Abstract
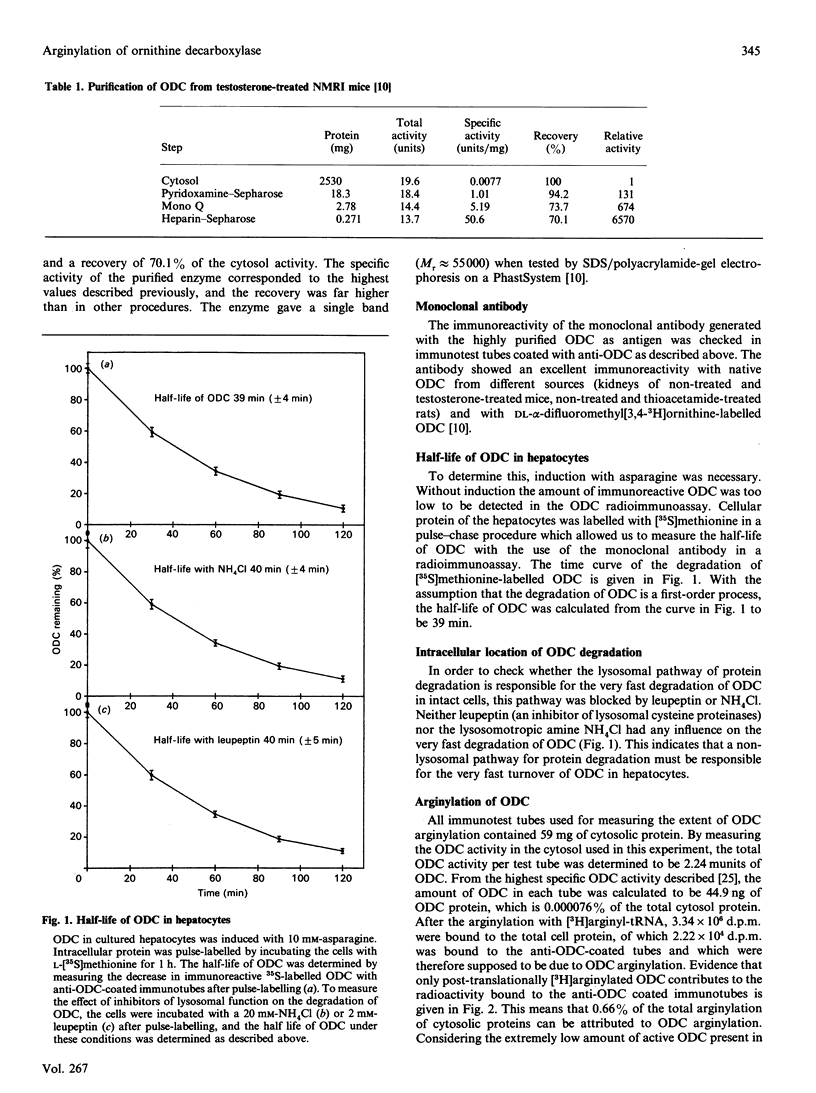
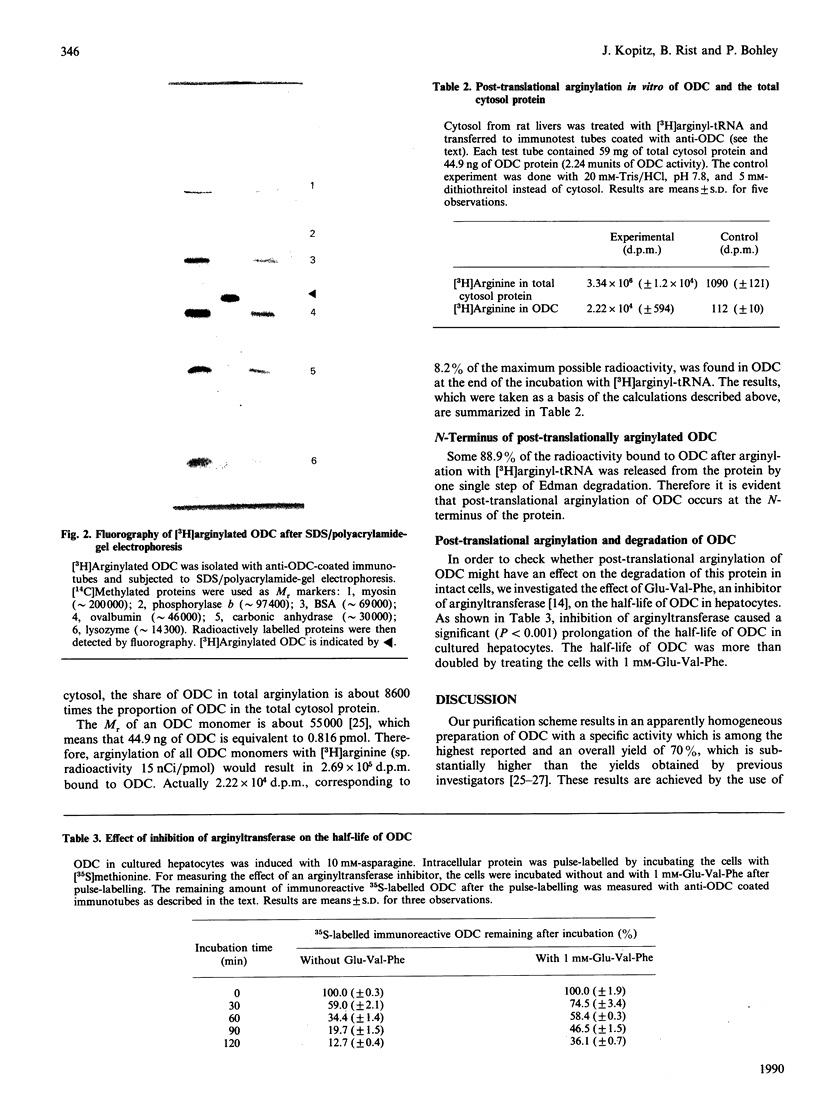
Ornithine decarboxylase (ODC) was purified 6500-fold from NMRI mouse kidneys under conditions designed to inhibit degradation by proteinases. The enzyme was homogeneous by SDS/polyacrylamide-gel electrophoresis, and the specific activity was among the highest reported. The yield was 70%. A monoclonal antibody against this preparation was generated and used in studies to investigate the half-life of ODC in cultured rat hepatocytes labelled with [35S]methionine. This value was 39 +/- 4 min and was unchanged when either NH4Cl (as a lysosomotropic agent) or leupeptin (as a lysosomal proteinase inhibitor) was added to the culture medium. Thus the intracellular turnover of ODC in cultured hepatocytes occurs mainly in extra-lysosomal compartments. Arginylation of rat ODC was investigated in vitro by incubation with L-[3H]arginyl-tRNA, and the incorporation of the label was compared with that of total cytosolic proteins. Arginylated ODC had a specific radioactivity 8600 times that of the bulk of cytosolic protein. Edman degradation of this ODC showed that the post-translational arginylation occurred only at the alpha-amino end of the enzyme. The inhibitor of arginyl-tRNA:protein arginyltransferase (EC 2.3.2.8), L-glutamyl-L-valyl-L-phenylalanine, increased the half-life of ODC in cultured hepatocytes from 39 min to more than 90 min. The possible significance of the preferential post-translational arginylation of ornithine decarboxylase to its rapid turnover is discussed.
Full text
PDF



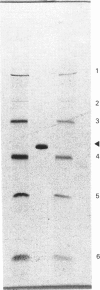
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arfin S. M., Bradshaw R. A. Cotranslational processing and protein turnover in eukaryotic cells. Biochemistry. 1988 Oct 18;27(21):7979–7984. doi: 10.1021/bi00421a001. [DOI] [PubMed] [Google Scholar]
- Bachmair A., Finley D., Varshavsky A. In vivo half-life of a protein is a function of its amino-terminal residue. Science. 1986 Oct 10;234(4773):179–186. doi: 10.1126/science.3018930. [DOI] [PubMed] [Google Scholar]
- Bachmair A., Varshavsky A. The degradation signal in a short-lived protein. Cell. 1989 Mar 24;56(6):1019–1032. doi: 10.1016/0092-8674(89)90635-1. [DOI] [PubMed] [Google Scholar]
- Bohley P., Kopitz J., Adam G. Surface hydrophobicity, arginylation and degradation of cytosol proteins from rat hepatocytes. Biol Chem Hoppe Seyler. 1988 May;369 (Suppl):307–310. [PubMed] [Google Scholar]
- Chau V., Tobias J. W., Bachmair A., Marriott D., Ecker D. J., Gonda D. K., Varshavsky A. A multiubiquitin chain is confined to specific lysine in a targeted short-lived protein. Science. 1989 Mar 24;243(4898):1576–1583. doi: 10.1126/science.2538923. [DOI] [PubMed] [Google Scholar]
