chloroplast genome sequences of the 15 Tetrastigma species, using species from the genera Pseudocayratia (Pseudocayratia pengiana, Pseudocayratia orientalisinensis), Cayratia (Cayratia geniculate, Cayratia cheniana), and Cyphostemma (Cyphostemma cymosum) as outgroups. The results (Figure 8) indicated that all four genera originated from the same clade. The Tetrastigma species clustered into a monophyletic group with 100% bootstrap support, indicating high confidence in the results. Within Tetrastigma, T. serrulatum, T. annamense, T. thorsborneorum, and T. pyriforme formed one clade, while the remaining species formed another clade, both with 100% bootstrap support. Additionally, T. voinierianm and T. planicaule formed a clade with T. hemsleyanum, and T. leucostaphylum and T. angustifolium formed a clade with T. cauliflorum, T. nilagiricum, and T. pachyphyllum.
Figure 8.
Maximum Likelihood phylogenetic tree based on complete chloroplast genomes, with Pseudocayratia pengiana, Pseudocayratia orientalisinensis, Cayratia geniculate, Cayratia cheniana, and Cyphostemma cymosum as outgroups. Bootstrap values are displayed on the branches.
2.7. Divergence Time Estimation
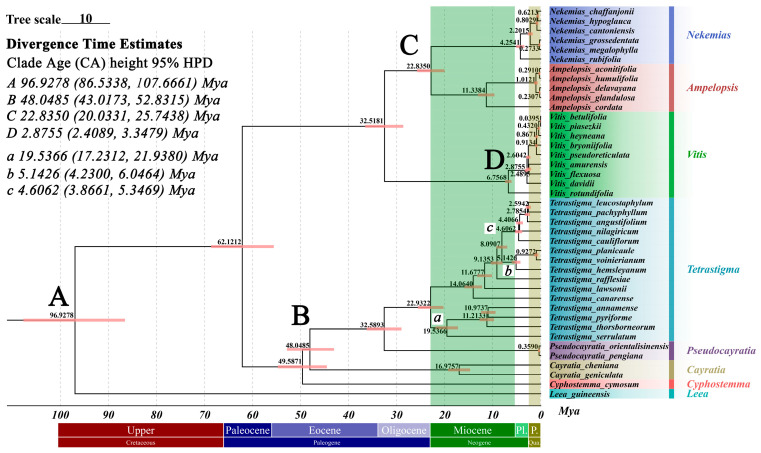
The Bayesian phylogenetic tree (Figure 9) constructed for 41 species within the Vitaceae family reveals detailed divergence times and significant evolutionary events within the family. This tree includes representatives from different genera: Nekemias, Ampelopsis, Vitis, Tetrastigma, Pseudocayratia, Cayratia, and Cyphostemma, with an outgroup reference from the Leeaceae family, Leea. The phylogenetic tree is time-calibrated, with the x-axis marking geological periods (Cretaceous, Paleogene, and Neogene). Nodes A-D represent clade nodes used for priors, where the values indicate the estimated divergence times in this analysis, not the actual priors. The outgroup, Leea guineensis, diverged from the Vitaceae family in the Late Cretaceous, around 96.93 Mya (95% HPD: 86.53–107.67 Mya), indicating a distant relationship and supporting its use as an outgroup. The phylogenetic tree shows clear genus-level separations. At 62.12 Mya, the Vitaceae family split into two branches, with Tetrastigma located in the Cayratieae subfamily. Within the Cayratieae subfamily, Pseudocayratia diverged from Tetrastigma around 32.59 Mya, closely following the divergence from Vitis. The divergence times for Cayratia and Cyphostemma suggest an older history, dating back to 49.59 Mya. For Tetrastigma, the topology of the Bayesian tree is largely consistent with that constructed using the Maximum Likelihood (ML) method, with minor differences. These differences appear mainly at nodes with low bootstrap values in the ML tree, such as the relationships among T. angustifolium, T. leucostaphylum, and T. pachyphyllum. In the Bayesian tree, T. leucostaphylum and T. pachyphyllum show a closer relationship, unlike in the ML tree where T. angustifolium is closer. Following the ML method results, we artificially divided the evolution of Tetrastigma into three clades: Subclade a: diverged around 19.54 Mya (95% HPD: 17.23–21.94 Mya) in the Miocene; Subclade b: diverged around 5.14 Mya (95% HPD: 4.23–6.05 Mya) in the Pliocene; Subclade c: diverged around 4.61 Mya (95% HPD: 3.87–5.35 Mya) in the Pliocene. Diverse divergences occurred in the Miocene, with recent diversification in the Pliocene. Overall, most Tetrastigma species in this study diverged before the Pleistocene, earlier than most of the selected Vitis species.
Figure 9.
Divergence times of Vitaceae and Tetrastigma species estimated based on the shared CDS sequences of the chloroplast genome, with Leea guineensis from the Leeaceae as the outgroup. The mean divergence times are shown next to the nodes, and the red bars correspond to the Clade Age height 95% HPD. (A–D) represent clade nodes used for priors, where the values indicate the results of estimated divergence times in this analysis, not priors. Specifically, (A) represents the deep calibration point for Leea and Vitaceae; (B) the existing fossil record of Tetrastigma; (C) for the genus Ampelopsis; (D) for the subgenus Vitis. (a–c) indicate the estimated divergence times within Tetrastigma, corresponding to the results in 2.6. The bottom of the figure shows the Geologic Time Scale, with the Lower Epoch of the Cretaceous Period (145–100.5 Mya) and the Holocene Epoch of the Quaternary Period (0.0117 Mya–present) omitted.
2.8. Selective Pressure Analyses
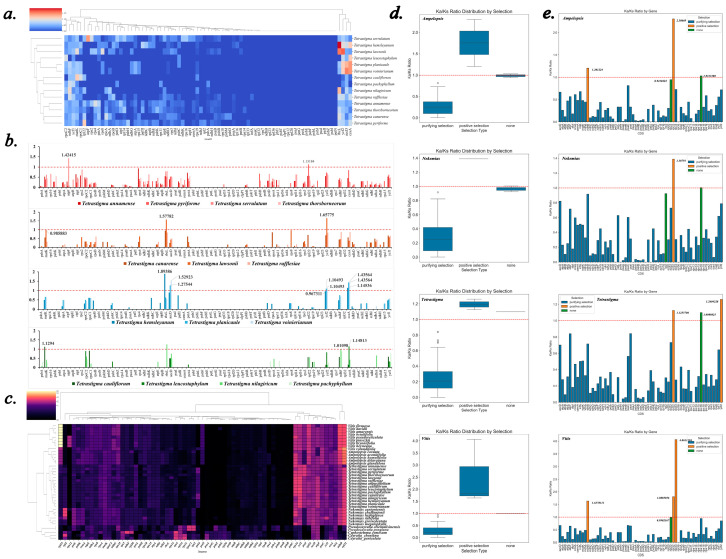
The Ka/Ks ratio was used to distinguish the types of selective pressures acting on genes, with Tetrastigma angustifolium as the reference. Ka and Ks values were calculated for the other 14 Tetrastigma species (Figure 10a). Based on the phylogenetic tree, selective pressure analysis was conducted by grouping the species accordingly (Figure 10b). The results indicated that some genes in Tetrastigma species are under positive selection, while most are under purifying selection.
Figure 10.
Selective pressure analysis results. (a) Cluster heatmap showing the Ka/Ks values of chloroplast genomes from 14 species, using Tetrastigma angustifolium as a reference, the Ka/Ks value varies between 0 and 2, corresponding to a color range of blue to red. (b) A set of graphs displaying the Ka/Ks values of each gene, dividing 14 species into four groups according to the result of the phylogenetic tree. (c) Cluster heatmap showing the Ka/Ks values of chloroplast genomes from 40 species, using Leea guineensis as a reference. The Ka/Ks value varies between 0 and 5, corresponding to a color range of black to yellow. (d) Box plots divided into four units by genus, taking the arithmetic mean of the Ka/Ks values of the common CDS of different species and obtaining a box plot to display the distribution of data. (e) Bar charts drawn by dividing the Ka/Ks values of the common CDS of different species into four units based on their genus, taking the arithmetic mean. Orange represents positive selection, blue represents purifying selection, and green represents Ka/Ks close to 1, indicating no significant selection.
In the clade consisting of T. serrulatum, T. annamense, T. thorsborneorum, and T. pyriforme, only the T. serrulatum’s atpF gene had a Ka/Ks ratio of 1.42415, indicating positive selection. Similarly, T. pyriforme’s rpl22 gene had a Ka/Ks ratio of 1.13184, also under positive selection. In contrast, the atpF and rpl22 genes in the remaining twelve species had Ka/Ks ratios of less than one, indicating purifying selection. Additionally, most genes across these species were under purifying selection. The remaining three clades (T. canarense, T. lawsonii, T. rafflesiae; T. voinierianm, T. planicaule, T. hemsleyanum; T. leucostaphylum, T. cauliflorum, T. nilagiricum, T. pachyphyllum) showed some common patterns. In T. lawsonii and T. hemsleyanum, the rbcL gene had Ka/Ks ratios of 1.57782 and 1.29386, respectively, indicating positive selection. The ccsA gene in T. voinierianm, T. planicaule, and T. hemsleyanum had Ka/Ks ratios greater than one, also suggesting positive selection.
