Abstract
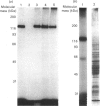
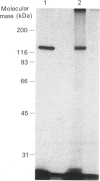
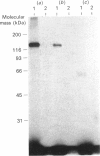
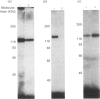
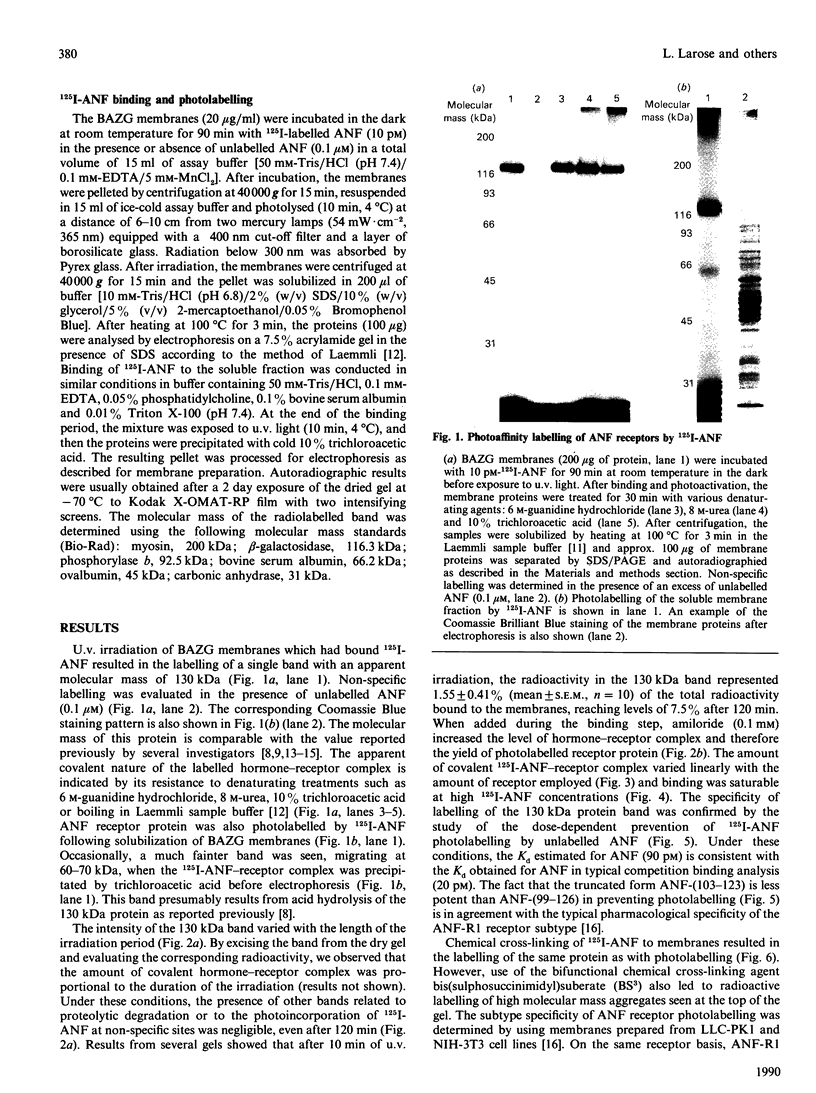
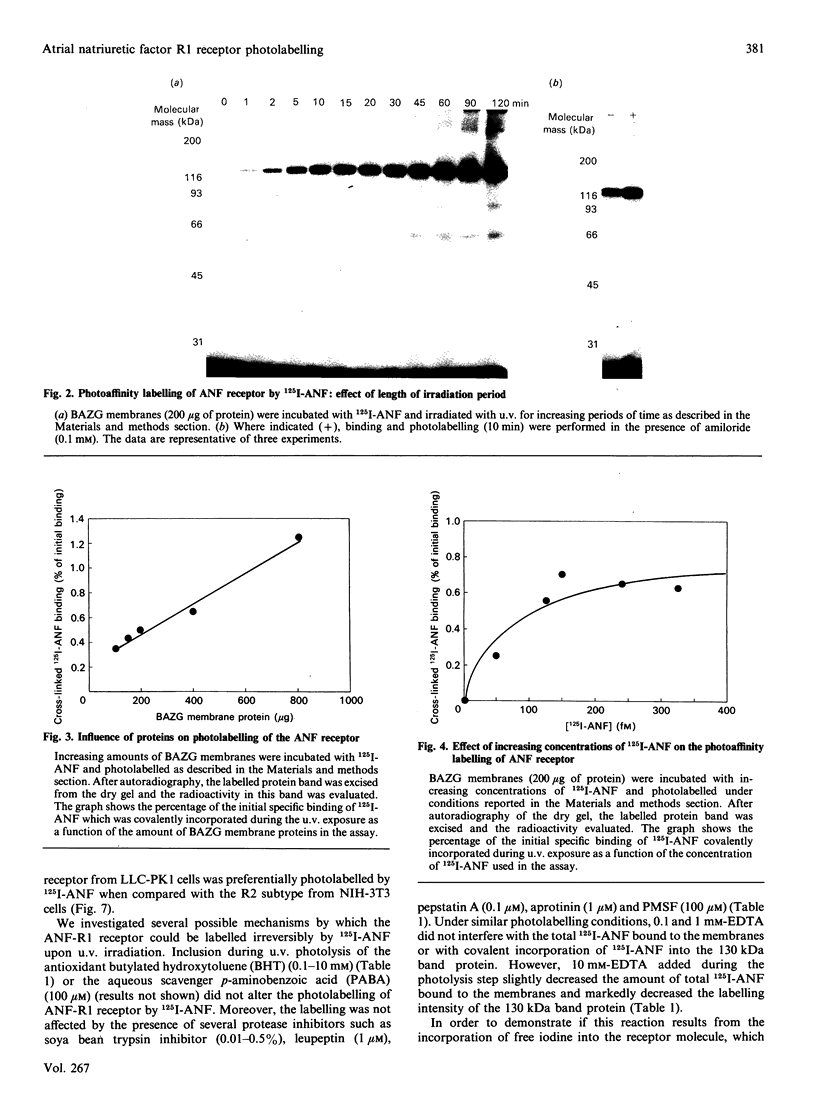
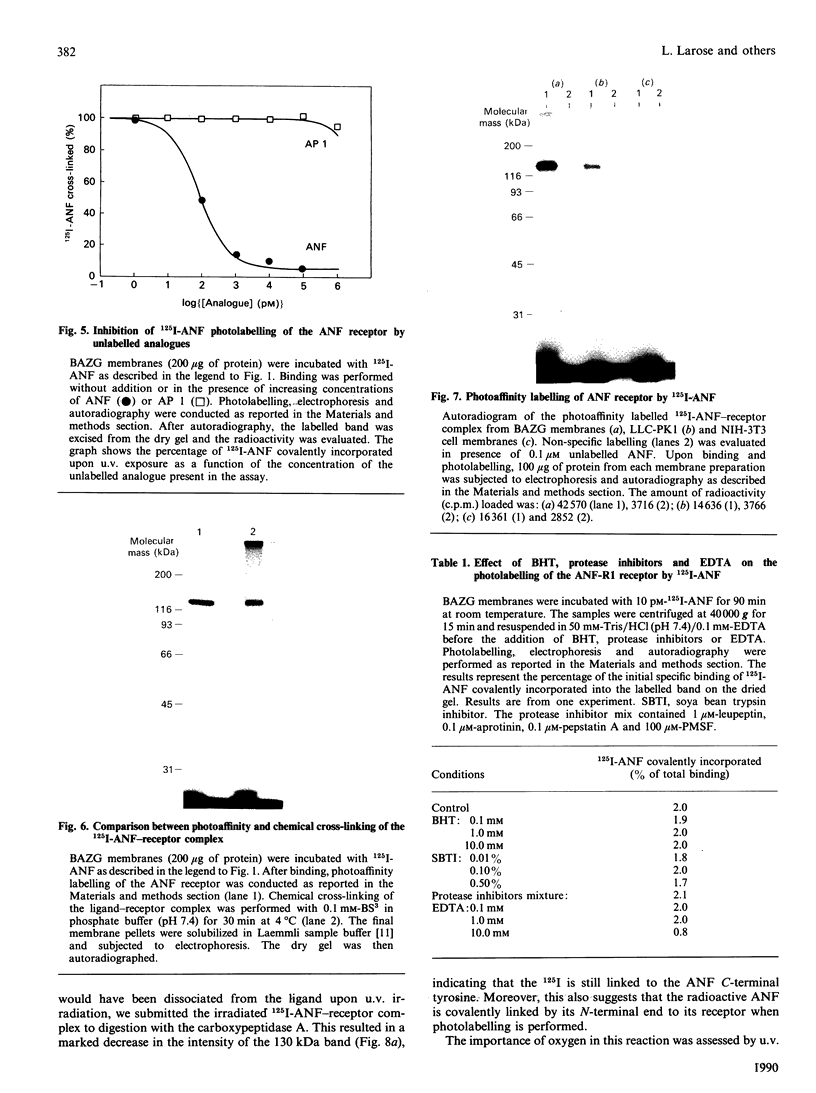
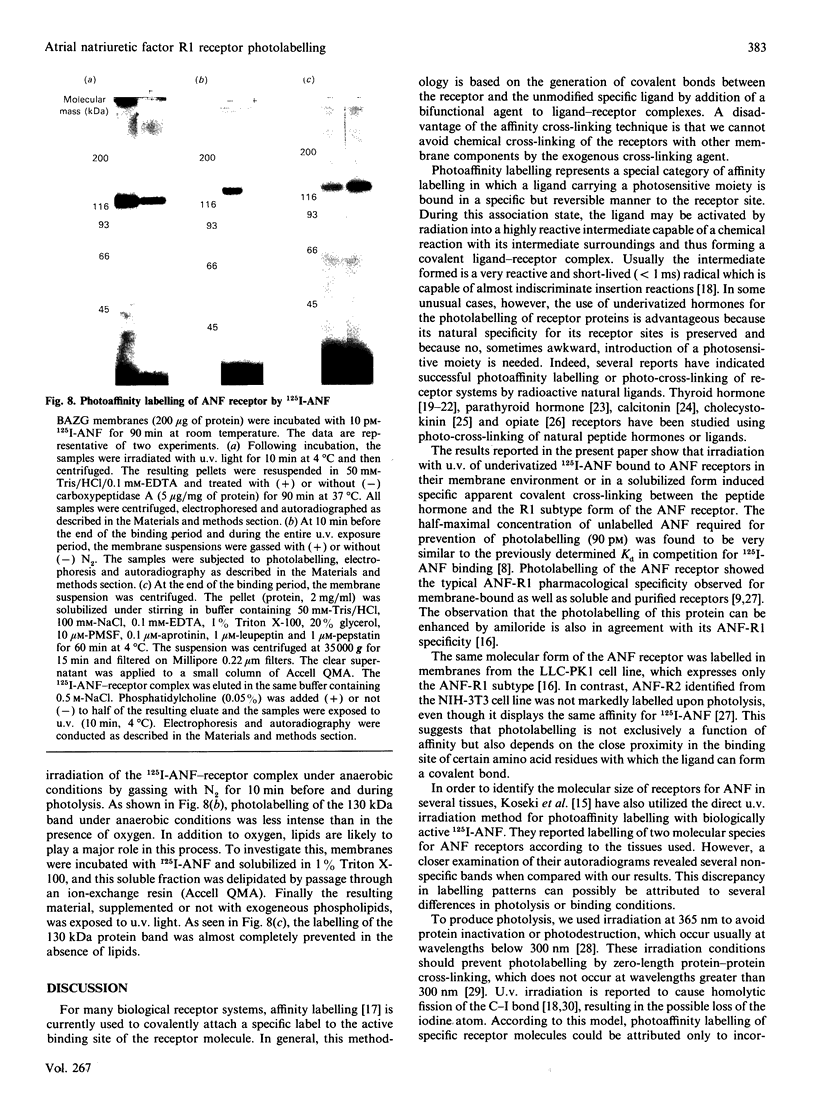
In bovine adrenal zona glomerulosa, atrial natriuretic factor (ANF) exerts its physiological effect through high-affinity binding to specific membrane receptors. On studying further the molecular properties of the ANF receptor binding domain, we have observed that incubation of intact or solubilized bovine adrenal zona glomerulosa membranes with 125I-ANF-(99-126) followed by u.v. irradiation results in the irreversible labelling of a 130 kDa protein corresponding to the ANF-RI receptor. This process is time-, protein- and 125I-ANF-dependent. The apparently covalent nature of this complex is documented by its resistance to heat, guanidine hydrochloride, urea and trichloroacetic acid denaturation. Photolabelling with underivatized 125I-ANF is much more efficient with the ANF-R1 than with the ANF-R2 receptor. After photolysis, the covalently linked 125I-ANF is still sensitive to digestion by carboxypeptidase A, suggesting that ANF is linked by its N-terminal end to the receptor upon u.v. irradiation and that its C-terminal end is still freely accessible. Aerobic conditions and lipids are required for the photolabelling, suggesting a role in this process for malondialdehyde, a highly reactive secondary product associated with u.v.-induced lipid peroxidation. This simple method should provide a powerful tool in the accurate characterization of the hormone-binding domain of the ANF receptor.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bone E. A., Rosenzweig S. A. Characterization of cholecystokinin receptors in toad retina. Peptides. 1988 Mar-Apr;9(2):373–381. doi: 10.1016/0196-9781(88)90273-2. [DOI] [PubMed] [Google Scholar]
