Abstract
Background
Acute heart failure (AHF) is a common emergency department (ED) presentation that may have poor outcomes but often does not require hospital admission. There is little evidence to guide dispositional decisions.
Objectives
The authors sought to create a risk score for predicting short-term serious outcomes (SSO) in patients with AHF.
Methods
We pooled data from 3 prospective cohorts: 2 published studies and 1 new cohort. The 3 cohorts prospectively enrolled patients who required treatment for AHF at 10 tertiary care hospital EDs. The primary outcome was SSO, defined as death <30 days, intubation or noninvasive ventilation (NIV), myocardial infarction, or relapse to ED <14 days. The logistic regression model evaluated 13 predictors, used an AIC-based step-down procedure, and bootstrapped internal validation.
Results
Of the 2,246 patients in the 3 cohorts (N = 559; 1,100; 587), the mean age was 77.4 years, 54.5% were male, 3.1% received intravenous nitroglycerin, 5.2% received ED NIV, and 48.6% were admitted to the hospital. There were 281 (12.5%) SSOs including 70 deaths (3.1%) with many in discharged patients. The final HEARTRISK6 Scale included 6 variables: valvular heart disease, tachycardia, need for NIV, creatinine, troponin, and failed reassessment (walk test). Choosing HEARTRISK6 total-point admission thresholds of ≥1 or ≥2 would yield, respectively, sensitivities of 88.3% (95% CI: 83.9%-91.8%) and 71.5% (95% CI: 65.9%-76.7%) and specificities of 24.7% (95% CI: 22.8%-26.7%) and 50.1% (95% CI: 47.9%-52.4%) for SSO.
Conclusions
Using 3 large prospectively collected datasets, we created a concise and sensitive risk scale for patients with AHF in the ED. Implementation of the HEARTRISK6 scale could lead to safer and more efficient disposition decisions.
Key words: acute heart failure, emergency department, patient safety
Central Illustration
Each year, more than 1 million patients with sudden dyspnea due to acute heart failure (AHF) present to U.S. and Canadian hospitals for treatment. AHF is a common and serious condition that frequently results in morbidity and death and is a leading cause of hospital admissions for seniors.1, 2, 3, 4 Physicians treating AHF patients in the emergency department (ED) must make the difficult decision of whether to admit or discharge them. Not all patients will benefit from hospitalization, as many will respond to therapy in the ED. Conversely, many AHF patients may go on to have adverse outcomes due to disease progression, ie, they die, require intensive care therapy, or suffer myocardial infarction (MI) while in the hospital. Further, some patients discharged from the ED after treatment die or relapse back to the ED and require admission. Efficient admission decisions are important because there is frequently a shortage of available beds in hospitals, and many EDs are severely overcrowded. We previously documented that <50% of AHF patients seen in Canadian EDs were admitted to hospitals and that 1 in 10 of those not admitted suffered short-term serious outcomes (SSO).5,6
While there are many excellent guidelines on the investigation and treatment of heart failure, there is very limited literature on how to determine if these patients should be admitted or discharged from the ED.3,7,8 Many risk scoring systems have been proposed for AHF patients, but few have been considered to have a low risk of bias or to be practical for ED use.9 A concise clinical tool that estimates the risk of poor outcomes could help clinicians with disposition decisions for patients with AHF.
We previously published 2 studies that sought to create a risk scoring tool to assist with disposition decisions for patients with AHF.5,6 These prospective cohort studies identified simple bedside criteria that could be used to estimate the subsequent risk of SSO. These derived models had 10 variables, which may be too many for optimal use in a busy ED. Consequently, our goal for the current study was to use a much larger patient sample to create a more concise and practical risk scale for AHF patients.
Methods
Design and setting
We conducted 3 prospective cohort studies of patients with AHF and have pooled the data for the current analysis. The first cohort study (RAD1: 2007-2010) enrolled 559 patients.5 The second cohort study (RAD2: 2011-2015) enrolled 1,100 patients.6 The third cohort (2015-2019) has not been published and enrolled 587 patients. All studies were conducted at 10 academic hospital EDs in Canada.
Study population
We included consecutive visits of patients ≥50 years of age who presented to the ED with shortness of breath (<7 days duration) due to AHF. Eligibility of patients was reviewed by the study steering committee. For all studies, we included both patients subsequently admitted to the hospital and those discharged from the ED, as inclusion of both groups of patients allows us to better model the risk of SSO for all patients. As there is no gold standard for the diagnosis of heart failure, we used the criteria recommended by the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology.2 To be eligible, patients had to have appropriate symptoms (shortness of breath or fatigue) with clinical signs of fluid retention (pulmonary or peripheral) in the presence of an underlying abnormality of cardiac structure or function. In instances where doubt remained, a beneficial response to treatment (eg, a brisk diuresis accompanied by improvement in breathlessness) was considered. We did not use NT-proBNP measurements as most Canadian EDs did not have access to this test, and same-day echocardiography is rarely available.