Additionally, an analysis was conducted on 40 species of the Vitaceae family, using Leea guineensis from the Leeaceae family as a reference species. Ka/Ks ratios for 70 CDS sequences from these 40 species were calculated, with genes having a high proportion of NA values filtered out, resulting in 66 CDS being analyzed. A cluster heatmap displayed the specific Ka/Ks values for species and genes (Figure 10c), showing that most genes were dark-colored, indicating significant purifying selection. Furthermore, box plots (Figure 10d) and bar charts (Figure 10e) were used to illustrate the distribution and specific values, as well as the type of selection.
Most Vitaceae species, except those from the Pseudocayratia, Cayratia, and Cyphostemma, exhibited significant bright areas in the rps16-rpl32 region, suggesting strong positive selective pressures in this region, particularly on rpl32. In Ampelopsis species, rpl32 and ndhB showed significant positive selection, with arithmetic mean Ka/Ks ratios of 2.307 and 1.201, respectively. Although the Ka/Ks ratio for the rps16 gene was greater than one, it was not significant, similar to the rpl22 gene, indicating that neither rps16 nor rpl22 underwent significant purifying or positive selection. In the Nekemias species, the Ka/Ks ratio for rpl32 was 1.388, indicating significant positive selection, while the Ka/Ks ratios for rps16 and rpl2 were not significantly different from 1. In the Tetrastigma species, both rpl32 and ycf2 showed significant positive selection, with arithmetic mean Ka/Ks ratios of 1.126 and 1.260, respectively. As in the other genera, the rps16 gene did not have a significantly higher Ka/Ks ratio than one. In the Vitis species, rpl33, rpl32, and ndhB were under significant positive selection, with arithmetic mean Ka/Ks ratios of 4.061, 1.809, and 1.638, respectively. The rpl22 gene, however, was not under significant positive or purifying selection after significance analysis. Genes under significant positive selection in the four genera are recorded (Table 4), along with data on normality and the tests used. Significance analysis was conducted in groups due to considerable inter-genera differences for some genes. The Pseudocayratia, Cayratia, and Cyphostemma exhibited more differentiated characteristics, particularly in the rps16-rpl32 region, which was inconsistent with other genera. In these genera, genes such as rps16, psaI, psaC, ndhB, and rpl32 might be under purifying selection. However, due to the small number of species, no significance analysis was performed for these genera.
Table 4.
CDS with significant positive selection among the four genera.
| Genus | CDS | Ka/Ks Mean | Selection | p-Value | Significant | Normality | Test |
|---|---|---|---|---|---|---|---|
| Ampelopsis | ndhB | 1.201224 | positive selection | 0.006501702 | TRUE | FALSE | Mann–Whitney U |
| rpl32 | 2.30669 | positive selection | 0 | TRUE | TRUE | t-test | |
| Nekemias | rpl32 | 1.38791 | positive selection | 0 | TRUE | TRUE | t-test |
| Tetrastigma | rpl32 | 1.125571 | positive selection | 9.51019 × 10−6 | TRUE | FALSE | Mann–Whitney U |
| ycf2 | 1.260423 | positive selection | 4.58275 × 10−6 | TRUE | TRUE | t-test | |
| Vitis | ndhB | 1.637811 | positive selection | 0.000122063 | TRUE | FALSE | Mann–Whitney U |
| rpl32 | 1.808506 | positive selection | 7.03107 × 10−5 | TRUE | FALSE | Mann–Whitney U | |
| rpl33 | 4.061119 | positive selection | 0.00206442 | TRUE | FALSE | Mann–Whitney U |
3. Discussion
3.1. Chloroplast Genome Basic Characteristics
Tetrastigma is renowned for its ornamental and medicinal significance, and there is a substantial market demand for it. Although the Tetrastigma genus encompasses a variety of species, most of research to date has focused on Tetrastigma hemsleyanum, which has limited resources. The breeding and development foundation of Tetrastigma species is weak. Previous studies on chloroplast genomic DNA regions (atp B-rbcL, atp F-atpH, matK, psbK-psbI, rbcL, rpoC1, rps16, trn C-petN, trn H-psbA, trn L-trnF) and the seed and plant morphological data of the Tetrastigma species [22] have laid a theoretical groundwork for the development of Tetrastigma. This study supplements the aforementioned research by analyzing the genomic characteristics and genetic relationships of fifteen species within the genus based on complete chloroplast genome data. The primary methodology employed was bioinformatics analysis, which involved the assembly of genomes from ten species and the examination of the chloroplast genomes from fifteen species. The size of the Tetrastigma chloroplast genome ranges from 159,387 (T. nilagiricum) to 160,736 bp (T. annamense). In terms of GC content, the GC content in the IR regions is generally higher than that in the LSC and SSC regions, a pattern commonly observed across other species [16,26]. This distribution of GC content may be related to the replication mechanism and gene expression regulation of the chloroplast genome. Šmarda et al. have studied the GC content in monocotyledonous plants and suggested that GC-rich DNA might have an advantage during cellular freezing and desiccation processes, which may imply a potential impact of GC content on the stability of gene expression under specific environmental conditions [27], which may enhance the plants’ resistance to drought and low-temperature stress. The structure of the chloroplast genome, composed of the LSC region, a pair of IR regions, and the SSC region, forms the typical quadripartite structure. The length of the IR regions varies from 26,288 bp (T. annamense) to 26,934 bp (T. thorsborneorum), while the length of the SSC region ranges from 18,649 bp (T. thorsborneorum) to 19,339 bp (T. annamense). This quadripartite structure of the chloroplast genome is an adaptive feature formed during the long-term evolutionary process of plants, contributing to the stability, replication efficiency, and genetic diversity of the genome. Palmer et al. found that when large inverted repeat sequences are lost, chloroplast DNA undergoes more frequent rearrangements [28], and Perry et al. reported that the synonymous substitution rate of IR genes in leguminous plants is 2.3 times lower than that of single-copy (SC) genes [29]. The IR regions may play a role in preventing or reducing harmful effects during genome rearrangements. Additionally, apart from the pseudogenes ψycf1 and ψrps19-fragment, the Tetrastigma chloroplast genome encodes a total of 130 genes, including 85 CDS, 8 rRNA genes, and 37 tRNA genes. The self-replicating genes are crucial for the replication and repair of chloroplast DNA, while the photosynthetic system genes are directly involved in the process of photosynthesis. Other genes and genes with unknown functions may play roles in the biosynthesis, energy conversion, and signal transduction processes within the chloroplast.
3.2. SSRs Analysis
SSRs are a common structural feature in the chloroplast genomes of plants. The results indicate that the predominant type of SSR is mononucleotide repeats, which are predominantly spatially located in the LSC region, with fewer other types of repeats, and the IR regions are highly conserved, mainly composed of A/T bp, similar to previous studies [30,31].
In both mononucleotide and dinucleotide repeats, the content of A/T (69.27%) and AT/AT (16.59%) is significantly higher than that of C/G (1.40%) and AG/CT (1.32%). This pattern is also observed in tetranucleotide repeats, where the number of SSRs without C/G is usually greater than those containing C/G, except for AAAG/CTTT and AATC/ATTG. However, looking at the total count, the number of trinucleotide repeats, pentanucleotide repeats, and similar types is too low to be representative, unlike mononucleotide and dinucleotide repeats.
The distribution and types of SSRs may vary among varied species due to a variety of factors, including genetic diversity, evolutionary pressure, and environmental adaptability. The number of repeats in SSRs can affect gene regulation, transcription, and protein function, providing a source of quantitative and qualitative variation [32]. Eukaryotic genomes contain a rich array of mononucleotide repeats (MNRs), and studies have shown that MNRs may have a certain relationship with single nucleotide polymorphisms (SNPs), possibly increasing the frequency of SNPs at adjacent positions [33]. The concentrated distribution of SSRs in the LSC region may be related to the higher gene density and functional diversity of this region, while the scarcity of SSRs in the IR regions may be related to their role in maintaining genomic stability. It is noteworthy that among the 15 Tetrastigma species, four species were detected to have pentanucleotide repeats: T. annamense, T. pachyphyllum, T. pyriforme, and T. serrulatum. These pentanucleotide repeats may play a role in the identification of these species, and in fact many studies have already used this approach for species identification [34], with related molecular markers also being developed. The diversity and distribution of SSRs are of significant importance for the adaptability and evolution of species and can serve as genetic markers to help study the genetic structure and intraspecific variation of species.