- Casanova J., Horowitz Z. D., Copp R. P., McIntyre W. R., Pascual A., Samuels H. H. Photoaffinity labeling of thyroid hormone nuclear receptors. Influence of n-butyrate and analysis of the half-lives of the 57,000 and 47,000 molecular weight receptor forms. J Biol Chem. 1984 Oct 10;259(19):12084–12091. [PubMed] [Google Scholar]
- Comporti M. Lipid peroxidation and cellular damage in toxic liver injury. Lab Invest. 1985 Dec;53(6):599–623. [PubMed] [Google Scholar]
- De Léan A., Racz K., Gutkowska J., Nguyen T. T., Cantin M., Genest J. Specific receptor-mediated inhibition by synthetic atrial natriuretic factor of hormone-stimulated steroidogenesis in cultured bovine adrenal cells. Endocrinology. 1984 Oct;115(4):1636–1638. doi: 10.1210/endo-115-4-1636. [DOI] [PubMed] [Google Scholar]
- De Léan A., Vinay P., Cantin M. Distribution of atrial natriuretic factor receptors in dog kidney fractions. FEBS Lett. 1985 Dec 2;193(2):239–242. doi: 10.1016/0014-5793(85)80160-5. [DOI] [PubMed] [Google Scholar]
- Féthière J., Meloche S., Nguyen T. T., Ong H., De Lean A. Distinct properties of atrial natriuretic factor receptor subpopulations in epithelial and fibroblast cell lines. Mol Pharmacol. 1989 May;35(5):584–592. [PubMed] [Google Scholar]
- Genest J., Cantin M. The atrial natriuretic factor: its physiology and biochemistry. Rev Physiol Biochem Pharmacol. 1988;110:1–145. doi: 10.1007/BFb0027530. [DOI] [PubMed] [Google Scholar]