We excluded patients who did not fit the definition of AHF or who were clearly too ill to be considered for discharge after 2 to 12 hours of ED management: 1) resting oxygen saturation <85% on room air or after being on home oxygen level for 20 minutes; 2) heart rate ≥120 beats/min; 3) systolic blood pressure <85 mm Hg; 4) confusion, disorientation, dementia; 5) primary presentation is for ischemic chest pain requiring treatment or with acute ischemic ST-T changes on initial electrocardiogram (ECG); 6) ST-segment elevation MI on initial ECG; 7) terminal status: death expected within weeks from chronic illness; 8) from nursing home or chronic care facility (not the senior’s residence); 9) enrolled in the previous 2 months; or 10) on chronic hemodialysis. No patients who were discharged home were excluded.
Data collection
Patients were assessed for standardized clinical variables by ED staff physicians (all certified in emergency medicine) or by supervised residents in emergency medicine training programs, who were trained by means of a 1-hour practical session. The variables were assessed and interpreted at a target of 2 to 8 hours after ED treatment (to a maximum of 12 hours) and recorded on a physician data form. Research assistants collected other clinical and laboratory results from the electronic patient records including standardized variables from the history, clinical examination, routine laboratory values, cardiac troponins (initial and repeat <6 hours within the ED), chest x-ray, and initial and repeat ECG. NT-proBNP values were not used in our modeling as they were only available for a minority of patients. Patients were reassessed after treatment and were considered “successful” if they could start and complete a walk test, during which they were asked to walk at their own pace in the ED for a period of 3 minutes, regardless of the distance covered.5 Patients were considered “unsuccessful” if they were too ill to start or complete the walk test due to abnormal vital signs (SaO2 < 90% on room air or usual O2, or HR >110 beats/min, or respiratory rate > 28).
Outcome measures
The primary outcome was the composite short-term serious outcome defined as:
a) Death from any cause within 30 days of the index ED visit, or
b) Any of the following within 14 days of the ED visit, regardless of whether initially admitted: 1) Endotracheal intubation or need for noninvasive ventilation (NIV) after hospital admission (not in the ED), unless on NIV at home; 2) MI diagnosed after admission (not in the ED), as defined by the Joint ESC/ACCF/AHA/WHF Task Force for the Third Universal Definition of Myocardial Infarction10 (the fourth was not published at the time of patient enrollment);11 3) major procedure defined as unplanned coronary artery bypass graft, percutaneous coronary intervention, cardiac valvular surgery, or new hemodialysis; 4) relapse and hospital admission for patients who were discharged on the initial ED visit, defined as a return to the ED for any related medical problem within 14 days resulting in admission to the hospital. We believe that this composite outcome is more meaningful to patients and ED physicians as it represents a pragmatic combination of death and other undesirable outcomes, which we hope can be prevented by admission to the hospital.
The presence of a SSO was verified by a subcommittee of senior investigators blinded to the predictors. This outcome was well defined and easy to verify from the source documents: 1) ED health records; 2) hospital health records; 3) computerized hospital patient tracking and record system; and 4) review of provincial death records. Any remaining patients were followed by telephone after 30 days.
Statistical analysis
We adhered to the principles of the TRIPOD Statement for reporting of multivariable prediction models.12 We conducted logistic regression modeling to predict SSO using the 13 variables that were prespecified as predictors for the rule (Supplemental Material). These variables were chosen a priori before model building, based upon those variables evaluated in the 2 prior studies and the clinical experience of the expert investigators.
Multicollinearity was examined using a variable clustering algorithm and variance inflation factors. Heart rate and creatinine were log-transformed due to their skewed distributions. Prior to logistic regression analysis, we generated 10 multiple imputation-completed datasets, employing the method of predictive mean matching and using the bootstrap to approximate the process of drawing predicted values from a full Bayesian predictive distribution.13 The imputation model consisted of all candidate predictor variables, the outcome, and auxiliary variables (see Supplemental Material). The fully prespecified model with 13 predictors (where age, CO2, and SaO2 are modeled using restricted cubic spline functions with 3 knots, and log heart rate, and log creatinine with 5 knots) was fitted separately to each of the 10 multiple imputation-completed datasets, and the results were combined across the datasets using Rubin’s rules. Further, we used an Akaike information criterion-based stepdown procedure to reduce the number of variables in the model.14,15 We used bootstrap internal validation, generating 1,000 bootstrap samples and calculating optimism-corrected performance. Using regression coefficients, we then created a scoring grid, assigning points for different levels of the predictor variables and evaluating the observed and estimated risks for each score total. Next, calibration plots were created for the final model. We then calculated the impact on sensitivity and specificity for SSO, as well as on potential hospital admissions. Finally, we compared various cut-points of the total score to current clinical practice.