3.3. Condon Usage Analysis
This study conducted an in-depth analysis of codon usage and amino acid frequency across fifteen varied species, aiming to explore the conservation and variability in codon usage preferences among varied species. The results indicate that despite certain differences, there is a low variation in the overall number of codon usages among varied species, which may be closely related to the efficiency and accuracy of gene expression. During the evolutionary process, to maintain the stability of protein function, natural selection may favor the retention of codon usage patterns that can optimize the translation process. Most codons tend to end with A or T, which may be related to the stability of mRNA and translation efficiency. If the third position of a codon is A or T, it may be more easily recognized by tRNA, thereby increasing the translation speed. In fact, compared with other codons, the optimal codons rich in AT can generally reduce the energy required for mRNA folding, thus improving translation efficiency [35]. Our research results are consistent with this theory, and this preference may be universally present across varied species. The analysis of RSCU values further reveals the variability in codon usage frequency. We found that the RSCU values of thirty codons are greater than one, indicating that the usage frequency of these codons is higher than the average level.
3.4. IR/SC Boundaries Analysis
This study utilized IRScope to conduct an in-depth analysis of the IR/SC boundary regions of the chloroplast genomes in 15 Tetrastigma species. By comparing the gene distribution and variability at the IR/SC boundaries among varied species, we revealed the conservation and variability of the chloroplast genome structure, as well as the potential impacts of these variations on gene annotation and functional integrity. We found that the rpl22 and psbA genes are located in the LSC region in all studied Tetrastigma species, further confirming the conservation of the LSC region in these species, consistent with the findings of Dong et al. [36]. In contrast, the rps19 gene spans the LSC/IRb boundary in all but one species, T. rafflesiae, which is also in line with the results of Dong et al., who found that even within the same species the spatial situation of the chloroplast genome’s IR/SC boundary can vary, especially in the distribution of rps19 and rpl2 [36], indicating that there may be certain variability within the Tetrastigma genus. At the IRa/LSC boundary, the spatial length from the trnH gene to the boundary varies from 1 to 94 bp. Notably, the distances of trnH to the boundary in T. lawsonii and T. voinierianum are 90 and 86 bp, respectively, showing a significant difference from the other species, and this variability may indicate the uniqueness of these two species in their evolutionary process. Additionally, the position of the ndhF gene in the SSC region also exhibits some variability. The chloroplast genome is highly conserved, especially in the IR regions, and genes spanning the IR and SC regions are prone to having incomplete gene fragments annotated on the opposite side (usually considered pseudogenes), such as the segment of ycf1 spanning SSC/IR that exists in IRa can be found in IRb, leading to the incorrect annotation of fragments of ycf1. This phenomenon is consistent with the results of Feng et al. [37,38].
3.5. Comparing the Polymorphism of Chloroplast Genomes
Based on the chloroplast genomes of 15 Tetrastigma species, the Pi value was calculated. In other species, such as those of the Pleione genus, 12 polymorphic sites were detected in the chloroplast genome at Pi > 0.011 [26]. However, even with a threshold set at Pi > 0.015, a considerable number of polymorphic sites (13 in total, some of which are formed by the connection of multiple genes) were still detected in the chloroplast genomes of Tetrastigma species. The results of mVISTA and Pi analysis together revealed polymorphic sites within the chloroplast genome such as rps16, trnS, and so on (rps16-trnRUCU, trnCGCA-trnTGGU, trnSUGA-trnGGCC, and ndhF-trnLUAG; 10 in LSC: rps16, rps16-trnQ, trnS, trnD, psbC-trnS-psbZ, accD-psaI, psbE-petL-petG, clpPI, rpl16, rps3-rps19; 2 in SSC: ndhF-ndhD and ycfI). Chloroplast markers developed based on polymorphic sites have a wide range of applications. Amar et al. used the ycf1-ndhF region as a plastid barcode for low-level taxonomic phylogenetic analysis of Prunus persica, validating the effectiveness of this site [39]. Therefore, based on the aforementioned detected polymorphic sites, corresponding chloroplast markers can be developed, which can accurately identify Tetrastigma species and are of great significance for their interspecific identification and phylogenetic analysis.
3.6. Phylogenetic Relationships and Divergence Time Estimation
3.6.1. Phylogenetic Relationships
The Tetrastigma genus holds immense medicinal value, and precise identification of Tetrastigma species is crucial for the effective use of their medicinal resources. Therefore, understanding the phylogenetic relationships within the Tetrastigma genus has become a focal point of interest. This study, which constructed a phylogenetic tree based on chloroplast genome sequences, provides new insights into the evolutionary history and classification of Tetrastigma plants. The monophyletic clustering and high support values for Tetrastigma species underscore the stability of their taxonomy and their shared evolutionary lineage.
Previous studies have also attempted to elucidate the phylogenetic relationships within Tetrastigma using various molecular markers. For instance, Habib et al. constructed a phylogenetic tree for the Vitaceae family using 10 chloroplast DNA markers (atpB-rbcL, atpF-atpH, matK, psbK-psbI, rbcL, rpoC1, rps16, trnC-petN, trnH-psbA, trnL-trnF) and studied the phylogenetic relationships within the Tetrastigma genus [22]. This study corroborates some of the findings of that research, confirming the close phylogenetic relationship between T. thorsborneorum and T. pyriforme. Moreover, in the classification by Habib et al., T. hemsleyanum, T. voinierianum, and T. planicaule, although clustered into a larger group, still show some distance. Phylogenetic trees constructed using chloroplast DNA markers and those based on complete chloroplast genomes may differ, especially when the relationships are close. In fact, the analysis presented in this study suggests that, in addition to the chloroplast DNA markers used by Habib et al., genes such as trnD and ycf1 are also suitable as markers [39,40]. Considering more factors comprehensively may lead to more refined results.
The clustering relationship of T. cauliflorum, T. nilagiricum, T. pachyphyllum, T. leucostaphylum, and T. angustifolium (with 100% support value) further supports their close phylogenetic relationship within the Tetrastigma genus. However, the lower support value (67.4%) between T. leucostaphylum and T. angustifolium suggests that the evolutionary history of these species may be more complex and could be influenced by gene flow or other factors. Phylogenetic analysis based on the chloroplast genome provides a reliable framework for understanding the genetic relationships among species within the Tetrastigma genus. However, the lower support values for some branches indicate that further research is needed to fully resolve the evolutionary history of these species. Future studies could include more molecular markers and expand taxonomic sampling, including more Tetrastigma species and related genera, to gain a more comprehensive understanding of their phylogenetic relationships.
3.6.2. Divergence Time Estimation
A time-calibrated tree allows for researchers to better understand the phylogenetic relationships, evolutionary history, and other aspects among species, playing a crucial role when conventional methods are ineffective [41]. This study, through the construction of a Bayesian phylogenetic tree, reveals detailed divergence times for 41 species within the Vitaceae family, providing profound insights into significant evolutionary events within the family. Notably, the topologies of the Bayesian tree and the Maximum Likelihood (ML) tree are generally consistent, with only minor differences at nodes with low bootstrap values. In the Bayesian tree, T. leucostaphylum and T. pachyphyllum show a closer relationship, unlike T. angustifolium in the ML tree. This might reflect the advantages of the Bayesian method in handling evolutionary rate variation and time calibration.
First, the divergence time between the outgroup Leea guineensis and the Vitaceae family is approximately 96.93 Mya (95% HPD: 86.53–107.67 Mya), occurring in the Late Cretaceous. This result not only confirms the validity of using Leea guineensis as an outgroup prior, but also emphasizes its distant relationship with the Vitaceae family, consistent with previous findings [42,43]. The classification of Leea has been debated; some scholars consider Leea a part of the Vitaceae family. However, modern perspectives based on morphological traits such as upright shrub habits and the absence of tendrils classify it as a separate family, Leeaceae. The divergence time between Leea and the Vitaceae family during the Cretaceous period, significantly earlier than within-family divergences, supports this separation.
