ABSTRACT
Most studies on anesthesia focus on the nervous system of mammals due to their interest in medicine. The fact that any life form can be anaesthetised is often overlooked although anesthesia targets ion channel activities that exist in all living beings. This study examines the impact of lidocaine on rice (Oryza sativa). It reveals that the cellular responses observed in rice are analogous to those documented in animals, encompassing direct effects, the inhibition of cellular responses, and the long-distance transmission of electrical signals. We show that in rice cells, lidocaine has a cytotoxic effect at a concentration of 1%, since it induces programmed reactive oxygen species (ROS) and caspase-like-dependent cell death, as already demonstrated in animal cells. Additionally, lidocaine causes changes in membrane ion conductance and induces a sharp reduction in electrical long-distance signaling following seedlings leaves burning. Finally, lidocaine was shown to inhibit osmotic stress-induced cell death and the regulation of Ca2+ homeostasis. Thus, lidocaine treatment in rice and tobacco (Nicotiana benthamiana) seedlings induces not only cellular but also systemic effects similar to those induced in mammals.
KEYWORDS: Anaesthesia, calcium, lidocaine, osmotic stress, programmed cell death, rice
Introduction
Since the 19th century, the use of anesthesia has revolutionized medicine so rapidly that it is now commonplace. Furthermore, the significant progress facilitated by anesthesia in both human and veterinary medicine has led to a general lack of awareness among the public and specialists alike of the fact that the ability to be anaesthetised, far from being specific to animal species, is a trait common to all living creatures.1–4 Many different chemicals with no structural similarities have been found to induce anaesthesia in animals.5 Anaesthetics were shown to act via specific proteins, notably Na+ channels that control neuronal excitability.5,6 However, anaesthetics appear to have effects on many other proteins, receptors, and channels.7 Defining the actions of anaesthetics at the molecular and cellular levels is still widely regarded as the sine qua non for understanding their complex set of clinical effects: unconsciousness, amnesia and immobility. Studies on alternative models could thus provide significant advances both in the understanding of anaesthetic cellular effects and in our theoretical understanding of this phenomenon common to all living beings.
In recent years, there has been renewed interest in the effects of anaesthetics on plants. Milne and Beamish showed that local anaesthetics inhibit rapid leaf movement in the plant Mimosa pudica.8 More recently, diethyl ether has been shown to cause inhibition of rapid trap closure mediated by inhibition of electrical signaling in the Venus fly trap (Dionaea muscipula), a carnivorous plant.9,10 Scherzer et al. have further shown that diethyl ether treatment of Venus fly trap suppresses the propagation of Ca2+ and long-distance signaling through action potentials (APs) triggering trap closure, and that glutamate receptors (GLRs) are likely targets of this anesthetic.11 Diethyl ether seems able to impair long-distance electrical signaling and Ca2+ signaling in the model plant Arabidopsis thaliana.12
Our study focuses on the effects of lidocaine, the most widely used local anaesthetic in medicine. Originally called lignocaine, from the Latin lignum (“wood”) or xylocaine from the ancient Greek root, xylon (“wood”) because the molecule made the limbs feel like wood, and from the end of the word “cocaine” since the latter recalls local anaesthetic properties and lidocaine presents a chemical structure similar to cocaine.13 In animals, water-soluble lidocaine is known as a local anaesthetic that blocks nonselective voltage-gated Na+ channels, inhibiting the propagation of APs.14–17 However, lidocaine could also desensitize other channels like TRPA1, a calcium permeable non‐selective cation channel.18 In animal models lidocaine can have an effect on cytosolic calcium levels, which may be an increase, or a decrease depending on the dose.19–21 The effect of lidocaine on Ca2+ homeostasis seems effectively complex and could further involve different Ca2+ regulations resulting from intracellular Ca2+ release or Ca2+ influx.22–24 Lidocaine could also be incorporated into the membrane altering membrane properties such as fluidity, permeability, and lipid packing order or disrupt raft-like ordered membrane domains which have different potency for channel blocking and neurotoxicity.25–27 Lidocaine can affect intra- and extra-cellular signalling pathways in both neuronal and non-neuronal cells, resulting in long-term modulation of biological functions, including cell growth and death.28 Indeed, lidocaine was shown to produce an increase in the amount of O2.– in vitro and in vivo.29,30 This reactive oxygen species (ROS) generation is related to activation of caspases and induction of apoptosis and necrosis in SH-SY5Y cells in a dose- and time-dependent manner.30
In plants, lidocaine was shown as other anaesthetics to inhibit the rapid movement of Mimosa pudica leaves.8,10,31 Yokawa et al. further demonstrated that lidocaine can induce ROS generation and slow the rate of endocytic vesicle recycling in plants that can alter membrane properties.10 Finally in plants as in animals, anaesthetics seem to impede many global biological functions over the long term. Indeed, the respiration, the seed dormancy breaking and the chlorophyll synthesis were altered after lidocaine treatments.10,32 This study focuses on the effect of lidocaine on rice (Oryza sativa), investigating whether it induces cellular responses in plants resembling those observed in animals.
Materials and methods
Two types of biological material were used in this study, cultured cells of rice for cellular approaches, and young seedlings of rice and tobacco for measurements of systemic electrical signals.
Rice culture conditions
Transformed rice cell suspensions (Oryza sativa L., cv. Nipponbare) expressing the gene encoding apo-aequorin (enabling quantification of cytosolic Ca2+) were maintained in AA culture medium complemented with 20 g.L−1 sucrose, 2 mg.L−1 2,4-dichlorophenoxy acetic acid (2,4 D), 0.1 mg.L−1 kinetin at 22 ± 2°C with continuous shaking (gyratory shaker at 150 rpm).33,34 Cell suspensions were sub-cultured weekly using a 1:35 dilution. All experiments were performed at 22 ± 2°C using log-phase cells.
For rice (Oryza sativa L., cv. Giglio) and tobacco (Nicotiana benthamiana) seedlings cultures, seeds were directly sown on soil in 7 cm2 pots and grown in a controlled-environment growth chamber in a 16 h light/8 h dark (1 plant per pot). The intensity of white light at the level of plant leaves was ∼50 μmol m−2s−1. The vegetation room was air-conditioned; the temperature was 24 ± 2°C and relative humidity 50–70%. Plants were watered twice per week with tap water and no other treatment was applied. After 1.5 or 2 months, seedlings with the same development characteristics are selected and placed in hydroponics in a nutrient solution (NF U 42–004, Canna), for 7 days before being used for experiments.
Cell viability assays
Cell viability in the cell suspension culture was determined by staining the dead cells with Evans blue dye (0.005%, w/v) by mixing and incubating the cells and the dye for 10 min under agitation.35 Cells were counted under a microscope and cells that accumulated Evans blue were considered dead. For each independent treatment 200 cells were counted and the procedure was repeated at least three times for each condition. The inhibitory agents actinomycin D (AD) and cycloheximide (Chx) were administered at a concentration of 20 µg.mL−1 each, 15 minutes prior to the application of lidocaine.