Research ethics
The research ethics boards of 2 hospitals determined that written informed consent was required, whereas those at the other sites waived the need for written consent for this observational study. Patients were not involved in the design, conduct, or interpretation of this study.
Results
In total, 2,246 patients from the 3 cohorts (n = 559, n = 1,100, and n = 587) (Supplement Figure 1) at 10 hospital sites were included. The patients had a mean age of 77.4 years, 54.5% were male, 41.1% arrived by ambulance, 37.3% were found to be in permanent atrial fibrillation or flutter, and the mean ejection fraction was 45.1% (Table 1). In the ED, most (89.4%) received IV diuretics, 5.2% received NIV, and 3.1% were given IV nitroglycerin. Almost one-half (48.6%) had initial ED troponin levels greater than the upper reference level. Another 2,132 eligible patients who were not enrolled were very similar except for a higher proportion admitted (Supplemental Table 1).
Table 1.
Characteristics of Acute Heart Failure Patient Visits (N = 2,246)
| Study (%) | |
| RAD1 | 559 (24.9) |
| RAD2 | 1,100 (49.0) |
| Cohort 3 | 587 (26.1) |
| Hospital site (%) | |
| Ottawa Hospital Civic Campus - Ottawa | 863 (38.4) |
| Ottawa Hospital General Campus - Ottawa | 316 (14.1) |
| Mount Sinai Hospital - Toronto | 304 (13.5) |
| Kingston General Hospital - Kingston | 279 (12.4) |
| University of Alberta - Edmonton | 229 (10.2) |
| Foothills Medical Centre - Calgary | 145 (6.5) |
| London Health Sciences Centre - London | 73 (3.3) |
| Jewish General Hospital - Montreal | 24 (1.1) |
| Hotel Dieu Hospital - Kingston | 8 (0.4) |
| North East Community Health Centre - Edmonton | 5 (0.2) |
| Demographics | |
| Age, y | 77.4 ± 10.6 |
| Range | 49-104 |
| Male (%) | 1,224 (54.5) |
| Arrival status | |
| Arrival by ambulance (%) | 924 (41.1) |
| Temperature, °C (N = 2,123) | 36.2 ± 0.7 |
| Heart rate, beats/min | 84.2 ± 20.1 |
| Respiratory rate | 22 ± 5.7 |
| Systolic blood pressure, mm Hg | 141 ± 27.3 |
| SaO2 by oximetry | 94.6 ± 4.8 |
| Duration of dyspnea, h | 62.2 ± 57.1 |
| Canadian Triage Acuity Scale (CTAS)a | 2 (2-3) |
| Past medical history (%) | |
| Heart failure | 1,638 (72.9) |
| Hypertension | 1,624 (72.3) |
| Diabetes | 903 (40.2) |
| Permanent atrial fibrillation | 850 (37.8) |
| Myocardial infarction | 736 (32.8) |
| Coronary artery bypass graft | 499 (22.2) |
| Chronic obstructive pulmonary disease | 487 (21.7) |
| Chronic renal failure | 479 (21.3) |
| Valvular heart disease | 420 (18.7) |
| Pacemaker | 358 (15.9) |
| Stroke or transient ischemic attack | 339 (15.1) |
| Angina | 219 (9.8) |
| Percutaneous coronary intervention | 144 (6.4) |
| Active cancer | 118 (5.3) |
| Peripheral vascular disease (intervention) | 103 (4.6) |
| Dementia | 78 (3.5) |
| Intubation for respiratory distress | 22 (1.0) |
| Smoker: current or former (%) | 789 (35.1) |
| Home oxygen (%) | 130 (5.8) |
| Ejection fraction from medical records (N = 1,617) | 45.1 ± 16.1 |
| Current cardiac medications (%) | |
| ACE inhibitors | 965 (43.0) |
| Antiarrhythmics | 164 (7.3) |
| Amiodarone | 147 (6.5) |
| Sotalol | 11 (0.5) |
| Propafenone | 3 (0.1) |
| Anticoagulants | 927 (41.3) |
| Warfarin | 672 (29.9) |
| New oral anticoagulant (dabigatran, rivaroxaban, apixaban) | 255 (11.4) |
| Antiplatelet medications | 1,019 (45.4) |
| Beta blockers | 1,517 (67.5) |
| Calcium channel blockers | 754 (33.6) |
| Digoxin | 267 (11.9) |
| Diuretics | 1,667 (74.2) |