- Ciechanover A., Ferber S., Ganoth D., Elias S., Hershko A., Arfin S. Purification and characterization of arginyl-tRNA-protein transferase from rabbit reticulocytes. Its involvement in post-translational modification and degradation of acidic NH2 termini substrates of the ubiquitin pathway. J Biol Chem. 1988 Aug 15;263(23):11155–11167. [PubMed] [Google Scholar]
- Dahlmann B., Kuehn L., Rutschmann M., Reinauer H. Purification and characterization of a multicatalytic high-molecular-mass proteinase from rat skeletal muscle. Biochem J. 1985 May 15;228(1):161–170. doi: 10.1042/bj2280161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkenburg P. E., Haass C., Kloetzel P. M., Niedel B., Kopp F., Kuehn L., Dahlmann B. Drosophila small cytoplasmic 19S ribonucleoprotein is homologous to the rat multicatalytic proteinase. Nature. 1988 Jan 14;331(6152):190–192. doi: 10.1038/331190a0. [DOI] [PubMed] [Google Scholar]
- Fausto N. RNA and amine synthesis in the liver of rats given injections of thioacetamide. Cancer Res. 1970 Jul;30(7):1947–1952. [PubMed] [Google Scholar]
- Ferber S., Ciechanover A. Role of arginine-tRNA in protein degradation by the ubiquitin pathway. Nature. 1987 Apr 23;326(6115):808–811. doi: 10.1038/326808a0. [DOI] [PubMed] [Google Scholar]
- Gebhardt R., Jung W. Biliary secretion of sodium fluorescein in primary monolayer cultures of adult rat hepatocytes and its stimulation by nicotinamide. J Cell Sci. 1982 Aug;56:233–244. doi: 10.1242/jcs.56.1.233. [DOI] [PubMed] [Google Scholar]
- Ghoda L., van Daalen Wetters T., Macrae M., Ascherman D., Coffino P. Prevention of rapid intracellular degradation of ODC by a carboxyl-terminal truncation. Science. 1989 Mar 17;243(4897):1493–1495. doi: 10.1126/science.2928784. [DOI] [PubMed] [Google Scholar]
- Glass J. R., Gerner E. W. Spermidine mediates degradation of ornithine decarboxylase by a non-lysosomal, ubiquitin-independent mechanism. J Cell Physiol. 1987 Jan;130(1):133–141. doi: 10.1002/jcp.1041300119. [DOI] [PubMed] [Google Scholar]
- Gonda D. K., Bachmair A., Wünning I., Tobias J. W., Lane W. S., Varshavsky A. Universality and structure of the N-end rule. J Biol Chem. 1989 Oct 5;264(28):16700–16712. [PubMed] [Google Scholar]
- Gravela E., Zuretti M. F., Papino F., Sartorio L. Relative in vitro stability of ornithine decarboxylase from liver preneoplastic nodules and hepatomas. Cancer Res. 1983 May;43(5):2298–2300. [PubMed] [Google Scholar]