Caspase-like activity
Caspase-like activity detection in suspension cultured cells was performed using the fluorescent caspase inhibitor FITC-VAD-fmk (CaspAceTM, Promega, Ex = 490 nm, Em = 528 nm), according to the manufacturer’s instructions. Briefly, cells were incubated with 10 μM FITC-VAD-fmk during 20 min before treatment. The increase in fluorescence represents an increase in caspase-like activity.36,37 For measurement, 45 µl of cell suspension were placed in each well, and fluorescence was recorded continuously for one hour by a plate spectrofluorometer (Tecan infinite TM M200) and expressed in relative fluorescence units (RFU).
Cytosolic calcium measurements
Rice cultured cells expressing apoaequorin in the cytosol were used to record cytoplasmic Ca2+ variations.33,38 Aequorin was reconstituted by overnight incubation of the cell suspensions or seedlings in AA medium containing 12.5 μM native coelenterazine. For cell suspensions, 500 μL aliquots of cell suspension were directly transferred carefully to a luminometer glass tube. Treatments with lidocaine were performed by gentle pipette injection in the luminometer tubes. The luminescence counts were recorded continuously at 0.2 s intervals with a FB12-Berthold luminometer. At the end of each experiment, the residual aequorin was discharged by addition of 1 mL of a 1 M CaCl2 solution dissolved in 100% methanol. The resulting luminescence was used to estimate the total amount of aequorin for each condition. Calibration of calcium levels was performed using the equation: pCa = 0.332588(–logk) + 5.5593, where k is a rate constant equal to luminescence counts per second divided by total remaining counts.38 Data are expressed as micromolar and are means ± s.e.
Monitoring of ROS production
The production of ROS was monitored by measuring the chemiluminescence of the Cypridina Luciferin Analog (CLA) as previously described.35,39 CLA is known to react mainly with O2•- and 1O2 by emitting light and allows measuring the ROS in plant cells. Chemiluminescence from CLA was monitored using a FB12-Berthold luminometer (with a signal integrating time of 0.2 s). The ROS scavengers 1,2-dihydroxybenzene-3,5-disulfonic acid disodium salt (Tiron) and 1,4 diazabicyclo[2.2.2]octane (DABCO) were added 15 min before the different treatments.
Measure of cell polarization
The Bis (1,3-dibarbituric acid)-trimethine (DiBAC4) was used to measure plasma membrane polarization by fluorescence emission.40,41 The rice cultured cells were incubated with this probe (2 μM) for 30 min in the dark, under agitation. Measurements were performed using a spectrofluorometer (Tecan infinite TM M200) with 45 µl of cell culture per well. Fluorescence is recorded continuously with 490 nm excitation and 516 nm emission filters. An increase in fluorescence indicates plasma membrane depolarization, and a decrease indicates hyperpolarization. Measurements are recorded for 900 cycles of measurement, and treatment solutions were injected at the 80th cycle. Polarization variations are expressed in RFU. For each condition, 3 replicates were performed.
Measure Na+ accumulation in rice cells
To analyze the Na+ accumulation in the rice cultured cells, the sodium indicator CoroNa-green was used (Molecular Probes, USA). CoroNa-Green indicator (0.5 μM) was incubated with the cells for 30 min then washed two times with de cell medium.42 The cells were treated with NaCl at the beginning of the measurement in a 96-well plate with 50 μL of cells per well (Tecan infinite TM M200 spectrophotometer). The excitation wavelength was set at 485 nm and the emission were detected at 516 nm.
Electrical signal measurements on seedlings
Numerous studies have demonstrated that burn-induced flame stimulation leads to the propagation of an electrical signal along the plant stem.43 We therefore chose this stimulus to test the possible inhibitory effects of lidocaine on such signals. Seedlings were maintained in hydroponics with or without 1% (˜37 mM) lidocaine for 1 h. The studies were carried out on 1.5 months-old seedlings presenting the same developmental stage. The changes in the electrical potential (EP) were measured in a Faraday cage with two extracellular Ag/AgCl electrodes (a silver wire, 0.5 mm diameter coated with silver chloride) inserted across leaves for rice and stem for tobacco with a 10 cm space. The electrical potential was recorded from tissues adjacent to the electrode, i.e., vascular bundles, parenchyma, and epidermis as described in various studies.44–47 Electrical potential real time measurements were implemented using a wireless universal unit HIOKI LR 8511 data acquisition system. The sample frequency of recording was 100 ms. EP measurements were made at room temperature (22 ± 2°C).
Lidocaine treatments
In conducting experiments on cultured cells, lidocaine hydrochloride monohydrate was diluted in culture medium and added directly to the experimental tubes or wells in varying volumes to achieve the expected final concentrations. The volume added was never exceeded by more than 10% of the final volume of the tubes or wells. An equivalent volume of cell culture medium was used as a control. In the case of experiments conducted on whole seedlings, the 1% lidocaine solution was replaced with water in the flask, and the roots were treated for a period of 1 hour.
Statistical analysis
The statistical approach consisted in using Welch t test with holm correction for multiple comparison on the various samples of three replicates at minima considering that they are representative of a population following a normal pattern. The R software (R version 4.1.3) was used and P-values <0.05 were considered significant.
Results
Lidocaine could induce a PCD in subset of the rice suspension cell population
Lidocaine was shown to induce cell death in both neuronal and non-neuronal animal cells.28,30 We first evaluated in rice cell culture the impact of the additions of lidocaine 0.25 to 1%, in the range of what is currently used in animal cells or plants studies. Addition of lidocaine in the culture medium induced a dose and time dependent increase in cell death reaching 30% after 24 h for 1% of lidocaine (Figure 1(a,b)). Dead cells displayed a large cell shrinkage as frequently observed during plant PCD process.48,49 In order to confirm whether this cell death was due to an active process requiring active gene expression and cellular metabolism, rice cultured cells were treated with actinomycin D (AD), an inhibitor of RNA synthesis, or with cycloheximide (Chx), an inhibitor of protein synthesis, at 20 µg.mL−1 each, 15 min prior to 1% lidocaine exposures. In both cases, AD and Chx strongly reduced cell death (Figure 1(c)). These results indicated that the lidocaine-induced cell death required active cell metabolism, namely gene transcription and de novo protein synthesis. Taken together, these data suggest that lidocaine at 1% induced a PCD in a subset of the rice cultured cell population.
Figure 1.