- Horowitz Z. D., Sahnoun H., Pascual A., Casanova J., Samuels H. H. Analysis of photoaffinity label derivatives to probe thyroid hormone receptor in human fibroblasts, GH1 cells, and soluble receptor preparations. J Biol Chem. 1988 May 15;263(14):6636–6642. [PubMed] [Google Scholar]
- Kikugawa K., Beppu M. Involvement of lipid oxidation products in the formation of fluorescent and cross-linked proteins. Chem Phys Lipids. 1987 Jul-Sep;44(2-4):277–296. doi: 10.1016/0009-3084(87)90054-5. [DOI] [PubMed] [Google Scholar]
- Kooper G. N., Levinson N. R., Copeland C. F., Bowen W. D. Photoaffinity labeling of opiate receptors using intrinsically photoactive 3H-opiates. Mol Pharmacol. 1988 Mar;33(3):316–326. [PubMed] [Google Scholar]
- Koseki C., Hayashi Y., Ohnuma N., Imai M. Difference in molecular size of receptors for alpha-rat atrial natriuretic polypeptide among the kidney, aorta, and adrenal gland as identified by direct UV-photoaffinity labeling. Biochem Biophys Res Commun. 1986 Apr 14;136(1):200–207. doi: 10.1016/0006-291x(86)90895-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liu B., Meloche S., McNicoll N., Lord C., De Léan A. Topographical characterization of the domain structure of the bovine adrenal atrial natriuretic factor R1 receptor. Biochemistry. 1989 Jun 27;28(13):5599–5605. doi: 10.1021/bi00439a039. [DOI] [PubMed] [Google Scholar]
- Meloche S., McNicoll N., Liu B., Ong H., De Léan A. Atrial natriuretic factor R1 receptor from bovine adrenal zona glomerulosa: purification, characterization, and modulation by amiloride. Biochemistry. 1988 Oct 18;27(21):8151–8158. doi: 10.1021/bi00421a025. [DOI] [PubMed] [Google Scholar]
- Meloche S., Ong H., Cantin M., De Léan A. Molecular characterization of the solubilized atrial natriuretic factor receptor from bovine adrenal zona glomerulosa. Mol Pharmacol. 1986 Dec;30(6):537–543. [PubMed] [Google Scholar]