| Nitrates | 640 (28.5) |
| Statins | 1,326 (59.0) |
| Vasodilators | 97 (4.3) |
| Emergency department treatment (%) | |
| Intravenous diuretic | 2,009 (89.4) |
| Sublingual nitroglycerin | 167 (7.4) |
| Intravenous nitroglycerin | 69 (3.1) |
| Noninvasive positive pressure ventilation | 116 (5.2) |
| Laboratory values | |
| Hemoglobin (g/L) | 119.2 ± 20.4 |
| White blood cell (109/L) | 8.9 ± 5.1 |
| Urea (mmol/L) (N = 2,109) | 10.5 ± 6.2 |
| Creatinine (μmol/L) | 120.9 ± 67.5 |
| Serum CO2 (mmol/L) | 25.7 ± 3.8 |
| Potassium (mmol/L) | 4.3 ± 2.9 |
| Glucose (mmol/L) | 7.7 ± 3.4 |
| pCO2 (mm Hg) (N = 981) | 46.5 ± 10.8 |
| pH (N = 980) | 7.4 ± 0.1 |
| Troponin on arrival > upper reference level (%)b | 1,091 ± 48.6 |
| Highest troponin >3× upper reference level (%)b | 386 ± 17.2 |
| Electrocardiogram findings (%) | |
| Atrial fibrillation or flutter | 837 (37.3) |
| A-V conduction disturbance | 734 (32.7) |
| Chest x-ray findings (%) | |
| Cardiomegaly | 1,162 (51.7) |
| Pulmonary congestion | 1,466 (65.3) |
| Pleural effusion | 1,248 (55.6) |
| Pneumonia | 130 (5.8) |
Values are n (%), mean ± SD, or median (IQR).
Canadian Triage Acuity Scale (CTAS) ranges from 1 (most urgent) to 5 (least urgent).
Unable to pool troponin values because of different assays used.
On the index ED visit, 48.6% were admitted to the hospital, and the remaining 51.4% were discharged home (Table 2). Among those admitted, 14.4% suffered a short-term serious outcome after admission (not in the ED) including death (3.6%), NIV (3.9%), intubation (1.4%), MI (3.0%), and/or major procedure (5.4%). Among those initially discharged, 10.7% experienced SSO including death (1.5%) and return visit with admission (10.4%).
Table 2.
Outcomes of Acute Heart Failure Patient Visits (N = 2,246)
| Admitted to hospital on index emergency department visit (%) | 1,091 (48.6) |
| Noninvasive ventilation required after admission (N = 1,091) | 43 (3.9) |
| Intubation required after admission (N = 1,091) | 15 (1.4) |
| Myocardial infarction after admission (N = 1,091) | 33 (3.0) |
| Major procedure (N = 1,091) | 59 (5.4) |
| Coronary artery bypass graft | 13 (1.2) |
| Percutaneous coronary intervention | 14 (1.3) |
| Valvular cardiac surgery | 17 (1.6) |
| New hemodialysis | 14 (1.3) |
| Death after admission (N = 1,091) | 39 (3.6) |
| Death after discharge within 30 days (N = 1,091) | 14 (1.3) |
| Discharged from the emergency department | 1,155 (51.4) |
| Relapse back to emergency department within 14 days (%) (N = 1,155) | 246 (21.3) |
| Dyspnea | 189 (16.4) |
| Chest pain | 45 (3.9) |
| Inability to ambulate | 12 (1.0) |
| Fever | 8 (0.7) |
| Sepsis | 2 (0.2) |
| Other | 80 (6.9) |
| Relapse and admitted to hospital within 14 days (%) (N = 1,155) | 120 (10.4) |
| Admitted to intensive care unit (N = 120) | 11 (9.2) |
| Death within 30 days (%) (N = 1,155) | 17 (1.5) |
| Short-term serious outcomes (%) | 281 (12.5) |
| Admitted patients (N = 1,091) | 157 (14.4) |
| Discharged patients (N = 1,155) | 124 (10.7) |
| Total deaths, inside and out of hospital (%) | 70 (3.1) |
Values are n (%).
A comparison of patients with and without SSO is shown in the Supplemental Table 2. The 281 (12.5%) patients with SSO were more likely to have arrived by ambulance, have a heart rate ≥110 on ED arrival, have a history of valvular heart disease or chronic kidney disease, have elevated creatinine and troponin levels, have received NIV or IV nitroglycerin in the ED, and have failed reassessment (walk test).