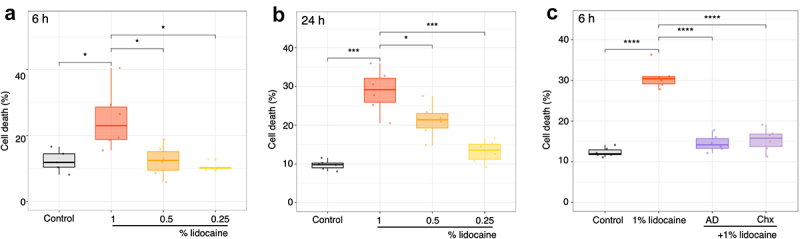
Lidocaine induces programmed cell death (PCD) in rice cells. Evolution of dose -dependent cell death induced by lidocaine treatments at 6 (a) and 24 h (b) for 3 different concentrations: 1%, 0.5% and 0.25%. (c) Effect of pretreatment with actinomycin D (AD, 20 μg.mL−1) or cycloheximide (chx, 20 μg.mL−1) on lidocaine-induced cell death at 1% after 6 h. Each point represents one replicate. Significance is represented by *: p < 0.05, ***: p < 0.001 and ****: p ≤ 0.0001.
Lidocaine-induced cell death in animal model seems dependent on caspase activations and ROS generation.30 Although, no true caspases have been found within plants, evidence suggests that caspase-like proteins participate in plant PCD.36 We thus tested on the cell death induced by 1% lidocaine the effect of pan-caspase fluorescent inhibitor (zVADfmk) known to interact with caspase-like activity in plant cells.36,37 No significant fluorescence was observed in control cells, whereas fluorescence increases appeared progressively in cells treated with 1% lidocaine (Figure 2(a)) suggesting that caspase-like proteins were activated in rice cells by lidocaine.
Figure 2.
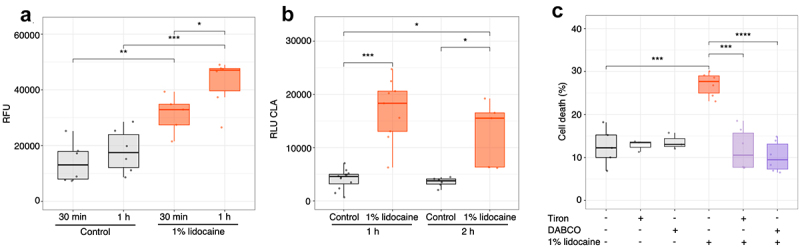
Caspase-like and ROS generation are involved in lidocaine-induced PCD in rice cells. (a) Induction of caspase-like activity revealed with the zVAD-fmk probe after treatment with 1% lidocaine at 30 min and 1 h. (b) Effect of 1% lidocaine treatment on ROS generation after 1 h and 2 h. (c) Effect of pretreatment with ROS scavengers, Tiron (5 mM) or DABCO (5 mM) on 1% lidocaine-induced cell death after 4 h. Each point represents one replicate. Significance is represented by *: p < 0.05, **: p < 0.01, ***: p < 0.001 and ****: p ≤ 0.0001.
We further checked for a putative lidocaine-induced ROS production in rice cultured cells by using the chemiluminescence of CLA which indicates the generation of O2•- and/or1O2. We could observe a ROS generation 1 h after application of 1% lidocaine (Figure 2(b)), when the culture medium used as control did not induce significant increase in chemiluminescence. We further checked the impact of the ROS scavengers (Tiron and DABCO, 5 mM each) on the 1% lidocaine-induced cell death. Both scavengers decreased the lidocaine-induced PCD (Figure 2(c)). These data suggest that, as observed in animal cells, caspase-like activity and ROS generation are involved in the lidocaine-induced PCD in rice cells.
Lidocaine could induce changes in membrane ion conductance
Despite its toxic effect lidocaine is used for anesthesia in animal. Its effects seem to function through perturbation of ionic conductance, mainly Na+ and Ca2+. In rice cells direct addition of 0.5% lidocaine did not significantly affect cell polarization analyzed with DIBAC4 fluorescence, when 1% seems to slightly hyperpolarized the cells (Figure 3(a)). We further checked if 0.5% lidocaine, a nontoxic dose in short term (Figure 1(a)) could affect Na+ conductance by analyzing the cytosolic Na+ accumulation using the sodium indicator CoroNa-green.42 Addition of 400 mM of NaCl effectively induced an accumulation of Na+ in the cytosol of rice cells in a few minutes (Figure 3(b)), indicating the activation of ion channels allowing the influx of Na+ into the cells. Pretreatment with 0.5% of lidocaine decreased the Na+ accumulation (Figure 3(b)) suggesting a putative role for lidocaine in Na+ conductance regulation in rice. To check for putative effect of lidocaine on Ca2+ conductance we used rice cells expressing aequorin in their cytosol.33,50 Addition of lidocaine induced a rapid decrease in [Ca2+]cyt in rice cells (Figure 3(c,d)) suggesting also a role for lidocaine in Ca2+ conductance regulation in rice cells.
Figure 3.
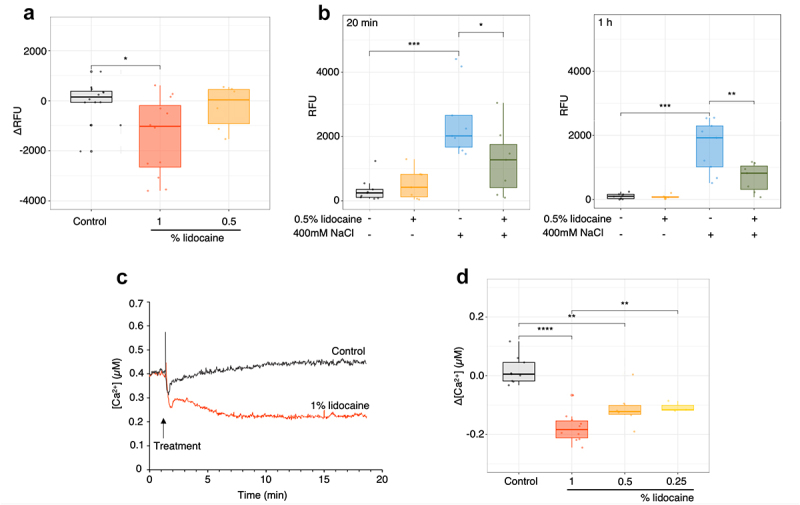
Effect of lidocaine on plasma membrane potential and Na+ and Ca2+ cytosolic levels. (a) Membrane potential recordings by DIBAC4 fluorescence after treatment with 0.5 or 1% lidocaine. (b) Decrease in Na+ accumulation at 20 min and 1 h by lidocaine at 0.5% after addition of 400 mM NaCl. (c) Decrease in cytosolic Ca2+ concentration by 1% lidocaine. Recording of one experiment representative to the others. (d) Mean cytosolic Ca2+ concentration after lidocaine treatments with 1, 0.5 and 0.25% lidocaine after 5 min. Each point represents one replicate. Significance is represented by *: p < 0.05, **: p < 0.01, ***: p < 0.001 and ****: p ≤ 0.0001.