Within the Vitaceae family, the Cayratieae subfamily includes the genera Tetrastigma, Pseudocayratia, Cayratia, and Cyphostemma. For Tetrastigma, results indicate that Pseudocayratia and Tetrastigma diverged during the Oligocene period. Previous reports mostly place this divergence in the Eocene period [23,24], earlier than our study, but consistent at the species level for specific divergence times, such as T. rafflesiae and T. lawsonii, diverging around 15 Mya, aligning with previous findings [42]. This suggests significant divergence events in Tetrastigma during both the Oligocene and Miocene periods. However, some studies analyzing the divergence time of Cyphostemma propose that the divergence of Tetrastigma can be traced back to the Cretaceous period [43], much earlier than the Oligocene period. This discrepancy is related to various factors, such as the choice of fossil calibration points, molecular clock models, and the type of molecular data used, such as nuclear or chloroplast gene markers. Empirical data have a limitation in that the actual divergence times are seldom known, making it difficult to judge the best method when age estimates differ [44]. Nonetheless, it is clear that significant events occurred between the Oligocene and Cretaceous periods. A recent study reveals a profound history of extinction and dispersal in the Neotropics, based on fossil seeds of Vitaceae from the Cenozoic, documenting the extinction patterns of the family [45]. Furthermore, this study found that multiple significant divergence events occurred before the Pleistocene. Specifically, three subclades of Tetrastigma diverged during the Miocene (19.54 Mya) and the Pliocene (5.14 Mya and 4.61 Mya), indicating that the genus underwent multiple adaptive radiations and evolutionary events throughout its geological history. Outside of the Cayratieae subfamily, the relationship between the genera Nekemias and Ampelopsis within the family Vitaceae has been estimated to diverge at a time close to that of the genus Vitis, consistent with a previous study [46].
3.7. Selective Pressure Analysis
3.7.1. Selective Pressure Analysis of the Tetrastigma Genus
Selective pressure, also known as evolutionary pressure, refers to the external forces exerted on an organism during its evolutionary process, thereby altering the direction of this process. Darwin’s concept of natural selection, or survival of the fittest, indicates that selective pressures from the natural environment enable those organisms best adapted to survive and reproduce. The Ka/Ks analysis is crucial in studying the molecular evolution of nucleic acids. It represents the ratio between the nonsynonymous substitution rate (Ka) and the synonymous substitution rate (Ks) of two protein-coding genes. This ratio can determine whether selective pressure is acting on a protein-coding gene.
| (1) |
| (2) |
The Ka/Ks ratio reveals the type of selection acting on a gene: Ka >> Ks or Ka/Ks >> 1 indicates positive selection; Ka = Ks or Ka/Ks = 1 indicates neutral evolution; Ka << Ks or Ka/Ks << 1 indicates purifying selection.
The selective pressure analysis for Tetrastigma angustifolium and other Tetrastigma species suggests that some genes in the Tetrastigma genus may play a significant role in adapting to environmental pressures. Purifying selection is widespread, with most genes in the Tetrastigma species showing a Ka/Ks ratio of less than one, indicating that they are primarily influenced by purifying selection, where natural selection tends to remove harmful non-synonymous mutations to maintain the stability of these genes. The positive selection of the atpF gene may be related to its function in photosynthesis, while the positive selection of the rpl22 gene may be related to the efficiency or accuracy of protein synthesis, which could be downregulated or upregulated under certain stress conditions to help cells adapt to environmental changes. Amanda et al.’s research has confirmed that rpl22 may be involved in the cell’s response to certain stress conditions, helping cells adapt to environmental changes by regulating its expression levels [47]. The positive selection of the rbcL and ccsA genes may be related to the adaptability of these species in specific ecological niches. The rbcL gene encodes a key enzyme in photosynthesis, and the ccsA gene participates in the biosynthesis of chloroplasts. The positive selection of these genes may indicate that the Tetrastigma species have undergone specific adaptive evolution in photosynthesis and chloroplast function. The results of the Ka/Ks analysis also correspond with the phylogenetic tree constructed in this study.
3.7.2. Selective Pressure Analysis of Vitaceae Family
This study found that the rpl32 gene in most Vitaceae species is under significant positive selection, excluding species from the genera Pseudochayratia, Cayratia, and Cyphosemma. Meanwhile, the rps16 gene exhibited a stable neutral situation, with Ka/Ks ratios close to one even in the Vitis species. Both rps16 and rpl32 encode ribosomal proteins that play critical roles in protein synthesis. A study on rice highlighted the importance of the rpl32 gene under abiotic stress conditions [48]. Although this study focused on rpl32 genes located on chromosomes 8 and 9 rather than the chloroplast genome, it suggests potential functions for chloroplast rpl32 due to gene transfer and exchange involving nuclear, mitochondrial, and chloroplast genes. A study on Euphorbia schimperi also revealed the evolutionary fate of rpl32 and rps16 [49]. Similarly, research has identified the transfer of the rpl32 gene from the chloroplast to the nuclear genome, accompanied by the acquisition of new transit peptides [50]. Studies on the Ranunculaceae family reported pseudogenization of the rpl32 gene, with deletions near the 5′ end leading to internal stop codons [51]. Additionally, chloroplast-encoded rps16 has been replaced by nuclear-encoded rps16 products, indicating possible pseudogenization of rps16 as well [52].
The Ka/Ks ratios in the Vitis genus are particularly noteworthy, especially for the rpl33 gene. The rpl33 gene exhibited exceptionally high Ka/Ks ratios, indicating strong positive selection, consistently observed among Vitis species sampled in this study. The rpl33 gene encodes another ribosomal protein, and a study on tobacco demonstrated that rpl33 is essential for maintaining adequate plastid translation capacity under low-temperature stress [53]. Similarly, in Arabidopsis, chloroplast-encoded rpl33 has been shown to be necessary for survival under cold stress [53]. These findings suggest that rpl33 may influence plant tolerance to cold stress. This unique feature of Vitis aligns with previous divergence time estimates, which suggest that Vitis diverged approximately 32.518 million years ago, during the Oligocene period, consistent with previous studies [54]. The reduction in atmospheric CO2 levels during the Oligocene likely caused global cooling [55]. The Eocene–Oligocene transition around 34 million years ago is one of the most significant cooling events in Cenozoic climate evolution. Given the high Ka/Ks ratio of the rpl33 gene, it is plausible that mutations in the ancestral rpl33 gene of Vitis enabled its survival during the Oligocene global cooling event. This timing aligns well with other evidence, such as geographic relationships, which require further investigation. However, it is certain that rpl33 in the genus Vitis is a key locus distinguishing it from other genera within the Vitaceae family. In addition to the genera involved in this study, previous research has found that rpl33 in the genera Cissus, Parthenocissus, and Clematicissus is under significant purifying selection [56,57]. Besides rpl33, the ndhB gene in Vitis also showed significant positive selection, possibly related to the photoperiod [58].
The genera Ampelopsis and Nekemias showed significantly reduced Ka/Ks ratios for the psaI gene, all below 0.5, indicating strong purifying selection. However, the study by Zhang et al. suggests that psaI in the genus Ampelopsis is under significant positive selection, attributing this to the creeping growth habit of the species, which benefits from enhanced competition for sunlight [57]. The precise function of the psaI gene remains unclear, as studies attempting to knock out psaI found that PSI redox reactions do not require psaI [59]. Unfortunately, the study does not specify the reference species used for selective pressure analysis, leading us to hypothesize that the differences may be due to the choice of reference species. Another similar study, which conducted a comparative analysis between the genera Vitis and Ampelopsis, concluded that psaI is under significant purifying selection, consistent with our findings [60]. For rpl22, a study comparing protein-coding genes between Ampelopsis and Vitis found that the Ka/Ks ratio of the rpl22 gene was significantly greater than one [61], contrary to our findings. Our study found that relative to the Leeaceae family, the Ka/Ks value for the rpl22 gene is generally close to one or zero, with no values significantly greater than one. This discrepancy may be due to pseudogenization of the rpl22 gene [62] or the choice of study samples.
Using Leea guineensis as a reference, this study identified common features among Vitaceae species, such as the significant positive selection of the rpl32 gene. The high Ka/Ks ratio of the ycf2 gene also distinguishes Tetrastigma species. The Ka/Ks ratios of ycf2 in species outside the genus Tetrastigma align with the findings of Zhang et al. In the genera Ampelopsis and Nekemias, the Ka/Ks ratios of ycf2 are all below one, indicating significant purifying selection [57]. Hypothetical chloroplast open reading frames (ycf) are sequences with unknown functions in the plastid genome [63], similar to ycf1. The high variability of ycf2 makes it a potential DNA barcode at the species level. However, like rpl22 and rps16, ycf2 may also undergo pseudogenization [64]. Previous divergence time estimates suggest that Tetrastigma diverged earlier than Vitis, Ampelopsis, and Nekemias, consistent with earlier studies [24]. The variability of ycf2 may have been beneficial for the evolution of Tetrastigma and thus retained. In contrast to Vitis, Tetrastigma showed significant purifying selection for genes like ndhB and rpl33.
Notable differences in selective pressure patterns were observed in certain genera, such as Pseudocayratia, Cayratia, and Cyphosemma, particularly in the rps16-rpl32 region. These genera exhibited purifying selection on several genes, including rps16, psaI, psaC, ndhB, and rpl32. Furthermore, a study calculated the Ka/Ks ratios for the genus Cissus and, similar to Pseudochayratia, Cayratia, and Cyphosemma, observed significant purifying selection at loci such as rps16 and psaI [56], contrasting with the positive or neutral selection generally found in other Vitaceae genera. In addition, for the genera Cissus and Cyphostemma, Zecca et al. identified their distinctiveness in terms of increased substitution rates. Our findings also support those of Zecca et al. [65]. Further research is needed to explore the reasons for these differences, given the limited chloroplast genome data for these species.