Multiple imputations were required for 3 predictor variables with missing values (SaO2 1%, CO2 1%, and troponin 4%; Supplemental Table 3). No problems with multicollinearity were detected among the 13 predictor variables (Supplemental Table 4). The full prediction model of the 13 variables before the stepdown procedure is shown in the Supplemental Tables 5 and 6 (C-statistic 0.69 [95% CI: 0.66-0.72]). Across the 1,000 bootstrap samples, a 6-variable model was identified 26% of the time (Supplemental Table 7). Table 3 shows the 6 independent predictors of SSO in the final clinical model (Supplemental Figure 3).
Table 3.
Independent Predictors of Short-Term Serious Outcomes as Determined by Multiple Logistic Regression Analysis for 2,246 Acute Heart Failure Patient Visits
| Beta (95% CI) | OR (95% CI) | Points | |
|---|---|---|---|
| History of valvular heart disease | 0.64 (0.34-0.94) | 1.90 (1.40-2.56) | 1 |
| Heart rate (log-transformed) | 0.34 (0.13-0.56) | 1.41 (1.14-1.75) | 2-3 |
| Treated with noninvasive ventilation | 0.95 (0.50-1.40) | 2.58 (1.65-4.04) | 2 |
| Creatinine (log-transformed) | 0.34 (−0.01 to 0.69) | 1.40 (0.99-1.99) | 2-3 |
| Troponin ≥1 × URL | 0.04 (−0.36 to 0.44) | 1.04 (0.70-1.56) | 0 |
| Troponin ≥2 × URL | 0.01 (−0.36 to 0.38) | 1.01 (0.70-1.46) | 0 |
| Troponin ≥3 × URL | 0.40 (−0.18 to 0.98) | 1.49 (0.84-2.66) | 1 |
| Troponin ≥4 × URL | 0.21 (−0.50 to 0.93) | 1.24 (0.60-2.54) | 1 |
| Troponin ≥5 × URL | 0.94 (0.55-1.32) | 2.55 (1.74-3.75) | 2 |
| Reassessment failed | 0.29 (0.02-0.56) | 1.34 (1.02-1.75) | 1 |
Area under receiver operating characteristic curve: 0.68 (95% CI: 0.65-0.71).
URL = upper reference level.
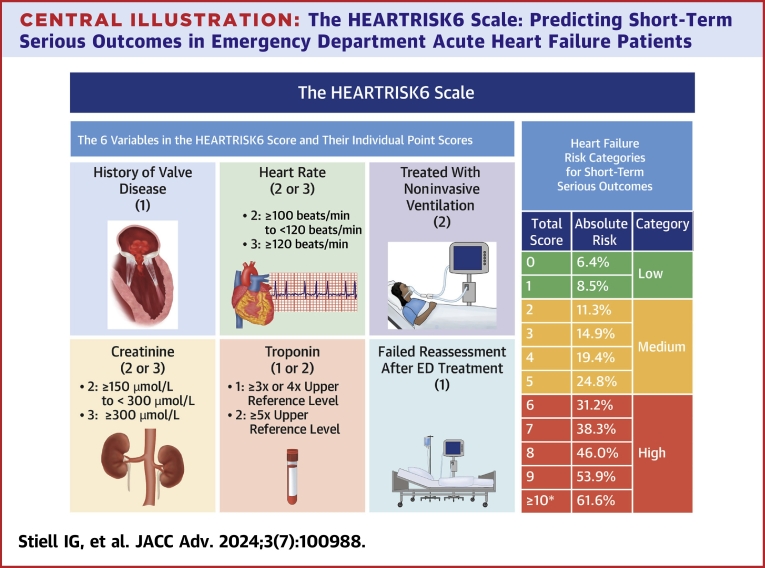
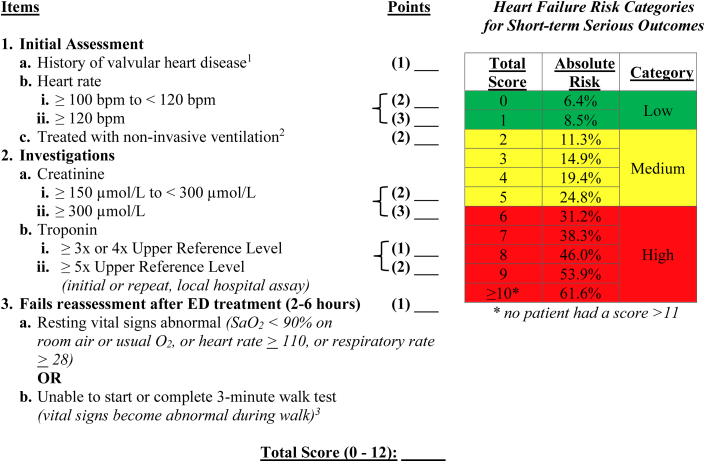
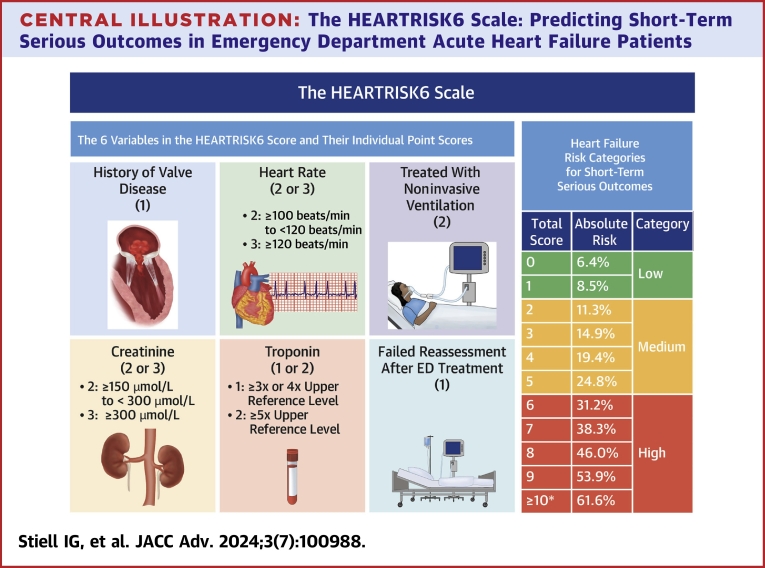