Lidocaine could inhibit signalling pathways in rice cells
Since it is difficult to evaluate anesthesia on plant cultured cells, we investigated whether lidocaine could inhibit stress-induced cellular responses. To this end, we applied osmotic stresses (hyper and hypo) to rice cells, the responses to which are already described in culture cells. Hyperosmotic stress was induced by adding 400 mM NaCl, diluted in fresh AA medium. Lidocaine was applied as a pretreatment at a concentration of 0.5%, a dose with low toxicity at 4 h (Figure 1(a)). Previous studies have demonstrated that saline and non-saline hyper-osmotic shocks induces PCD in various cultured cells.35,39,50 We first confirmed that the addition of NaCl induced cell death as early as 4 h in rice cells (Figure 4(a)). We thus further could show that a 15-minutes pre-treatment with 0.5% lidocaine drastically reduced this NaCl induced PCD (Figure 4(a)). Since such hyperosmotic shocks are also known to rapidly increase cytosolic Ca2+ levels39 and lidocaine seems to decrease [Ca2+]cyt in rice cells (Figure 3(b,c,d)), we checked the impact of lidocaine on NaCl induced increase in [Ca2+]cyt in rice cells. The addition of 400 mM NaCl effectively induces a biphasic and transient increase in cytosolic calcium levels (Figure 4(b)). When this hyperosmotic shock was applied to a rice cells pretreated for 15 min with 0.5% lidocaine, the NaCl-induced increase in [Ca2+]cyt. was reduced (Figure 4(b,c)) as it could be expected from the decrease in [Ca2+]cyt induced by lidocaine alone. This confirms the ability of lidocaine in interfering with Ca2+ signaling and signaling pathway leading to PCD in rice cells.
Figure 4.
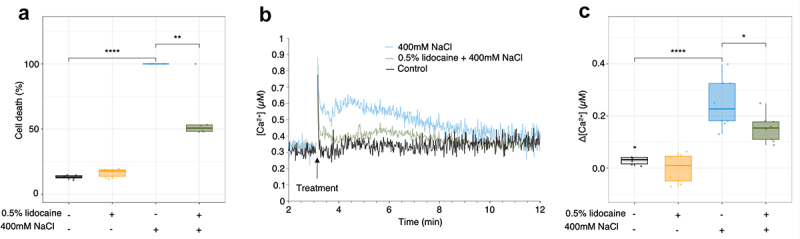
Lidocaine effect on the 400 mM NaCl-induced cell death and Ca2+ variations. (a) Mean cell death amplitude after hyper-osmotic shock induced by 400 mM NaCl, in the presence or absence of 0.5% lidocaine, after 4 h. (b) Decrease of the 400 mM NaCl -induced increase in cytosolic Ca2+ levels by lidocaine (0.5%). Recording of one experiment representative to the others. (c) Mean cytosolic Ca2+ concentration after 400 mM NaCl added, in the presence or absence of 0.5% lidocaine. Each point is one replicate. Significance is represented by *: p < 0.05, **: p < 0.01 and ****: p ≤ 0.0001.
To test the effect of lidocaine on hypoosmotic shock we added demineralized water to halve the osmolarity of the culture medium for cells, with or without a 15 min pre-treatment with 0.5% lidocaine. We observed that lidocaine reduced the cell death induced by the hypoosmotic shock (Figure 5(a)). On the contrary, the well-known rapid increase in [Ca2+]cyt induced by hypoosmotic shock51,52 was exhausted after 0.5% lidocaine pretreatment (Figure 5(b,c)). These last data confirm the ability of lidocaine in interfering in Ca2+ signaling in rice cells but also highlight the probable complex interactions of lidocaine in Ca2+ homeostasis regulation and suggest several cellular targets for lidocaine.53
Figure 5.
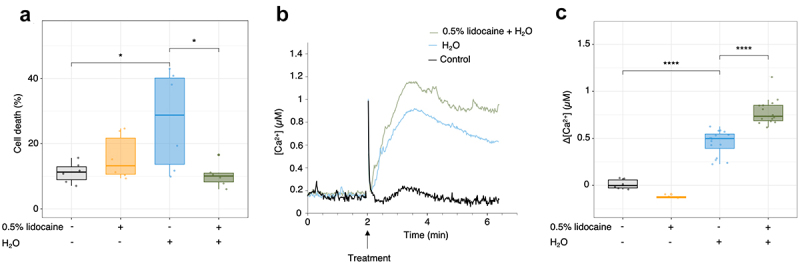
Lidocaine effect on the hypo-osmotic shock-induced cell death and Ca2+ variations. (a) Mean cell death amplitude after hypo-osmotic shock, in the presence or absence of 0.5% lidocaine, after 4 h. (b) Increase of the hypo-osmotic shock-induced increase in cytosolic Ca2+ levels by lidocaine 0.5%. Recording of one experiment representative to the others. (c) Mean cytosolic Ca2+ concentration after H2O added, in the presence or absence of 0.5% lidocaine at the maximal amplitude variations. Each point is one replicate. Significance is represented by *: p < 0.05 and ****: p ≤ 0.0001.
Lidocaine inhibits flame-induced long distance electrical signalling in seedlings
In animal models, lidocaine is known to decrease the amplitude of APs and increase their duration.54 Other anaesthetics such as diethyl ether were also shown to inhibit APs in plant.,9–11 To push forward our studies on rice we tested whether lidocaine could similarly have an effect on the well-known long distance electrical signals elicited upon leaf burning.43 We recorded EP variations propagating along the leaves of young rice seedlings after leaf burning with or without root pretreatment by 1% lidocaine. As expected from the literature, burning induces hyperpolarization (negative voltage shift recorded extracellularly, representing intracellular depolarization) (Figure 6(a,b)). These hyperpolarisations present characteristics of slow wave potentials (SWPs).55 Pretreatment with 1% lidocaine for 1 h reduced the extent of the hyperpolarisations following flame stimulation (Figure 6(a,b)). Since the extent of the hyperpolarisations were quite weak, as already observed for SWPs in rice,56 we further reproduced the experiments on tobacco seedlings known to present large SWPs in response to leaves burning. As observed in rice seedlings, in tobacco seedlings burning-induced hyperpolarisations were drastically reduced upon root pretreatment with 1% lidocaine (Figure 6(c,d)).
Figure 6.