4. Materials and Methods
4.1. Sequence Assembly and Annotation
Genomic sequencing data for 10 Tetrastigma species were downloaded from NCBI, and the SRA files were converted into paired-end sequencing files using NCBI’s sra toolkit (v2.11.3). Data quality control of the raw data was performed using Fastp (v0.12.4) [66], resulting in clean_data. The filter_reads.pl script from NOVOplasty (v4.3.1) [67], with the published chloroplast genome of Tetrastigma hemsleyanum as the reference sequence, was utilized to filter chloroplast genome reads from the clean_data of the 10 species. Assembly was conducted using Getorganelle (v1.7.7.0) [21], producing circular assemblies for all 10 species that conform to the quadripartite structure of chloroplast genomes. The assembled sequences were used as references for alignment with the corresponding raw data, with a kmer setting of 125, ensuring good coverage of chloroplast genome reads for all ten species and resulting in assemblies without N-base.
Theoretically, there are homologous regions between the chloroplast genome and the nuclear genome. By aligning with reference sequences, homologous regions in the nuclear genome can also be identified. However, these homologous sequences do not affect the assembly process. This is because the copy number of these sequences in the chloroplast genome is significantly higher than in the nuclear genome, resulting in a much higher sequencing depth for the chloroplast genome in the same dataset. Typically, the data used have low sequencing depth for the nuclear genome, which makes it easy for these sequences to be discarded during assembly, thereby directly yielding a complete assembly result. Even when using high-depth data, the depth of the chloroplast genome remains much higher than that of the nuclear genome, facilitating the removal of nuclear genome fragments during assembly.
Chloroplast genome data for an additional five Tetrastigma species were downloaded from NCBI. Sequencing data for five species within the Vitaceae family were also downloaded, filtered, and assembled for the construction of a phylogenetic tree. GeSeq [68] was used to annotate the chloroplast genomes of 15 Tetrastigma species, and annotations were manually refined using the Geneious (R9.0.2) [69]. Physical circular maps of the plant chloroplast genomes were created using the OGDRAW (v1.3.1) [70].
4.2. Sequence Analysis and Statistics
Based on the assembly and annotation results, the lengths, GC content, and other basic information of the chloroplast genomes for the 15 Tetrastigma species were calculated. The MISA microsatellite finder (v2.1, 25 August 2020) [71] was used to identify Simple Sequence Repeats (SSRs) in the Tetrastigma chloroplast genomes. The repeat units included mononucleotide, dinucleotide, trinucleotide, tetranucleotide, pentanucleotide, and hexanucleotide repeats, with minimum repeat parameters set at 10, 5, 4, 3, 3, and 3, respectively. The shared CDS sequences of 15 Tetrastigma species were extracted, codon usage and relative synonymous codon usage (RSCU) were analyzed, with RSCU values calculated and visualized.
4.3. Genome Comparison and Sequence Divergence Analyses
Information extracted from the annotation files was used for chloroplast genome comparison analysis with mVISTA (v2.0). DnaSP V6 [72] was utilized to calculate the nucleotide diversity (Pi) values for the 15 species, with a sliding window of 600 bp and a step size of 200 bp. The results were visualized using Excel, and the genes corresponding to the diversity hotspots were annotated. IRScope (16 March 2018) [73] was employed to illustrate the contraction and expansion of the IR/SC region boundaries.
4.4. Phylogenetic Analysis
The raw sequencing data for five species within the Vitaceae family were downloaded from NCBI, and their corresponding chloroplast genomes were assembled. MAFFT (v7.310) [74] was used to perform multiple sequence alignment of the chloroplast genomes for 20 species. The aligned sequences were then imported into MEGA11 [75] to determine the best DNA/protein model (ML). Using the Maximum Likelihood method, the GTR+G+I model was selected (Table S3), with the Bootstrap set to 1000, to construct the phylogenetic tree.
4.5. Divergence Time Estimation
A total 41 species were used for analysis, including those from the genera Ampelopsis, Vitis, and Tetrastigma in the Vitaceae family, along with an outgroup reference from the genus Leea. The common CDS sequences of the 41 species were extracted and aligned using MAFFT (v7.310), resulting in an alignment length of 64,116 bp. BEAST (v2.7.7) was used to estimate the divergence time of the Tetrastigma genus. Phylosuite (v1.2.3) was employed to calculate the best model, and the TVM+F+G4 model was chosen as the substitution model. The Gamma Category Count was set to 4, Shape to 1.1, and Frequencies to Empirical. Strict molecular clock was used. The prior tree was determined to be the Yule model, with four calibration points (a–d) set to normal distributions: (a) the deep calibration point for Leea and Vitaceae, with a mean of 85 Mya and a SD of 4 Mya, providing a 95% confidence interval (CI) of 77.6–92.6 Mya [76]; (b) based on the existing fossil record of Tetrastigma (https://paleobiodb.org/classic/checkTaxonInfo?taxon_no=418545&is_real_user=1, accessed on 12 July 2024.), with a mean of 61 Mya and a SD of 2.55 Mya, providing a 95% CI of 56.0–66.0 Mya; (c) for the genus Ampelopsis, with a mean of 41.2 Mya and a SD of 9.59 Mya, providing a 95% CI of 23.4–61.0 Mya [23]; (d) for the subgenus Vitis, with a mean of 3.2 Mya and a SD of 0.5 Mya, providing a 95% CI of 2.22–4.18 Mya [77].
We performed two runs, each consisting of 50,000,000 generations, and used Tracer to check for convergence. The results of the runs were combined using LogCombiner (v2.7.7). Finally, the resulting tree was obtained using TreeAnnotator (v2.7.7) with 20% burn-in.
4.6. Selective Pressure Analysis
Coding sequences (CDSs) and protein sequences were extracted from the GenBank files of 15 Tetrastigma chloroplast genomes. BLASTN (v2.14.0+) was utilized to compare other protein sequences against a reference protein sequence to identify the best matches, thereby obtaining homologous protein sequences. MAFFT (v7.310) [75] was employed for automatic alignment of the homologous protein sequences. The aligned protein sequences were mapped back to the coding sequences to obtain aligned CDS. The Ka and Ks values were computed using KaKs_Calculator3 [78] based on the MLWL method.
Python (v3.9.6) was used to analyze Ka/Ks values for multiple genes from Excel files. The pandas library (v2.2.2) was utilized to read the data. For statistical analysis, a normality test on the Ka/Ks values of each gene was conducted using the Shapiro–Wilk test, implemented in the scipy.stats.shapiro (v1.9.1) function. If the p-value was greater than the significance level (α = 0.05), the data were considered normally distributed. Subsequently, hypothesis testing was performed. For normally distributed data, a one-sample t-test (scipy.stats.ttest_1samp) was used to determine whether the mean Ka/Ks value significantly differed from one. For non-normally distributed data, the Mann–Whitney U test (scipy.stats.mannwhitneyu) was applied to assess whether the Ka/Ks values significantly differed from one. For confidence interval estimation, the t-distribution (scipy.stats.t.ppf) was used to calculate the 95% confidence interval for normally distributed data. For non-normally distributed data, the interquartile range (25th and 75th percentiles) was used as a non-parametric confidence interval. In the interpretation of results, significance was determined if the p-value was less than the significance level (α = 0.05). If the t-test statistic was greater than zero and the result was significant, or if the Mann–Whitney U test indicated that the mean Ka/Ks value was greater than one and the result was significant, positive selective pressure was inferred. Conversely, if the t-test statistic was less than zero and the result was significant, or if the Mann–Whitney U test indicated that the mean Ka/Ks value was less than one and the result was significant, purifying selective pressure was inferred. Otherwise, no significant selective pressure was concluded.