The “HEARTRISK6” scoring scale, ranging from 0 to 12 total points (Figure 1), included a history of moderate-severe valvular heart disease, elevated heart rate on arrival, need for NIV in the first hour, increased creatinine and troponin levels in the ED, and failure of reassessment after ED treatment. “Moderate-severe valvular disease” was based on a prior diagnosis from cardiology notes, echocardiograph reports, or discharge records.
Figure 1.
The HEARTRISK6 Acute Heart Failure Risk Scale
1History of valvular heart disease: moderate or severe valvular heart disease from prior cardiology or imaging notes. 2Treated with noninvasive ventilation: BiPAP within 1 hour of initial assessment. 3Unable to start or complete a 3-minute walk test (vital signs become abnormal during the walk test): score if patient’s O2 drops below 90%, heart rate ≥110 beats/min, respiratory rate ≥28 during walk test, or if patient is unable to complete due to fatigue or dyspnea. ED = emergency department.
Table 4 shows the observed incidence of SSO at each cut-point level, as well as sensitivity, specificity, and projected admission levels, compared to the actual practice of the treating physicians (Supplemental Table 8). For example, if admission to the hospital was suggested for patients with a total score of ≥1, then the sensitivity for SSO would be 88.3%, the specificity 24.7%, and the proportion admitted 76.9%. Alternately, a threshold score of ≥2 would yield sensitivity 71.5%, specificity 50.1%, and proportion admitted 52.6%. These compare to existing Canadian clinical practice, which had a much lower sensitivity of 55.9%, a similar specificity of 52.5%, and lower admissions of 48.6%. We also demonstrated good calibration for the clinical model with the slope of the observed vs expected graph very close to 1.0 (Supplemental Figure 2).
Table 4.