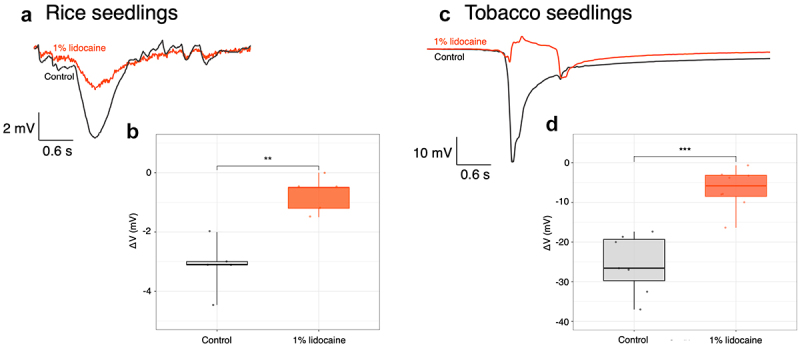
Inhibition of systemic electrical signaling induced by leaf burning in rice and tobacco seedlings by 1% lidocaine. Kinetics obtained following tip leaf burns of rice (a) or tobacco (c) with (orange line) or without (black line) root treatment with 1% lidocaine for 1 h. Representative voltage recording of one experiment. (b-d) difference between the potentials at the point of maximum variation (ΔV) for untreated and lidocaine treated plants. Each point is one replicate. Significance is represented by **: p < 0.01 and ***: p < 0.001.
Discussion
In animal cells lidocaine could have cytotoxic activity in addition to its anaesthetic activity.28,30 In this study, we first check for putative toxic effects of lidocaine on rice cell cultures. At 1%, lidocaine effectively induced the death of some rice cells, these last presenting large cell shrinkage. The cell death extent was of about 30% of the cultured cells after 24 h. Lower doses didn’t induce significant death, at least in the short term. The decrease in lidocaine-induced cell death after pretreatment with inhibitors of RNA synthesis (AD) and protein synthesis (Chx), suggested that active processes are needed to achieve this cell death. These cellular events are reminiscent of what is observed during the development of PCD in response to numerous stimuli in plant as in animal. Furthermore, the dependence of lidocaine-induced PCD to caspase-like activity and ROS generation in rice cells resembles to what was described for the cytotoxicity of lidocaine in some animal cells.30 As a whole, these data suggest that the toxic effect of lidocaine resemble the one observed in animal cells and suggest conserved targets in animal and plant cells.
Besides this toxic effect, in animal cells lidocaine seems to regulate plasma membrane properties possibly through different mechanisms,19,20,25 even if the blockage of sodium channels preventing conduction of the electrical impulse is the most frequent mechanism described to explain lidocaine anaesthetic effect in animals.14–17,57 Addition of lidocaine to rice cultured cells did not strongly modified the cell polarization even if a slight hyperpolarization could be observed at 1% lidocaine. However, pretreatment with lidocaine seems to limit the Na+ uptake by rice cells. Although specific sodium channels are not present in plant cells,4 Na+ could be taken up through various cation channels (cyclic nucleotide gated channel (CNGC), non-selective cation channels (NSCC), glutamate-like receptors (GLR) or transporters from the HKT family,58 what suggests that lidocaine could affect the activity of some of these channels in rice. Lidocaine was also shown to reduce the cytosolic Ca2+ level in rice cells, what could also be related to the blockage of non-specific cations channels limiting the Ca2+ influx with maintained activity of Ca2+ efflux transporters. The decrease in cytosolic Ca2+ may be related to data of Trotta et al., who observed a decrease in calcium uptake with lidocaine in rabbit brain microsomal fraction.21 However, in this model the effect seems to be dose dependent, higher concentrations increasing calcium uptake. Increase in cytosolic Ca2+ was also observed in mouse neuronal cells possibly through depletion of Ca2+ store (via an IP3R- and RYR-independent manner) and suppression of store-operated Ca2+ influx,24 even if lidocaine was also found to stimulate ryanodine binding to ryanodine receptors, Ca2+ release channels.23 In cultured neurons of Lymnaea stagnalis the increase in cytosolic Ca2+ concentrations by lidocaine seems furthermore related to morphological damage and shrinkage.20 Since lidocaine seems able to regulate various cationic conductance in various membranes, we could not exclude that in rice cells the decrease in cytosolic Ca2+ by lidocaine could also be due to a stimulation of Ca2+ transporters allowing Ca2+ export out of the cells or towards internal stocks. Furthermore, we found upon stimulation by hyper- and hypoosmotic shocks that cells pretreated with lidocaine present altered cytosolic Ca2+ variations but in opposite directions, a decrease in response to saline hyperosmotic shock but an increase for hypoosmotic shock. These data highlight the complex effect of lidocaine on Ca2+ homeostasis in rice cells probably involving different targets. It is also worth noting that when hypo-osmotic stresses were applied, the membrane depolarization observed in rice cells with the DIBAC4 probe was amplified by lidocaine pretreatment (not shown), as was the cytosolic Ca2+ level confirming the effects of lidocaine on membrane properties.
In another way our data showing the reduction of PCD by lidocaine triggered by hyper- and hypoosmotic shocks clearly indicate its putative role in controlling signaling pathways known to involve early ion channels regulations in animal,59,60 as in plants.61 Since anaesthetics were also shown in plant to inhibit APs in D. muscipula possibly through inhibition of a Ca2+ dependent GLR,9,11 we further check if lidocaine could impair burn-induced SWPs in whole seedlings of rice and tobacco.62 We could observe in these two models an inhibition of SWPs induced upon burning of the leaf tips after a pretreatment of roots with lidocaine. These data suggest that lidocaine could limit long distance signaling in plants not only through AP inhibition but also through SWP inhibition. The SWPs induce by wounding in rice were shown to be dependent on the activation of the glutamate receptor OsGLR3.4 allowing Ca2+ influx in rice.56 SWPs and GLRs dependent Ca2+ signaling was also shown to be inhibited by diethyl-ether in A. thaliana.12 Lidocaine could thus regulate the burn-mediated systemic SWPs by inhibiting the Ca2+ influx through such GLRs as do diethyl ether in D. muscipula and A. thaliana.,11,12
While this study clearly shows that Ca2+ signaling is probably a target of lidocaine in plants, further work is needed to identify the target(s) of lidocaine, both at the plasma membrane level and with regard to the Ca2+ stores potentially involved in response to lidocaine. In any case, these preliminary data suggest that lidocaine are capable of modifying a certain number of cellular responses likely to inhibit the transmission of electrical signals in plants, as observed in animals. Moreover, our data are also to be compared with the effects of other anaesthetics in plants, effectively when we showed that lidocaine reduces the cytosolic Ca2+ level in rice cells, it was shown to increase in response to diethyl ether application A. thaliana63 and not modified in ketamine-treated plants.64 Continuing this work could enable us to verify whether or not the mechanisms involved in plants correspond to evolutionarily conserved properties and/or could open up prospects for the search for new cellular targets for lidocaine.