5. Conclusions
This study assembled the complete chloroplast genomes of ten species within the genus Tetrastigma and annotated and analyzed the chloroplast genomes of fifteen species within the genus. The results indicate that: (1) through SSR analysis, we detected a total of 1139 SSRs in the chloroplast genomes, with a higher proportion of A/T repeats, and identified unique pentanucleotide repeats in five plants, which have potential as SSR markers. Comparative analysis identified several polymorphic hotspots in the chloroplast genomes of Tetrastigma species (rps16-trnRUCU, trnCGCA-trnTGGU, trnSUGA-trnGGCC, and ndhF-trnLUAG; 10 in LSC: rps16, rps16-trnQ, trnS, trnD, psbC-trnS-psbZ, accD-psaI, psbE-petL-petG, clpPI, rpl16, rps3-rps19; 2 in SSC: ndhF-ndhD and ycf1). These loci and the detected SSRs can be used to develop DNA markers. (2) Phylogenetic analysis provided a high-confidence understanding of the evolutionary relationships within the Tetrastigma genus. Phylogenetic analysis based on the entire chloroplast genome grouped Tetrastigma species into a monophyletic clade, with T. serrulatum, T. annamense, T. thorsborneorum, and T. pyriforme showing close relationships. (3) We estimated the divergence times based on 40 Vitaceae species. The differentiation of the Vitaceae family can be traced back to 96.96 Mya, while Tetrastigma species can be traced back to the Oligocene, with diversification occurring during the Miocene and Pliocene, and possibly divergence events before the Eocene. This timeline is crucial for comparing the evolutionary history of Tetrastigma with other genera in the Vitaceae family. Additionally, this study used a strict molecular clock for analysis, showing good convergence; the evaluation of other molecular clocks requires further study. (4) This study investigated the selective pressures experienced by key genes in the Tetrastigma genus, finding significant positive selection in genes such as atpF, rbcL, and accD. Furthermore, at the broader Vitaceae family level, selective pressure analysis highlighted evolutionary features distinguishing the Tetrastigma genus from other genera, with most genes under purifying selection. The high Ka/Ks ratio of the rpl32 gene is a notable feature of Vitaceae evolution. Additionally, significant positive selection in the rpl33 and ndhB genes played an important role in the evolution of the Vitis genus, while significant positive selection in the ycf2 gene is a characteristic of Tetrastigma evolution. This part of the study helps to understand how the Tetrastigma genus adapts to its environment and how these evolutionary pressures compare to those in other genera of the Vitaceae family. Furthermore, the analysis of the rpl22 gene’s results differed from previous studies; additionally, this study did not explore the unique selective pressures in the genera Pseudocayratia, Cayratia, and Cyphosemma, which are worth further research. The results of this study provide valuable data on SSR and SNP markers, phylogenetic relationships, divergence times, and selective pressures. These findings enhance our understanding of the genetic diversity, evolutionary history, and adaptive mechanisms of the Tetrastigma genus, laying a solid foundation for future research in population genetics, species identification, and conservation biology of this genus.
Acknowledgments
We thank Nanjing GenePioneer Biotech Co., Ltd. for providing the free online analysis platform. Some parts of this study were conducted using their tools, which significantly assisted research. We also want to express gratitude to Xiaojian Qu for sharing the statistical scripts. These scripts can be found on GitHub (https://github.com/quxiaojian/Bioinformatic_Scripts, accessed on 8 April 2024.).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms25158290/s1.
Author Contributions
Conceptualization, P.X. and J.Z.; methodology, J.Z.; validation, J.Z., Y.H. and P.X.; formal analysis, J.Z. and W.C.; data curation, J.Z., Y.H. and W.C.; writing—original draft preparation, J.Z.; writing—review and editing, Y.H., W.C. and P.X.; visualization, J.Z.; supervision, P.X.; project administration, P.X.; funding acquisition, P.X. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
All raw data from this study can be downloaded from NCBI, with the corresponding accession numbers listed in the Supplementary Materials (Table S1). Additionally, data generated during the analysis can be obtained by contacting the corresponding author.
Conflicts of Interest
The authors declare no known competing interests or personal relationships which could have appeared to influence the work reported in this paper.
Funding Statement
This work was supported by the Zhejiang Provincial Natural Science Foundation of China (LY23H280013) and Zhejiang Province “Three agriculture nine side” agricultural science and technology cooperation program (2023SNJF031).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Gong W., Liu T., Zhou Z., Wu D., Shu X., Xiong H. Physicochemical characterizations of starches isolated from Tetrastigma hemsleyanum Diels et Gilg. Int. J. Biol. Macromol. 2021;183:1540–1547. doi: 10.1016/j.ijbiomac.2021.05.117. [DOI] [PubMed] [Google Scholar]
- 2.Dao P.T.A., Quan T.L., Mai N.T.T. Antioxidant Constituents from the Stem of Tetrastigma erusbescense Planch. (Vitaceae) [(accessed on 12 July 2024)];Nat. Prod. Sci. 2014 20:22–28. Available online: https://koreascience.kr/article/JAKO201413240715681.page. [Google Scholar]
- 3.Liao S., Cai W., Chen D., Xie P., Huang J., Zhu X., Ma G. Anti-inflammatory and Analgesic Effects of the Extracts of Tetrastigmatis Hemsleyanum’s Aerial Parts from Fujian In Vivo. Chin. J. Mod. Appl. Pharm. 2017;34:319–324. doi: 10.13748/j.cnki.issn1007-7693.2017.03.004. [DOI] [Google Scholar]
- 4.Feng Z., Hao W., Lin X., Fan D., Zhou J. Antitumor activity of total flavonoids from Tetrastigma hemsleyanum Diels et Gilg is associated with the inhibition of regulatory T cells in mice. OncoTargets Ther. 2014;7:947–956. doi: 10.2147/ott.S61794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han B., Zhai Y., Li X., Zhao H., Sun C., Zeng Y., Zhang W., Lu J., Kai G. Total flavonoids of Tetrastigma hemsleyanum Diels et Gilg inhibits colorectal tumor growth by modulating gut microbiota and metabolites. Food Chem. 2023;410:135361. doi: 10.1016/j.foodchem.2022.135361. [DOI] [PubMed] [Google Scholar]
- 6.Li Q., Chai W., Xia P. Explore the genetic diversity of the genus Pleione based on ITS and SCoT marker. Biochem. Syst. Ecol. 2022;103:104450. doi: 10.1016/j.bse.2022.104450. [DOI] [Google Scholar]
- 7.Bocianowski J., Nowosad K., Wróbel B., Szulc P. Identification of Associations between SSR Markers and Quantitative Traits of Maize (Zea mays L.) Agronomy. 2021;11:182. doi: 10.3390/agronomy11010182. [DOI] [Google Scholar]
- 8.Pidigam S., Thuraga V., Munnam S.B., Amarapalli G., Kuraba G., Pandravada S.R., Nimmarajula S., Sudini H.K. Genetic diversity, population structure and validation of SSR markers linked to Sw-5 and I-2 genes in tomato germplasm. Physiol. Mol. Biol. Plants. 2021;27:1695–1710. doi: 10.1007/s12298-021-01037-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choudhury A., Deb S., Kharbyngar B., Rajpal V.R., Rao S.R. Dissecting the plant genome: Through new generation molecular markers. Genet. Resour. Crop Evol. 2022;69:2661–2698. doi: 10.1007/s10722-022-01441-3. [DOI] [Google Scholar]
- 10.Boakyewaa Adu G., Badu-Apraku B., Akromah R., Garcia-Oliveira A.L., Awuku F.J., Gedil M. Genetic diversity and population structure of early-maturing tropical maize inbred lines using SNP markers. PLoS ONE. 2019;14:e0214810. doi: 10.1371/journal.pone.0214810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daniell H., Lin C.S., Yu M., Chang W.J. Chloroplast genomes: Diversity, evolution, and applications in genetic engineering. Genome Biol. 2016;17:134. doi: 10.1186/s13059-016-1004-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang J., Kan S., Liao X., Zhou J., Tembrock L.R., Daniell H., Jin S., Wu Z. Plant organellar genomes: Much done, much more to do. Trends Plant Sci. 2024;29:754–769. doi: 10.1016/j.tplants.2023.12.014. [DOI] [PubMed] [Google Scholar]