Estimated Probability of Having Short-Term Serious Outcomes for 2,246 Acute Heart Failure Patients Visits
| Current Practice | Number of Visits |
Score-Specific Incidence of SSO (%) |
Threshold Sensitivity % |
Threshold Specificity % |
Threshold Admission |
|---|---|---|---|---|---|
| (N = 2,246) | (n = 281, 12.5%) | 55.9% (95% CI: 49.9%-61.8%) | 52.5% (95% CI: 50.2%-54.7%) | (N = 48.6%) | |
| Score | 2,246 | ||||
| 0 | 518 | 33 (6.4) | 100 (98.7-100) | - | 100 |
| 1 | 547 | 47 (8.5) | 88.3 (83.9-91.8) | 24.7 (22.8-26.7) | 76.9 |
| 2 | 379 | 43 (11.3) | 71.5 (65.9-76.7) | 50.1 (47.9-52.4) | 52.6 |
| 3 | 396 | 59 (14.9) | 55.2 (49.1-61.1) | 67.1 (65.0-69.2) | 35.7 |
| 4 | 206 | 40 (19.4) | 37.0 (31.4-43.0) | 84.6 (84.6-86.2) | 18.1 |
| 5 | 116 | 29 (24.8) | 24.2 (19.3-29.6) | 93.3 (92.1-94.4) | 8.9 |
| 6 | 45 | 14 (31.2) | 10.0 (6.7-14.1) | 97.2 (96.3-97.8) | 3.7 |
| 7 | 25 | 10 (38.3) | 5.0 (2.8-8.2) | 98.7 (98.1-99.2) | 1.7 |
| 8 | 7 | 3 (46.0) | 1.4 (0.4-3.6) | 99.5 (99.1-99.8) | 0.6 |
| 9 | 5 | 3 (53.9) | 1.1 (0.2-3.1) | 99.8 (99.5-99.9) | 0.3 |
| ≥10 | 2 | 1 (61.6) | - | 100 (99.8-100) | 0.1 |
Values are n, n (%), or % (95% CI).
SSO = short-term serious outcomes.
Discussion
We included 2,246 patients seen in multiple EDs with a wide range of severity of acute heart failure. With a large number of serious short-term outcome cases, we were able to derive a robust predictive model that demonstrated good calibration and potentially better performance than current practice. Over 10% of those initially discharged from the ED went on to have serious short-term outcomes. The derived HEARTRISK6 Scale, comprised of 6 routine ED criteria, can be applied very quickly, estimates the risk of a poor outcome, and gives attending physicians important medical information upon which to make disposition decisions. In settings where admission for AHF is relatively high, using a cut-point total score of 1 or more as an indication for admission would yield high sensitivity while allowing one-quarter of patients to be discharged home. In hospitals with typically lower admission rates, using a cut-point of 2 or more would significantly improve sensitivity for SSO with only a slight increase in hospital admissions. To our knowledge, no other risk tools are routinely in use for ED patients with AHF.
Previous studies
A systematic review by Michaud in 2018 highlighted 9 scales with the purpose of assigning risk for ED patients with AHF.5,6,9,16, 17, 18, 19, 20, 21, 22 The authors conclude that the scales created by Lee and our group had the most robust body of evidence but had important differences between them. The EHMRG scale published by Lee has very recently undergone an implementation trial (COACH), which combined the prediction of risk with rapid cardiology follow-up for intermediate-risk patients.19,23,24 Concerns with the EHMRG scale include its complexity with 11 variables and the fact that it only predicts death and 30-day return visits without considering serious outcomes that occur within hospitals. The HEARTRISK6 Scale is the successor to our prior models, but it performs better and has fewer variables making it more clinically useful.5,6 Sister studies to create the Ottawa Chronic Obstructive Pulmonary Disease Scale were larger and more successful.25, 26, 27 Miro, Gil, and Spanish colleagues published an additional paper not included in the Michaud review that analyzed registry data and, interestingly, did not present patient characteristics.28,29 This scale only predicted mortality and was comprised of 13 variables including NT-proBNP levels.
Society guidelines offer excellent advice on management for patients with AHF but little guidance on short-term risk stratification and disposition from the ED. The European Society of Cardiology gives extensive advice on AHF treatment but does not discuss disposition from the ED.7 The 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure implies that all patients with AHF will be admitted and does not discuss alternative dispositions.8 The Canadian Cardiovascular Society guidelines provide detailed instruction on the diagnosis and management of AHF but do not address ED disposition decisions.3
Study Limitations and strengths
Our study has some limitations. While we have demonstrated the potential for improved patient outcomes with the use of the HEARTRISK6 Scale, this has yet to be shown in an implementation trial. We used robust internal validation rather than split-sample validation, which is an inefficient and outdated approach,30 but future external validation by an independent group is recommended. We note that our 2 most recent cohorts prospectively evaluated all 6 variables in the final HEARTRISK6 Scale, ie, we have introduced no new criteria. Finally, we acknowledge that when the study was first designed, we did not solicit patient or caregiver input.
We have discussed other issues above that some may consider to be limitations: why we chose a composite outcome, that troponin drawn on ED arrival is not both a predictor and an outcome, why we studied both admitted and discharged patients, and how we compare the scale to current clinical practice, which has much lower sensitivity.
Strengths of our study include data that were wholly collected prospectively and a very large cohort. We focused on AHF patients undergoing treatment in the ED, and this is the only study to evaluate response to treatment. Further, our primary outcome includes not just mortality but morbidity at 14 days, which is known to predict poor prognosis. Finally, all 6 components are easy to collect for bedside clinicians and do not require testing such as echocardiography or NT-proBNP levels, which may not be readily available.