- Meloche S., Ong H., De Léan A. Functional heterogeneity of atrial natriuretic factor receptor in bovine adrenal zona glomerulosa is explained by an amiloride-sensitive high affinity molecular complex. J Biol Chem. 1987 Jul 25;262(21):10252–10258. [PubMed] [Google Scholar]
- Misono K. S., Grammer R. T., Rigby J. W., Inagami T. Photoaffinity labeling of atrial natriuretic factor receptor in bovine and rat adrenal cortical membranes. Biochem Biophys Res Commun. 1985 Aug 15;130(3):994–1001. doi: 10.1016/0006-291x(85)91713-9. [DOI] [PubMed] [Google Scholar]
- Moseley J. M., Smith P., Martin T. J. Identification of the calcitonin receptor by chemical cross-linking and photoaffinity labeling in human cancer cell lines. J Bone Miner Res. 1986 Jun;1(3):293–297. doi: 10.1002/jbmr.5650010308. [DOI] [PubMed] [Google Scholar]
- Napier M. A., Vandlen R. L., Albers-Schönberg G., Nutt R. F., Brady S., Lyle T., Winquist R., Faison E. P., Heinel L. A., Blaine E. H. Specific membrane receptors for atrial natriuretic factor in renal and vascular tissues. Proc Natl Acad Sci U S A. 1984 Oct;81(19):5946–5950. doi: 10.1073/pnas.81.19.5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell M. J., Peters T. J. Ferritin and haemosiderin in free radical generation, lipid peroxidation and protein damage. Chem Phys Lipids. 1987 Nov-Dec;45(2-4):241–249. doi: 10.1016/0009-3084(87)90067-3. [DOI] [PubMed] [Google Scholar]
- Pascual A., Casanova J., Samuels H. H. Photoaffinity labeling of thyroid hormone nuclear receptors in intact cells. J Biol Chem. 1982 Aug 25;257(16):9640–9647. [PubMed] [Google Scholar]
- Schenk D. B., Johnson L. K., Schwartz K., Sista H., Scarborough R. M., Lewicki J. A. Distinct atrial natriuretic factor receptor sites on cultured bovine aortic smooth muscle and endothelial cells. Biochem Biophys Res Commun. 1985 Mar 15;127(2):433–442. doi: 10.1016/s0006-291x(85)80179-0. [DOI] [PubMed] [Google Scholar]
