Abstract
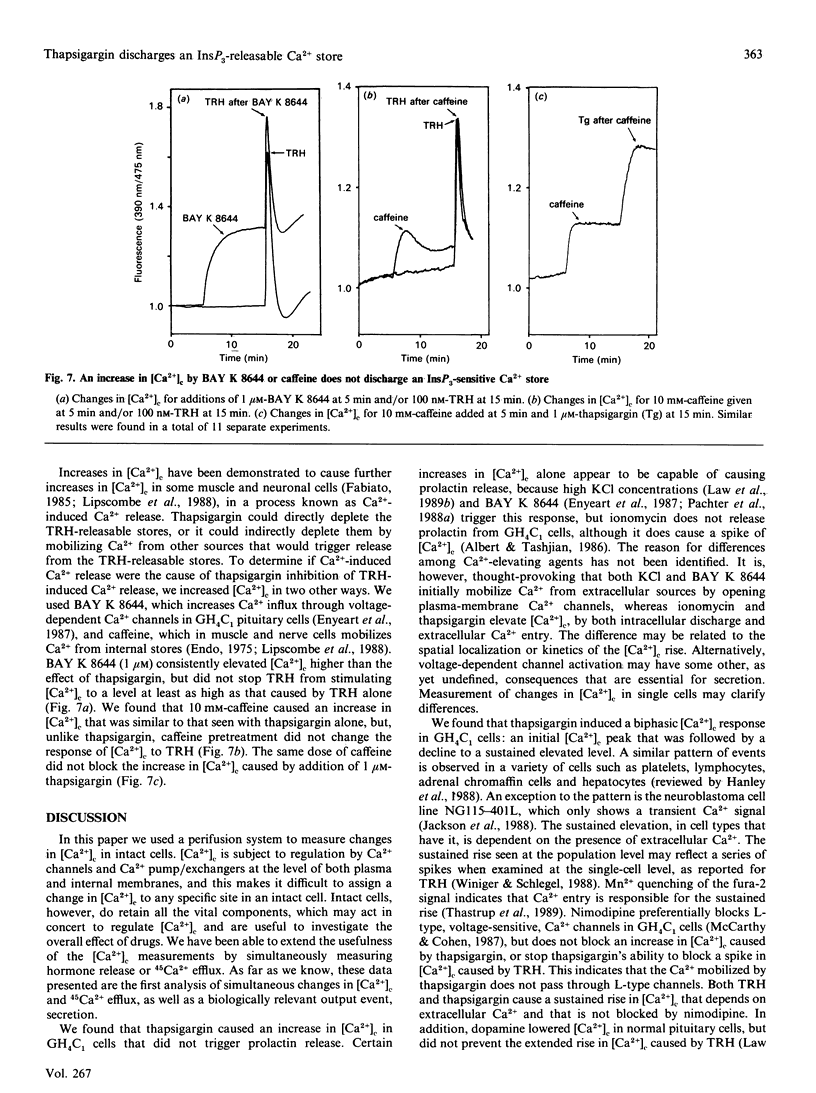
Thapsigargin stimulates an increase of cytosolic free Ca2+ concentration [( Ca2+]c) in, and 45Ca2+ efflux from, a clone of GH4C1 pituitary cells. This increase in [Ca2+]c was followed by a lower sustained elevation of [Ca2+]c, which required the presence of extracellular Ca2+, and was not inhibited by a Ca2(+)-channel blocker, nimodipine. Thapsigargin had no effect on inositol phosphate generation. We used thyrotropin-releasing hormone (TRH) to mobilize Ca2+ from an InsP3-sensitive store. Pretreatment with thapsigargin blocked the ability of TRH to cause a transient increase in both [Ca2+]c and 45Ca2+ efflux. The block of TRH-induced Ca2+ mobilization was not caused by a block at the receptor level, because TRH stimulation of InsP3 was not affected by thapsigargin. Rundown of the TRH-releasable store by Ca2(+)-induced Ca2+ release does not appear to account for the action of thapsigargin on the TRH-induced spike in [Ca2+]c, because BAY K 8644, which causes a sustained rise in [Ca2+]c, did not block Ca2+ release caused by TRH. In addition, caffeine, which releases Ca2+ from intracellular stores in other cell types, caused an increase in [Ca2+]c in GH4C1 cells, but had no effect on a subsequent spike in [Ca2+]c induced by TRH or thapsigargin. TRH caused a substantial decrease in the amount of intracellular Ca2+ released by thapsigargin. We conclude that in GH4C1 cells thapsigargin actively discharges an InsP3-releasable pool of Ca2+ and that this mechanism alone causes the block of the TRH-induced increase in [Ca2+]c.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert P. R., Tashjian A. H., Jr Ionomycin acts as an ionophore to release TRH-regulated Ca2+ stores from GH4C1 cells. Am J Physiol. 1986 Dec;251(6 Pt 1):C887–C891. doi: 10.1152/ajpcell.1986.251.6.C887. [DOI] [PubMed] [Google Scholar]
- Ali H., Christensen S. B., Foreman J. C., Pearce F. L., Piotrowski W., Thastrup O. The ability of thapsigargin and thapsigargicin to activate cells involved in the inflammatory response. Br J Pharmacol. 1985 Jul;85(3):705–712. doi: 10.1111/j.1476-5381.1985.tb10567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen C. J., McCarthy R. T. Nimodipine block of calcium channels in rat anterior pituitary cells. J Physiol. 1987 Jun;387:195–225. doi: 10.1113/jphysiol.1987.sp016570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enyeart J. J., Sheu S. S., Hinkle P. M. Dihydropyridine modulators of voltage-sensitive Ca2+ channels specifically regulate prolactin production by GH4C1 pituitary tumor cells. J Biol Chem. 1987 Mar 5;262(7):3154–3159. [PubMed] [Google Scholar]