- Hölttä E., Sistonen L., Alitalo K. The mechanisms of ornithine decarboxylase deregulation in c-Ha-ras oncogene-transformed NIH 3T3 cells. J Biol Chem. 1988 Mar 25;263(9):4500–4507. [PubMed] [Google Scholar]
- Isomaa V. V., Pajunen A. E., Bardin C. W., Jänne O. A. Ornithine decarboxylase in mouse kidney. Purification, characterization, and radioimmunological determination of the enzyme protein. J Biol Chem. 1983 Jun 10;258(11):6735–6740. [PubMed] [Google Scholar]
- Kanamoto R., Boyle S. M., Oka T., Hayashi S. Molecular mechanisms of the synergistic induction of ornithine decarboxylase by asparagine and glucagon in primary cultured hepatocytes. J Biol Chem. 1987 Oct 25;262(30):14801–14805. [PubMed] [Google Scholar]
- Kanamoto R., Utsunomiya K., Kameji T., Hayashi S. Effects of putrescine on synthesis and degradation of ornithine decarboxylase in primary cultured hepatocytes. Eur J Biochem. 1986 Feb 3;154(3):539–544. doi: 10.1111/j.1432-1033.1986.tb09432.x. [DOI] [PubMed] [Google Scholar]
- Kanamoto R., Utsunomiya K., Kameji T., Hayashi S. Effects of putrescine on synthesis and degradation of ornithine decarboxylase in primary cultured hepatocytes. Eur J Biochem. 1986 Feb 3;154(3):539–544. doi: 10.1111/j.1432-1033.1986.tb09432.x. [DOI] [PubMed] [Google Scholar]
- Kaye A. M. Ornithine decarboxylase. Purification and properties of ornithine decarboxylase. Cell Biochem Funct. 1984 Jan;2(1):2–6. doi: 10.1002/cbf.290020102. [DOI] [PubMed] [Google Scholar]
- Kitani T., Fujisawa H. Purification and properties of ornithine decarboxylase from rat liver. J Biol Chem. 1983 Jan 10;258(1):235–239. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mayer A., Gropper R., Schwartz A. L., Ciechanover A. Purification, characterization, and rapid inactivation of thermolabile ubiquitin-activating enzyme from the mammalian cell cycle mutant ts85. J Biol Chem. 1989 Feb 5;264(4):2060–2068. [PubMed] [Google Scholar]
- Minchiotti L., Galliano M., Iadarola P., Meloni M. L., Ferri G., Porta F., Castellani A. A. The molecular defect in a COOH-terminal-modified and shortened mutant of human serum albumin. J Biol Chem. 1989 Feb 25;264(6):3385–3389. [PubMed] [Google Scholar]
- Murakami Y., Marumo M., Hayashi S. Ornithine decarboxylase antizyme in kidneys of male and female mice. Biochem J. 1988 Sep 1;254(2):367–372. doi: 10.1042/bj2540367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami Y., Nishiyama M., Hayashi S. Involvement of antizyme in stabilization of ornithine decarboxylase caused by inhibitors of polyamine synthesis. Eur J Biochem. 1989 Mar 1;180(1):181–184. doi: 10.1111/j.1432-1033.1989.tb14630.x. [DOI] [PubMed] [Google Scholar]
- Rivett A. J. The multicatalytic proteinase of mammalian cells. Arch Biochem Biophys. 1989 Jan;268(1):1–8. doi: 10.1016/0003-9861(89)90558-4. [DOI] [PubMed] [Google Scholar]