- 13.Magdy M., Ou L., Yu H., Chen R., Zhou Y., Hassan H., Feng B., Taitano N., van der Knaap E., Zou X., et al. Pan-plastome approach empowers the assessment of genetic variation in cultivated Capsicum species. Hortic. Res. 2019;6:108. doi: 10.1038/s41438-019-0191-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xia L., Wang H., Zhao X., Obel H.O., Yu X., Lou Q., Chen J., Cheng C. Chloroplast Pan-Genomes and Comparative Transcriptomics Reveal Genetic Variation and Temperature Adaptation in the Cucumber. Int. J. Mol. Sci. 2023;24:8943. doi: 10.3390/ijms24108943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Go S., Koo H., Jung M., Hong S., Yi G., Kim Y.M. Pan-chloroplast genomes for accession-specific marker development in Hibiscus syriacus. Sci. Data. 2024;11:246. doi: 10.1038/s41597-024-03077-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo X., Wang Z., Cai D., Song L., Bai J. The chloroplast genome sequence and phylogenetic analysis of Apocynum venetum L. PLoS ONE. 2022;17:e0261710. doi: 10.1371/journal.pone.0261710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qian R., Ye Y., Hu Q., Ma X., Zheng J. Complete Chloroplast Genome of Gladiolus gandavensis (Gladiolus) and Genetic Evolutionary Analysis. Genes. 2022;13:1599. doi: 10.3390/genes13091599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang L., Zhang S., Fang J., Jin X., Mamut R., Li P. The Chloroplast Genome of the Lichen Photobiont Trebouxiophyceae sp. DW1 and Its Phylogenetic Implications. Genes. 2022;13:1840. doi: 10.3390/genes13101840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li M., Chen Q., Yang B., Ma J., Li B., Zhang L. The complete chloroplast genome sequence of Tetrastigma hemsleyanum Diels at Gilg. Mitochondrial DNA Part A DNA Mapp. Seq. Anal. 2016;27:3729–3730. doi: 10.3109/19401736.2015.1079878. [DOI] [PubMed] [Google Scholar]
- 20.Huang X., Zhou Q., Qin C., Mao C., Sun K., Qin B., Tang Q. The complete chloroplast genome of Tetrastigma planicaule, one important folk medicinal plant in China. Mitochondrial DNA Part B Resour. 2021;6:1745–1746. doi: 10.1080/23802359.2021.1930598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin J.J., Yu W.B., Yang J.B., Song Y., dePamphilis C.W., Yi T.S., Li D.Z. GetOrganelle: A fast and versatile toolkit for accurate de novo assembly of organelle genomes. Genome Biol. 2020;21:241. doi: 10.1186/s13059-020-02154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Habib S., Dang V.C., Ickert-Bond S.M., Zhang J.L., Lu L.M., Wen J., Chen Z.D. Robust Phylogeny of Tetrastigma (Vitaceae) Based on Ten Plastid DNA Regions: Implications for Infrageneric Classification and Seed Character Evolution. Front. Plant Sci. 2017;8:590. doi: 10.3389/fpls.2017.00590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nie Z.-L., Sun H., Manchester S.R., Meng Y., Luke Q., Wen J. Evolution of the intercontinental disjunctions in six continents in the Ampelopsis clade of the grape family (Vitaceae) BMC Evol. Biol. 2012;12:17. doi: 10.1186/1471-2148-12-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zecca G., Grassi F., Tabidze V., Pipia I., Kotorashvili A., Kotaria N., Beridze T. Dates and rates in grape’s plastomes: Evolution in slow motion. Curr. Genet. 2020;66:123–140. doi: 10.1007/s00294-019-01004-7. [DOI] [PubMed] [Google Scholar]
- 25.Sheikh-Assadi M., Naderi R., Kafi M., Fatahi R., Salami S.A., Shariati V. Complete chloroplast genome of Lilium ledebourii (Baker) Boiss and its comparative analysis: Lights into selective pressure and adaptive evolution. Sci. Rep. 2022;12:9375. doi: 10.1038/s41598-022-13449-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Q., Chen X., Yang D., Xia P. Genetic relationship of Pleione based on the chloroplast genome. Gene. 2023;858:147203. doi: 10.1016/j.gene.2023.147203. [DOI] [PubMed] [Google Scholar]
- 27.Šmarda P., Bureš P., Horová L., Leitch I.J., Mucina L., Pacini E., Tichý L., Grulich V., Rotreklová O. Ecological and evolutionary significance of genomic GC content diversity in monocots. Proc. Natl. Acad. Sci. USA. 2014;111:E4096–E4102. doi: 10.1073/pnas.1321152111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palmer J.D., Thompson W.F. Chloroplast DNA rearrangements are more frequent when a large inverted repeat sequence is lost. Cell. 1982;29:537–550. doi: 10.1016/0092-8674(82)90170-2. [DOI] [PubMed] [Google Scholar]
- 29.Perry A.S., Wolfe K.H. Nucleotide substitution rates in legume chloroplast DNA depend on the presence of the inverted repeat. J. Mol. Evol. 2002;55:501–508. doi: 10.1007/s00239-002-2333-y. [DOI] [PubMed] [Google Scholar]
- 30.Liu X., Bai Y., Wang Y., Chen Y., Dong W., Zhang Z. Complete Chloroplast Genome of Hypericum perforatum and Dynamic Evolution in Hypericum (Hypericaceae) Int. J. Mol. Sci. 2023;24:16130. doi: 10.3390/ijms242216130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Niu T., Tian C., Yang Y., Liu Q., Liu L., Tao Q., Li Z., Wu Z. Complete Chloroplast Genome of Corethrodendron fruticosum (Papilionoideae: Fabaceae): Comparative and Phylogenetic Analysis. Genes. 2023;14:1289. doi: 10.3390/genes14061289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kashi Y., King D.G. Simple sequence repeats as advantageous mutators in evolution. Trends Genet. 2006;22:253–259. doi: 10.1016/j.tig.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 33.Siddle K.J., Goodship J.A., Keavney B., Santibanez-Koref M.F. Bases adjacent to mononucleotide repeats show an increased single nucleotide polymorphism frequency in the human genome. Bioinformatics. 2011;27:895–898. doi: 10.1093/bioinformatics/btr067. [DOI] [PubMed] [Google Scholar]
- 34.Wang X.T., Zhang Y.J., Qiao L., Chen B. Comparative analyses of simple sequence repeats (SSRs) in 23 mosquito species genomes: Identification, characterization and distribution (Diptera: Culicidae) Insect Sci. 2019;26:607–619. doi: 10.1111/1744-7917.12577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hanson G., Coller J. Codon optimality, bias and usage in translation and mRNA decay. Nat. Rev. Mol. Cell Biol. 2018;19:20–30. doi: 10.1038/nrm.2017.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dong S., Zhou M., Zhu J., Wang Q., Ge Y., Cheng R. The complete chloroplast genomes of Tetrastigma hemsleyanum (Vitaceae) from different regions of China: Molecular structure, comparative analysis and development of DNA barcodes for its geographical origin discrimination. BMC Genom. 2022;23:620. doi: 10.1186/s12864-022-08755-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feng J.L., Wu L.W., Wang Q., Pan Y.J., Li B.L., Lin Y.L., Yao H. Comparison Analysis Based on Complete Chloroplast Genomes and Insights into Plastid Phylogenomic of Four Iris Species. BioMed Res. Int. 2022;2022:2194021. doi: 10.1155/2022/2194021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li M., Gong Q., Zhou M., Liu Q., Cheng R. Characterization of the complete chloroplast genome of the medicinal herb Eleutherococcus nodiflorus and its phylogenetic implications. Mitochondrial DNA Part B Resour. 2022;7:1890–1892. doi: 10.1080/23802359.2022.2116954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amar M.H. ycf1-ndhF genes, the most promising plastid genomic barcode, sheds light on phylogeny at low taxonomic levels in Prunus persica. J. Genet. Eng. Biotechnol. 2020;18:42. doi: 10.1186/s43141-020-00057-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee C., Wen J. Phylogeny of Panax using chloroplast trnC-trnD intergenic region and the utility of trnC-trnD in interspecific studies of plants. Mol. Phylogenet. Evol. 2004;31:894–903. doi: 10.1016/j.ympev.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 41.Chen J.-J., Cui B.-K., Zhou L.-W., Korhonen K., Dai Y.-C. Phylogeny, divergence time estimation, and biogeography of the genus Heterobasidion (Basidiomycota, Russulales) Fungal Divers. 2015;71:185–200. doi: 10.1007/s13225-014-0317-2. [DOI] [Google Scholar]
- 42.Wang Y.-H., Jiang W.-M., Comes H.P., Hu F.S., Qiu Y.-X., Fu C.-X. Molecular phylogeography and ecological niche modelling of a widespread herbaceous climber, Tetrastigma hemsleyanum (Vitaceae): Insights into Plio–Pleistocene range dynamics of evergreen forest in subtropical China. New Phytol. 2015;206:852–867. doi: 10.1111/nph.13261. [DOI] [PubMed] [Google Scholar]
- 43.Hearn D.J., Evans M., Wolf B., McGinty M., Wen J. Dispersal is associated with morphological innovation, but not increased diversification, in Cyphostemma (Vitaceae) J. Syst. Evol. 2018;56:340–359. doi: 10.1111/jse.12417. [DOI] [Google Scholar]
- 44.Britton T., Anderson C.L., Jacquet D., Lundqvist S., Bremer K. Estimating Divergence Times in Large Phylogenetic Trees. Syst. Biol. 2007;56:741–752. doi: 10.1080/10635150701613783. [DOI] [PubMed] [Google Scholar]
- 45.Herrera F., Carvalho M.R., Stull G.W., Jaramillo C., Manchester S.R. Cenozoic seeds of Vitaceae reveal a deep history of extinction and dispersal in the Neotropics. Nat. Plants. 2024;10:1091–1099. doi: 10.1038/s41477-024-01717-9. [DOI] [PubMed] [Google Scholar]