- Fabiato A. Time and calcium dependence of activation and inactivation of calcium-induced release of calcium from the sarcoplasmic reticulum of a skinned canine cardiac Purkinje cell. J Gen Physiol. 1985 Feb;85(2):247–289. doi: 10.1085/jgp.85.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershengorn M. C., Geras E., Purrello V. S., Rebecchi M. J. Inositol trisphosphate mediates thyrotropin-releasing hormone mobilization of nonmitochondrial calcium in rat mammotropic pituitary cells. J Biol Chem. 1984 Sep 10;259(17):10675–10681. [PubMed] [Google Scholar]
- Hanley M. R., Jackson T. R., Cheung W. T., Dreher M., Gatti A., Hawkins P., Patterson S. I., Vallejo M., Dawson A. P., Thastrup O. Molecular mechanisms of phospholipid signaling pathways in mammalian nerve cells. Cold Spring Harb Symp Quant Biol. 1988;53(Pt 1):435–445. doi: 10.1101/sqb.1988.053.01.051. [DOI] [PubMed] [Google Scholar]
- Jackson T. R., Patterson S. I., Thastrup O., Hanley M. R. A novel tumour promoter, thapsigargin, transiently increases cytoplasmic free Ca2+ without generation of inositol phosphates in NG115-401L neuronal cells. Biochem J. 1988 Jul 1;253(1):81–86. doi: 10.1042/bj2530081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaide H., Shogakiuchi Y., Nakamura M. The norepinephrine-sensitive Ca2+-storage site differs from the caffeine-sensitive site in vascular smooth muscle of the rat aorta. FEBS Lett. 1987 Apr 6;214(1):130–134. doi: 10.1016/0014-5793(87)80027-3. [DOI] [PubMed] [Google Scholar]
- Law G. J., Pachter J. A., Dannies P. S. Ability of repetitive Ca2+ spikes to stimulate prolactin release is frequency dependent. Biochem Biophys Res Commun. 1989 Feb 15;158(3):811–816. doi: 10.1016/0006-291x(89)92794-0. [DOI] [PubMed] [Google Scholar]
- Law G. J., Pachter J. A., Dannies P. S. Ca2+ transients induced by thyrotropin-releasing hormone rapidly lose their ability to cause release of prolactin. Mol Endocrinol. 1989 Mar;3(3):539–546. doi: 10.1210/mend-3-3-539. [DOI] [PubMed] [Google Scholar]
- Law G. J., Pachter J. A., Dannies P. S. Dopamine has no effect on thyrotropin-releasing hormone mobilization of calcium from intracellular stores in rat anterior pituitary cells. Mol Endocrinol. 1988 Oct;2(10):966–972. doi: 10.1210/mend-2-10-966. [DOI] [PubMed] [Google Scholar]
- Lipscombe D., Madison D. V., Poenie M., Reuter H., Tsien R. W., Tsien R. Y. Imaging of cytosolic Ca2+ transients arising from Ca2+ stores and Ca2+ channels in sympathetic neurons. Neuron. 1988 Jul;1(5):355–365. doi: 10.1016/0896-6273(88)90185-7. [DOI] [PubMed] [Google Scholar]