Acknowledgments
The authors thank Pr. Tomonori Kawano (Kitakyūshū University) for the kind gift of the rice culture cells.
Funding Statement
This study was supported by funds from Ministère de l’Enseignement supérieur, de la Recherche et de l’Innovation to LIED.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Author contributions
LSB, DAB and FF carried out and analyzed the experiments. CL participated in data analysis. AR helped maintaining the cultures. FB supervised the project with the help of PL, EG and PM. LSB and FB wrote the manuscript. The manuscript was written through contributions of all authors. All authors have read and agreed to the published version of the manuscript.
References
- 1.Bernard C. Leçons sur les phénomènes de la vie, communs aux animaux et aux végétaux [Lectures on Phenomena of Life Common to Animals and Plants]. Paris: Balllière and Son; 1878. [Google Scholar]
- 2.Grémiaux A, Yokawa K, Mancuso S, Baluška F. Plant anesthesia supports similarities between animals and plants: Claude Bernard’s forgotten studies. Plant Signaling and Behav. 2044;9(1):e27886. doi: 10.4161/psb.27886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kelz MB, Mashour GA. The biology of General anesthesia from paramecium to primate. Curr Biol. 2019;29(22):R1199–9. doi: 10.1016/j.cub.2019.09.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sylvain-Bonfanti L, Page J, Arbelet-Bonnin D, Meimoun P, Grésillon G, Bouteau F, Laurenti P. L’anesthésie, un processus commun à tout le vivant. médecine/sciences. 2023;39(10):738–743. doi: 10.1051/medsci/2023123. [DOI] [PubMed] [Google Scholar]
- 5.Sonner JM, Cantor RS. Molecular mechanisms of drug action: an emerging view. Annu Rev Biophys. 2013;42(1):143–167. doi: 10.1146/annurev-biophys-083012-130341. [DOI] [PubMed] [Google Scholar]
- 6.Franks NP, Lieb WR. Do general anaesthetics act by competitive binding to specific receptors? Nature. 1984;310(5978):599–601. doi: 10.1038/310599a0. [DOI] [PubMed] [Google Scholar]
- 7.Stamenic T, Todorovic SM. Thalamic T-Type calcium channels as targets for hypnotics and general anesthetics. 2022. Int J Mol Sci. 2022;23(4):2349. doi: 10.3390/ijms23042349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Milne A, Beamish T. Inhalational and local anesthetics reduce tactile and thermal responses inmimosa pudica. Can J Anesth/J Can Anesth. 1999;46(3):287–289. doi: 10.1007/BF03012612. [DOI] [PubMed] [Google Scholar]
- 9.Pavlovič A, Libiaková M, Bokor B, Jakšová J, Petřík I, Novák O, Baluška F. Anaesthesia with diethyl ether impairs jasmonate signalling in the carnivorous plant venus flytrap (dionaea muscipula). Ann Of Botany. 2020;125(1):173–183. doi: 10.1093/aob/mcz177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yokawa K, Kagenishi T, Pavlovič A, Gall S, Weiland M, Mancuso S, Baluška F. Anaesthetics stop diverse plant organ movements, affect endocytic vesicle recycling and ROS homeostasis, and block action potentials in venus flytraps. Ann Botany. 2017; doi: 10.1093/aob/mcx155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scherzer S, Huang S, Iosip A, Kreuzer I, Yokawa K, AL-Rasheid KAS, Heckmann M, Hedrich R. Ether anesthetics prevents touch-induced trigger hair calcium-electrical signals excite the venus flytrap. Sci Rep. 2022;12(1):2851. doi: 10.1038/s41598-022-06915-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jakšová J, Rác M, Bokor B, Petřík I, Novák O, Reichelt M, Mithöfer A, Pavlovič A. Anesthetic diethyl ether impairs long-distance electrical and jasmonate signallingin Arabidopsis thaliana. Plant Physiol And Biochem. 2021;169:311–321. doi: 10.1016/j.plaphy.2021.11.019. [DOI] [PubMed] [Google Scholar]
- 13.Tsuchiya H. Anesthetic agents of plant origin: a review of phytochemicals with anesthetic activity. Molecules. 2017;22(8):1369. doi: 10.3390/molecules22081369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chevrier P, Vijayaragavan K, Chahine M. Differential modulation of Nav1.7 and Nav1.8 peripheral nerve sodium channels by the local anesthetic lidocaine. Br J Pharmacol. 2004;142(3):576–584. doi: 10.1038/sj.bjp.0705796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaneda K, Oyama Y, Ikemoto Y, Akaike N. Blockade of the voltage-dependent sodium current in isolated rat hippocampal neurons by tetrodotoxin and lidocaine. Brain Res. 1989;484(1–2):348–351. doi: 10.1016/0006-8993(89)90379-X. [DOI] [PubMed] [Google Scholar]
- 16.Loser D, Schaefer J, Danker T, Möller C, Brüll M, Suciu I, Ückert A-K, Klima S, Leist M, Kraushaar U. et al. Human neuronal signalling and communication assays to assess functional neurotoxicity. Archiv Toxicol. 2021;95(1):229–252. doi: 10.1007/s00204-020-02956-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tikhonov DB, Bruhova I, Zhorov BS. Atomic determinants of state‐dependent block of sodium channels by charged local anesthetics and benzocaine. FEBS Lett. 2006;580(26):6027–6032. doi: 10.1016/j.febslet.2006.10.035. [DOI] [PubMed] [Google Scholar]
- 18.Docherty RJ, Ginsberg L, Jadoon S, Orrell RW, Bhattacharjee A. TRPA1 insensitivity of human sural nerve axons after exposure to lidocaine. Pain. 2013;154(9):1569–1577. doi: 10.1016/j.pain.2013.04.030. [DOI] [PubMed] [Google Scholar]
- 19.Doan LV, Eydlin O, Piskoun B, Kline RP, Recio-Pinto E, Rosenberg AD, Blanck TJJ, Xu F. Despite differences in cytosolic calcium regulation, lidocaine toxicity is similar in adult and neonatal rat dorsal root ganglia in vitro. Anesthesiology. 2014;120(1):50–61. doi: 10.1097/ALN.0b013e3182a2a561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kasaba T, Onizuka S, Kashiwada M, Takasaki M. Increase in intracellular Ca2+ concentration is not the only cause of lidocaine-induced cell damage in the cultured neurons of Lymnaea stagnalis. J Anesth. 2006;20(3):196–201. doi: 10.1007/s00540-006-0397-6. [DOI] [PubMed] [Google Scholar]