- 46.Lu L., Wang W., Chen Z., Wen J. Phylogeny of the non-monophyletic Cayratia Juss. (Vitaceae) and implications for character evolution and biogeography. Mol. Phylogenet. Evol. 2013;68:502–515. doi: 10.1016/j.ympev.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 47.Ng A.Y.E., Chan S.N., Pek J.W. Genetic compensation between ribosomal protein paralogs mediated by a cognate circular RNA. Cell Rep. 2024;43:114228. doi: 10.1016/j.celrep.2024.114228. [DOI] [PubMed] [Google Scholar]
- 48.Mukhopadhyay P., Reddy M.K., Singla-Pareek S.L., Sopory S.K. Transcriptional downregulation of rice rpL32 gene under abiotic stress is associated with removal of transcription factors within the promoter region. PLoS ONE. 2011;6:e28058. doi: 10.1371/journal.pone.0028058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alqahtani A.A., Jansen R.K. The evolutionary fate of rpl32 and rps16 losses in the Euphorbia schimperi (Euphorbiaceae) plastome. Sci. Rep. 2021;11:7466. doi: 10.1038/s41598-021-86820-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ueda M., Fujimoto M., Arimura S., Murata J., Tsutsumi N., Kadowaki K. Loss of the rpl32 gene from the chloroplast genome and subsequent acquisition of a preexisting transit peptide within the nuclear gene in Populus. Gene. 2007;402:51–56. doi: 10.1016/j.gene.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 51.Park S., Jansen R.K., Park S. Complete plastome sequence of Thalictrum coreanum (Ranunculaceae) and transfer of the rpl32 gene to the nucleus in the ancestor of the subfamily Thalictroideae. BMC Plant Biol. 2015;15:40. doi: 10.1186/s12870-015-0432-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roy S., Ueda M., Kadowaki K., Tsutsumi N. Different status of the gene for ribosomal protein S16 in the chloroplast genome during evolution of the genus Arabidopsis and closely related species. Genes Genet. Syst. 2010;85:319–326. doi: 10.1266/ggs.85.319. [DOI] [PubMed] [Google Scholar]
- 53.Rogalski M., Schöttler M.A., Thiele W., Schulze W.X., Bock R. Rpl33, a nonessential plastid-encoded ribosomal protein in tobacco, is required under cold stress conditions. Plant Cell. 2008;20:2221–2237. doi: 10.1105/tpc.108.060392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wan Y., Schwaninger H.R., Baldo A.M., Labate J.A., Zhong G.Y., Simon C.J. A phylogenetic analysis of the grape genus (Vitis L.) reveals broad reticulation and concurrent diversification during neogene and quaternary climate change. BMC Evol. Biol. 2013;13:141. doi: 10.1186/1471-2148-13-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.O’Brien C.L., Huber M., Thomas E., Pagani M., Super J.R., Elder L.E., Hull P.M. The enigma of Oligocene climate and global surface temperature evolution. Proc. Natl. Acad. Sci. USA. 2020;117:25302–25309. doi: 10.1073/pnas.2003914117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Senapati A., Chetri B.K., Mitra S., Shelke R.G., Rangan L. Decoding the complete chloroplast genome of Cissus quadrangularis: Insights into molecular structure, comparative genome analysis and mining of mutational hotspot regions. Physiol. Mol. Biol. Plants. 2023;29:709–724. doi: 10.1007/s12298-023-01312-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang L., Meng Y., Wang D., He G.-H., Zhang J.-M., Wen J., Nie Z.-L. Plastid genome data provide new insights into the dynamic evolution of the tribe Ampelopsideae (Vitaceae) BMC Genom. 2024;25:247. doi: 10.1186/s12864-024-10149-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ramadan A.M. Light/heat effects on RNA editing in chloroplast NADH-plastoquinone oxidoreductase subunit 2 (ndhB) gene of Calotropis (Calotropis procera) J. Genet. Eng. Biotechnol. 2020;18:49. doi: 10.1186/s43141-020-00064-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schöttler M.A., Thiele W., Belkius K., Bergner S.V., Flügel C., Wittenberg G., Agrawal S., Stegemann S., Ruf S., Bock R. The plastid-encoded PsaI subunit stabilizes photosystem I during leaf senescence in tobacco. J. Exp. Bot. 2017;68:1137–1155. doi: 10.1093/jxb/erx009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yu X., Tan W., Zhang H., Gao H., Wang W., Tian X. Complete Chloroplast Genomes of Ampelopsis humulifolia and Ampelopsis japonica: Molecular Structure, Comparative Analysis, and Phylogenetic Analysis. Plants. 2019;8:410. doi: 10.3390/plants8100410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Raman G., Park S. The Complete Chloroplast Genome Sequence of Ampelopsis: Gene Organization, Comparative Analysis, and Phylogenetic Relationships to Other Angiosperms. Front. Plant Sci. 2016;7:341. doi: 10.3389/fpls.2016.00341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Namgung J., Do H.D.K., Kim C., Choi H.J., Kim J.H. Complete chloroplast genomes shed light on phylogenetic relationships, divergence time, and biogeography of Allioideae (Amaryllidaceae) Sci. Rep. 2021;11:3262. doi: 10.1038/s41598-021-82692-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Machado L.O., Vieira L.D.N., Stefenon V.M., Faoro H., Pedrosa F.O., Guerra M.P., Nodari R.O. Molecular relationships of Campomanesia xanthocarpa within Myrtaceae based on the complete plastome sequence and on the plastid ycf2 gene. Genet. Mol. Biol. 2020;43:e20180377. doi: 10.1590/1678-4685-gmb-2018-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wolf P.G., Der J.P., Duffy A.M., Davidson J.B., Grusz A.L., Pryer K.M. The evolution of chloroplast genes and genomes in ferns. Plant Mol. Biol. 2011;76:251–261. doi: 10.1007/s11103-010-9706-4. [DOI] [PubMed] [Google Scholar]
- 65.Zecca G., Panzeri D., Grassi F. Detecting signals of adaptive evolution in grape plastomes with a focus on the Cretaceous–Palaeogene (K/Pg) transition. Ann. Bot. 2022;130:965–980. doi: 10.1093/aob/mcac128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen S., Zhou Y., Chen Y., Gu J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics. 2018;34:i884–i890. doi: 10.1093/bioinformatics/bty560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dierckxsens N., Mardulyn P., Smits G. NOVOPlasty: De novo assembly of organelle genomes from whole genome data. Nucleic Acids Res. 2017;45:e18. doi: 10.1093/nar/gkw955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tillich M., Lehwark P., Pellizzer T., Ulbricht-Jones E.S., Fischer A., Bock R., Greiner S. GeSeq–versatile and accurate annotation of organelle genomes. Nucleic Acids Res. 2017;45:W6–W11. doi: 10.1093/nar/gkx391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kearse M., Moir R., Wilson A., Stones-Havas S., Cheung M., Sturrock S., Buxton S., Cooper A., Markowitz S., Duran C. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28:1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Greiner S., Lehwark P., Bock R. OrganellarGenomeDRAW (OGDRAW) version 1.3. 1: Expanded toolkit for the graphical visualization of organellar genomes. Nucleic Acids Res. 2019;47:W59–W64. doi: 10.1093/nar/gkz238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Beier S., Thiel T., Münch T., Scholz U., Mascher M. MISA-web: A web server for microsatellite prediction. Bioinformatics. 2017;33:2583–2585. doi: 10.1093/bioinformatics/btx198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rozas J., Sánchez-DelBarrio J.C., Messeguer X., Rozas R. DnaSP, DNA polymorphism analyses by the coalescent and other methods. Bioinformatics. 2003;19:2496–2497. doi: 10.1093/bioinformatics/btg359. [DOI] [PubMed] [Google Scholar]
- 73.Amiryousefi A., Hyvönen J., Poczai P. IRscope: An online program to visualize the junction sites of chloroplast genomes. Bioinformatics. 2018;34:3030–3031. doi: 10.1093/bioinformatics/bty220. [DOI] [PubMed] [Google Scholar]
- 74.Katoh K., Standley D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tamura K., Stecher G., Kumar S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021;38:3022–3027. doi: 10.1093/molbev/msab120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wikström N., Savolainen V., Chase M.W. Evolution of the angiosperms: Calibrating the family tree. Proc. Biol. Sci. 2001;268:2211–2220. doi: 10.1098/rspb.2001.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zecca G., Abbott J.R., Sun W.B., Spada A., Sala F., Grassi F. The timing and the mode of evolution of wild grapes (Vitis) Mol. Phylogenet. Evol. 2012;62:736–747. doi: 10.1016/j.ympev.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 78.Zhang Z. KaKs_Calculator 3.0: Calculating Selective Pressure on Coding and Non-coding Sequences. Genom. Proteom. Bioinform. 2022;20:536–540. doi: 10.1016/j.gpb.2021.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All raw data from this study can be downloaded from NCBI, with the corresponding accession numbers listed in the Supplementary Materials (Table S1). Additionally, data generated during the analysis can be obtained by contacting the corresponding author.