- Schenk D. B., Phelps M. N., Porter J. G., Scarborough R. M., McEnroe G. A., Lewicki J. A. Identification of the receptor for atrial natriuretic factor on cultured vascular cells. J Biol Chem. 1985 Dec 5;260(28):14887–14890. [PubMed] [Google Scholar]
- Sen I. Identification and solubilization of atrial natriuretic factor receptors in human placenta. Biochem Biophys Res Commun. 1986 Mar 13;135(2):480–486. doi: 10.1016/0006-291x(86)90019-7. [DOI] [PubMed] [Google Scholar]
- Sweet F., Murdock G. L. Affinity labeling of hormone-specific proteins. Endocr Rev. 1987 May;8(2):154–184. doi: 10.1210/edrv-8-2-154. [DOI] [PubMed] [Google Scholar]
- Wills E. D., Wilkinson A. E. Release of enzymes from lysosomes by irradiation and the relation of lipid peroxide formation to enzyme release. Biochem J. 1966 Jun;99(3):657–666. doi: 10.1042/bj0990657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip C. C., Laing L. P., Flynn T. G. Photoaffinity labeling of atrial natriuretic factor receptors of rat kidney cortex plasma membranes. J Biol Chem. 1985 Jul 15;260(14):8229–8232. [PubMed] [Google Scholar]
- van der Walt B., Cahnmann H. J. Synthesis of thyroid hormone metabolites by photolysis of thyroxine and thyroxine analogs in the near UV. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1492–1496. doi: 10.1073/pnas.79.5.1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Walt B., Nikodem V. M., Cahnmann H. J. Use of un-derivatized thyroid hormones for photoaffinity labeling of binding proteins. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3508–3512. doi: 10.1073/pnas.79.11.3508. [DOI] [PMC free article] [PubMed] [Google Scholar]