- 21.Trotta EE, Freire GL, Godinho CS. The mode of action of local anesthetics on the calcium pump of brain. J Pharmacol And Exp Ther. 1980;214:670–674. [PubMed] [Google Scholar]
- 22.D’Agostino G, Saporito A, Cecchinato V, Silvestri Y, Borgeat A, Anselmi L, Uguccioni M. Lidocaine inhibits cytoskeletal remodelling and human breast cancer cell migration. Br J Anaesthesiology. 2018;121(4):962–968. doi: 10.1016/j.bja.2018.07.015. [DOI] [PubMed] [Google Scholar]
- 23.Martin C, Ashley R, Shoshan-Barmatz V. The effect of local anaesthetics on the ryanodine receptor/Ca 2+ release channel of brain microsomal membranes. FEBS Lett. 1993;328(1–2):77–81. doi: 10.1016/0014-5793(93)80969-2. [DOI] [PubMed] [Google Scholar]
- 24.Wu KC, Wong KL, Shiao LR, Chen CY, Chan P, Leung YM. Perturbation of Ca2+ stores and store-operated Ca2+ influx by lidocaine in neuronal N2A and NG108-15 cells. Eur J Pharmacol. 2021;904:174115. doi: 10.1016/j.ejphar.2021.174115. [DOI] [PubMed] [Google Scholar]
- 25.Choi W, Ryu H, Fuwad A, Goh S, Zhou C, Shim J, Takagi M, Kwon S, Kim SM, Jeon T-J. et al. Quantitative analysis of the membrane affinity of local anesthetics using a Model cell membrane. Membranes 2021. 2021;11(8):579. doi: 10.3390/membranes11080579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kamata K, Manno S, Takakuwa Y, Ozaki M. Reversible effect of lidocaine on raft formation. Int Congr Ser. 2005. 1283:1283:281–282. doi: 10.1016/j.ics.2005.06.077. [DOI] [Google Scholar]
- 27.Kinoshita M, Chitose T, Matsumori N. Mechanism of local anesthetic-induced disruption of raft-like ordered membrane domains. Biochemica Et Biophys Acta - Gener Subj. 2019. 1863:1863(9):1381–1389. doi: 10.1016/j.bbagen.2019.06.008. [DOI] [PubMed] [Google Scholar]
- 28.Werdehausen R, Braun S, Essmann F, Schulze-Osthoff K, Walczak H, Lipfert P, Stevens MF. Lidocaine induces apoptosis via the mitochondrial pathway independently of death receptor signaling. Anesthesiology. 2007;107(1):136–143. doi: 10.1097/01.anes.0000268389.39436.66. [DOI] [PubMed] [Google Scholar]
- 29.Gulyaeva NV, Obidin AB, Marinov BS. Modulation of superoxide dismutase by electron donors and acceptors. FEBS Lett. 1987;211(2):211–214. doi: 10.1016/0014-5793(87)81438-2. [DOI] [PubMed] [Google Scholar]
- 30.Okamoto A, Tanaka M, Sumi C, Oku K, Kusunoki M, Nishi K, Matsuo Y, Takenaga K, Shingu K, Hirota K. et al. The antioxidant N-acetyl cysteine suppresses lidocaine-induced intracellular reactive oxygen species production and cell death in neuronal SH-SY5Y cells. BMC Anesthesiol. 2016;16(1):104. doi: 10.1186/s12871-016-0273-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Luccia TP. Mimosa pudica, dionaea muscipula and anesthetics. Plant Signaling & Behav. 1722;7(9):1163–1167. doi: 10.4161/psb.21000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thomas HS. Comparative studies on respiration: IV. The effect of etger on the respiration of wheat. J Gener Physiol. 1918;1(2):203–207. doi: 10.1085/jgp.1.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nguyen HTN, Umemura K, Kawano T. Indole-3-acetic acid-induced oxidative burst and an increase in cytosolic calcium ion concentration in rice suspension culture. Biosci Biotechnol Biochem. 2016;80(8):1546–1554. doi: 10.1080/09168451.2016.1179094. [DOI] [PubMed] [Google Scholar]
- 34.Toriyama K, Hinata K. Cell suspension and protoplast culture in rice. Plant Sci. 1985;41(3):179–183. doi: 10.1016/0168-9452(85)90086-X. [DOI] [Google Scholar]
- 35.Zhao T, Arbelet-Bonnin D, Tran D, Monetti E, Lehner A, Meimoun P, Kadono T, Dauphin A, Errakhi R, Reboutier D. et al. Biphasic activation of survival and death pathways in Arabidopsis thaliana cultured cells by sorbitol-induced hyperosmotic stress. Plant Sci. 2021;305:110844. doi: 10.1016/j.plantsci.2021.110844. [DOI] [PubMed] [Google Scholar]
- 36.Elbaz M, Avni A, Weil M. Constitutive caspase-like machinery executes programmed cell death in plant cells. Cell Death Differ. 2002;9(7):726–733. doi: 10.1038/sj.cdd.4401030. [DOI] [PubMed] [Google Scholar]
- 37.Tran D, Rossi M, Biligui B, Kawano T, Mancuso S, Bouteau F. Ozone-induced caspase-like activities are dependent on early ion channel regulations and ROS generation in Arabidopsis thaliana cells. Plant Signaling And Behav. 2013;8(8):e25170. doi: 10.4161/psb.25170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Knight MR, Read ND, Campbell AK, Trewavas AJ. Imaging calcium dynamics in living plants using semi-synthetic recombinant aequorins. J Cell Biol. 1993;121(1):83–90. doi: 10.1083/jcb.121.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Monetti E, Kadono T, Tran D, Azzarello E, Arbelet-Bonnin D, Biligui B, Briand J, Kawano T, Mancuso S, Bouteau F. et al. Deciphering early events involved in hyperosmotic stress-induced programmed cell death in tobacco BY-2 cells. J Exp Botany. 2014;65(5):1361–1375. doi: 10.1093/jxb/ert460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gauthier A, Lamotte O, Reboutier D, Bouteau F, Pugin A, Wendehenne D. Cryptogein-induced anion effluxes: electrophysiological properties and analysis of the mechanisms through which they contribute to the elicitor-triggered cell Death. Plant Signaling And Behav. 2007;2(2):86–95. doi: 10.4161/psb.2.2.4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamada A, Gaja N, Ohya S, Muraki K, Narita H, Ohwada T, Imaizumi Y. Usefulness and limitation of DiBAC4(3), a voltage-sensitive fluorescent dye, for the measurement of membrane potentials regulated by recombinant large conductance Ca2±Activated K+ channels in HEK293 cells. Jpn J Pharmacol. 2001;86(3):342–350. doi: 10.1254/jjp.86.342. [DOI] [PubMed] [Google Scholar]
- 42.Arbelet-Bonnin D, Ben Hamed-Laouti I, Laurenti P, Abdelly C, Ben Hamed K, Bouteau F. Cellular mechanisms to survive salt in the halophyte cakile maritima. Plant Sci. 2018;272:173–178. doi: 10.1016/j.plantsci.2018.04.018. [DOI] [PubMed] [Google Scholar]