- Rogers S. W., Rechsteiner M. Degradation of structurally characterized proteins injected into HeLa cells. Basic measurements. J Biol Chem. 1988 Dec 25;263(36):19833–19842. [PubMed] [Google Scholar]
- Rogers S. W., Rechsteiner M. Degradation of structurally characterized proteins injected into HeLa cells. Effects of intracellular location and the involvement of lysosomes. J Biol Chem. 1988 Dec 25;263(36):19843–19849. [PubMed] [Google Scholar]
- Rogers S. W., Rechsteiner M. Degradation of structurally characterized proteins injected into HeLa cells. Tests of hypotheses. J Biol Chem. 1988 Dec 25;263(36):19850–19862. [PubMed] [Google Scholar]
- Schulz W. A., Gebhardt R., Mecke D. Dexamethasone restores hormonal inducibility of ornithine decarboxylase in primary cultures of rat hepatocytes. Eur J Biochem. 1985 Feb 1;146(3):549–553. doi: 10.1111/j.1432-1033.1985.tb08686.x. [DOI] [PubMed] [Google Scholar]
- Seely J. E., Pegg A. E. Effect of 1,3-diaminopropane on ornithine decarboxylase enzyme protein in thioacetamide-treated rat liver. Biochem J. 1983 Dec 15;216(3):701–707. doi: 10.1042/bj2160701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seely J. E., Pegg A. E. Effect of 1,3-diaminopropane on ornithine decarboxylase enzyme protein in thioacetamide-treated rat liver. Biochem J. 1983 Dec 15;216(3):701–707. doi: 10.1042/bj2160701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seely J. E., Persson L., Sertich G. J., Pegg A. E. Comparison of ornithine decarboxylase from rat liver, rat hepatoma and mouse kidney. Biochem J. 1985 Mar 1;226(2):577–586. doi: 10.1042/bj2260577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seely J. E., Pösö H., Pegg A. E. Purification of ornithine decarboxylase from kidneys of androgen-treated mice. Biochemistry. 1982 Jul 6;21(14):3394–3399. doi: 10.1021/bi00257a023. [DOI] [PubMed] [Google Scholar]
- Soffer R. L. Peptide acceptors in the arginine transfer reaction. J Biol Chem. 1973 Apr 25;248(8):2918–2921. [PubMed] [Google Scholar]
- Soffer R. L. Peptide acceptors in the leucine, phenylalanine transfer reaction. J Biol Chem. 1973 Dec 25;248(24):8424–8428. [PubMed] [Google Scholar]
- Williams G. M., Gunn J. M. Long-term cell culture of adult rat liver epithelial cells. Exp Cell Res. 1974 Nov;89(1):139–142. doi: 10.1016/0014-4827(74)90196-7. [DOI] [PubMed] [Google Scholar]
- Zuretti M. F., Gravela E. Studies on the mechanisms of ornithine decarboxylase in vitro inactivation. Biochim Biophys Acta. 1983 Jan 26;742(2):269–277. doi: 10.1016/0167-4838(83)90311-4. [DOI] [PubMed] [Google Scholar]