- Michell R. H., Kirk C. J., Maccallum S. H., Hunt P. A. Inositol lipids: receptor-stimulated hydrolysis and cellular lipid pools. Philos Trans R Soc Lond B Biol Sci. 1988 Jul 26;320(1199):239–246. doi: 10.1098/rstb.1988.0074. [DOI] [PubMed] [Google Scholar]
- Pachter J. A., Law G. J., Dannies P. S. Bombesin stimulates inositol polyphosphate production in GH4C1 pituitary tumor cells: comparison with TRH. Biochem Biophys Res Commun. 1988 Jul 29;154(2):654–659. doi: 10.1016/0006-291x(88)90189-1. [DOI] [PubMed] [Google Scholar]
- Pachter J. A., Law G. J., Dannies P. S. TRH and BAY K 8644 synergistically stimulate prolactin release but not 45Ca2+ uptake. Am J Physiol. 1988 Nov;255(5 Pt 1):C633–C640. doi: 10.1152/ajpcell.1988.255.5.C633. [DOI] [PubMed] [Google Scholar]
- Scharff O., Foder B., Thastrup O., Hofmann B., Møller J., Ryder L. P., Jacobsen K. D., Langhoff E., Dickmeiss E., Christensen S. B. Effect of thapsigargin on cytoplasmic Ca2+ and proliferation of human lymphocytes in relation to AIDS. Biochim Biophys Acta. 1988 Dec 9;972(3):257–264. doi: 10.1016/0167-4889(88)90200-5. [DOI] [PubMed] [Google Scholar]
- Thastrup O., Dawson A. P., Scharff O., Foder B., Cullen P. J., Drøbak B. K., Bjerrum P. J., Christensen S. B., Hanley M. R. Thapsigargin, a novel molecular probe for studying intracellular calcium release and storage. Agents Actions. 1989 Apr;27(1-2):17–23. doi: 10.1007/BF02222186. [DOI] [PubMed] [Google Scholar]
- Thastrup O., Foder B., Scharff O. The calcium mobilizing tumor promoting agent, thapsigargin elevates the platelet cytoplasmic free calcium concentration to a higher steady state level. A possible mechanism of action for the tumor promotion. Biochem Biophys Res Commun. 1987 Feb 13;142(3):654–660. doi: 10.1016/0006-291x(87)91464-1. [DOI] [PubMed] [Google Scholar]
- Thastrup O., Linnebjerg H., Bjerrum P. J., Knudsen J. B., Christensen S. B. The inflammatory and tumor-promoting sesquiterpene lactone, thapsigargin, activates platelets by selective mobilization of calcium as shown by protein phosphorylations. Biochim Biophys Acta. 1987 Jan 19;927(1):65–73. doi: 10.1016/0167-4889(87)90066-8. [DOI] [PubMed] [Google Scholar]
- Thayer S. A., Perney T. M., Miller R. J. Regulation of calcium homeostasis in sensory neurons by bradykinin. J Neurosci. 1988 Nov;8(11):4089–4097. doi: 10.1523/JNEUROSCI.08-11-04089.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winiger B. P., Schlegel W. Rapid transient elevations of cytosolic calcium triggered by thyrotropin releasing hormone in individual cells of the pituitary line GH3B6. Biochem J. 1988 Oct 1;255(1):161–167. doi: 10.1042/bj2550161. [DOI] [PMC free article] [PubMed] [Google Scholar]