- 43.Blyth MG, Morris RJ. Shear-enhanced dispersion of a wound substance as a Candidate mechanism for variation potential transmission. Front Plant Sci. 2019;10:1393. doi: 10.3389/fpls.2019.01393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dziubińska H, Trębacz K, Zawadzki T. Transmission route for action potentials and variation potentials in Helianthus annuus L. J Plant Physiol 1167–1172.2001;158(9):1167–1172. doi: 10.1078/S0176-1617(04)70143-1. [DOI] [Google Scholar]
- 45.Gil PM, Gurovich L, Schaffer B, García N, Iturriaga R. Electrical signaling, stomatal conductance, ABA and Ethylene content in avocado trees in response to root hypoxia. Plant Signaling And Behav 2009. 2009;4(2):100–108. doi: 10.4161/psb.4.2.7872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gurovich L, Hermosilla P. Electric signalling in fruit trees in response to water applications and light–darkness conditions. J Plant Physiol. 2009;166(3):290–300. doi: 10.1016/j.jplph.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 47.Oyarce P, Gurovich L. Electrical signals in avocado trees: responses to light and water availability conditions. Plant Signaling And Behav. 2010;5(1):34–41. doi: 10.4161/psb.5.1.10157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reape TJ, McCabe PF. Apoptotic-like regulation of programmed cell death in plants. Apoptosis. 2010;15(3):249–256. doi: 10.1007/s10495-009-0447-2. [DOI] [PubMed] [Google Scholar]
- 49.Van Doorn WG, Beers EP, Dangl JL, Franklin-Tong VE, Gallois P, Hara-Nishimura I, Jones AM, Kawai-Yamada M, Lam E, Mundy J. et al. Morphological classification of plant cell deaths. Cell Death And Differentiation. 2011;18(8):1241–1246. doi: 10.1038/cdd.2011.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hamada H, Kurusu T, Okuma E, Nokajima H, Kiyoduka M, Koyano T, Sugiyama Y, Okada K, Koga J, Saji H. et al. Regulation of a proteinaceous elicitor-induced Ca2+ influx and production of phytoalexins by a putative voltage-gated cation channel, OsTPC1, in cultured Rice cells. J Biol Chem. 2012;287(13):9931–9939. doi: 10.1074/jbc.M111.337659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ben Hamed-Laouti I, Arbelet-Bonnin D, De Bont L, Biligui B, Gakière B, Abdelly C, Ben Hamed K, Bouteau F. Comparison of NaCl-induced programmed cell death in the obligate halophyte cakile maritima and the glycophyte Arabidospis thaliana. Plant Sci. 2016;247:49–59. doi: 10.1016/j.plantsci.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 52.Cessna SG, Chandra S, Low PS. Hypo-osmotic shock of tobacco cells stimulates Ca2+Fluxes deriving first from external and then internal Ca2+Stores. J Biol Chem. 1998;273(42):27286–27291. doi: 10.1074/jbc.273.42.27286. [DOI] [PubMed] [Google Scholar]
- 53.Nguyen NTH, Bouteau F, Mazars C, Kuse M, Kawano T. Enhanced elevations of hypo-osmotic shock-induced cytosolic and nucleic calcium concentrations in tobacco cells by pretreatment with dimethyl sulfoxide. Biosci, Biotechnol Biochem. 2019;83(2):318–321. doi: 10.1080/09168451.2018.1533801. [DOI] [PubMed] [Google Scholar]
- 54.Wolff M, Schnöbel-Ehehalt R, Mühling J, Weigand MA, Olschewski A. Mechanisms of Lidocaine’s action on subtypes of spinal dorsal horn neurons subject to the diverse roles of Na+ and K+ channels in action potential generation. Anesth And Analgsia. 2014;119(2):463–470. doi: 10.1213/ANE.0000000000000280. [DOI] [PubMed] [Google Scholar]
- 55.Farmer EE, Gao Y, Lenzoni G, Wolfender JW, Wu, Q Q. Wound- and mechanostimulated electrical signals control hormone responses. New Phytol. 2020;227(4):1037–1050. doi: 10.1111/nph.16646. [DOI] [PubMed] [Google Scholar]
- 56.Yu B, Wu Q, Li X, Zeng R, Min Q, Huang J. GLUTAMATE receptor-like gene OsGLR3.4 is required for plant growth and systemic wound signaling in rice(oryza sativa). New Phytol. 2022;233(3):1238–1256. doi: 10.1111/nph.17859. [DOI] [PubMed] [Google Scholar]
- 57.Fozzard HA, Sheets MF, Hanck DA. The sodium channel as a target for local anesthetic drugs. Front Pharmacol. 2011;2. doi: 10.3389/fphar.2011.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maathuis FJM, Ahmad I, Patishtan J. Regulation of Na+ fluxes in plants. Front Plant Sci. 2014. 5. 5. doi: 10.3389/fpls.2014.00467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bortner CD, Cidlowski JA. Cell shrinkage and monovalent cation fluxes: role in apoptosis. Archives In Biochem And Biophysic. 2007;462(2):176–188. doi: 10.1016/j.abb.2007.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Okada Y, Maeno E, Mori S. Anion channel involved in induction of apoptosis and necrosis. In: Lauf P, Adragna NC. editors, in Advances in Experimental Medicine and Biology, vol. 559. Cell volume and signaling. Springer US; 2005. p. 205–209. doi: 10.1007/0-387-23752-6_19. [DOI] [PubMed] [Google Scholar]
- 61.Bouteau F, Reboutier D, Tran D, Laurenti P. Ion transport in plant cell shrinkage during death. Front Cell Dev Biol. 2020;8:566606. doi: 10.3389/fcell.2020.566606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Evans MJ, Morris RJ. Chemical agents transported by xylem mass flow propagate variation potentials. The Plant J. 2017;91(6):1029–1037. doi: 10.1111/tpj.13624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pavlovič A, Jakšová J, Kučerová Z, Špundová M, Rác M, Roudnický P, Mithöfer A. Diethyl ether anesthesia induces transient cytosolic [Ca2+] increase, heat shock proteins, and heat stress tolerance of photosystem II in arabidopsis. Front Plant Sci. 2022;13:995001. doi: 10.3389/fpls.2022.995001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pavlovič A, Ševčíková L, Hřivňacký M, Rác M. Effect of the general anaesthetic ketamine on electrical and Ca2+ signal propagation in Arabidopsis thaliana. Plants (Basel). 2024;13(6):894. doi: 10.3390/plants13060894. [DOI] [PMC free article] [PubMed] [Google Scholar]