Clinical implications
Physicians dealing with AHF patients in the ED often must make difficult disposition decisions. The severity of the acute episode may mandate admission to the hospital. Many patients, however, improve rapidly with diuresis and may be well enough to go home. The HEARTRISK6 Scale provides additional information to the physician in the ED trying to decide whether to admit or discharge (Central Illustration). With rapid assessment of the 6 component variables, the attending physician can estimate the risk that their patient will suffer a short-term serious outcome that might be prevented by hospital admission. We recognize that there are other factors for clinicians to consider such as the degree of home support the patient may have and the potential of optimizing medical therapy such as increasing the dosage of diuretics. Moreover, patients may not have early access to either a family doctor or a specialist for prompt reassessments to consider further investigations and treatments.31
Central Illustration.
The HEARTRISK6 Scale: Predicting Short-Term Serious Outcomes in Emergency Department Acute Heart Failure Patients
Components of the HEARTRISK6 scale. ∗No patient had a score >11. ED = emergency department.
We maintain that the components of the HEARTRISK6 Scale have been sufficiently tested in our 3 cohorts that physicians managing AHF patients in the ED can safely use the scale now to assist with their decisions. The final decision to admit or discharge, of course, relies on the best judgment of the most responsible physician in consultation with the patient and family. The scale can assist cardiologists, internists, hospitalists, and emergency physicians.
Research implications
Important next steps include an implementation trial to evaluate the actual impact of using the HEARTRISK6 Scale on patient outcomes. This could be accompanied by the development of best practice guidelines for the management of AHF patients in the ED.
Conclusions
Using 3 large, prospectively collected datasets, we created a more concise and sensitive risk scale to assist with complex admission decisions for patients with acute heart failure in the ED. Implementation of the HEARTRISK6 Scale could lead to safer and more efficient disposition decisions, with more high-risk patients being appropriately admitted and more low-risk patients being safely discharged.
PERSPECTIVES.
COMPETENCY IN PATIENT CARE: The HEARTRISK6 Scale identified determinants of SSO in patients with AHF in the ED. It could be used for disposition decisions, with more high-risk patients being appropriately admitted and more low-risk patients being safely discharged.
TRANSLATIONAL OUTLOOK: There are opportunities to evaluate the potential impact and physician acceptability of incorporating the HEARTRISK6 Scale into clinical care. Implementation studies could evaluate the impact on patient outcomes as well as barriers to use in practice.
Funding support and author disclosures
This study was funded by a Canadian Institutes of Health Research (CIHR) Foundation grant held by IGS. The funding agency had no role in the study design, collection, analysis, or manuscript preparation. The study was sponsored by the Ottawa Hospital Research Institute. Dr Perry is supported by a mid-career salary award from the Heart and Stroke Foundation of Ontario. Dr Rowe’s research is supported by a Scientific Director’s Grant (SOP 168483) from the CIHR (Ottawa, Ontario). All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors gratefully acknowledge Angela Marcantonio from the Ottawa Hospital Research Institute for her contributions to this project and Jennifer Brinkhurst for help with original data collection. They are very grateful to the emergency department physicians, nurses, and respiratory therapists for their invaluable and tireless assistance.
Footnotes
Author Contributions: I.S. and J.P. conceived the idea and prepared the manuscript. I.S. secured research funding. C.C. managed the budget, contracts, and personnel. E.B. coordinated the study and supervised data collection. A.M., B.R., J.D., J.Y., and B.B. were responsible for the acquisition of data at sites. M.T. and M.-J.N. conducted the statistical analyses. All authors supervised the conduct of the study and data collection, drafted the manuscript and/or contributed to its revision, and all approved the final version.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental tables and figures, please see the online version of this paper.
Supplementary data
References
- 1.Yancy C.W., Jessup M., Bozkurt B., et al. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2014;62(16):1495–1539. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 2.Ponikowski P., Voors A.A., Anker S.D., et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 3.Ezekowitz J.A., O'Meara E., McDonald M.A., et al. 2017 comprehensive update of the Canadian Cardiovascular Society guidelines for the Management of Heart Failure. Can J Cardiol. 2017;33(11):1342–1433. doi: 10.1016/j.cjca.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 4.McDonald M.A., Ashley E.A., Fedak P.W.M., et al. Mind the gap: current challenges and future state of heart failure care. Can J Cardiol. 2017;33(11):1434–1449. doi: 10.1016/j.cjca.2017.08.023. [DOI] [PubMed] [Google Scholar]
- 5.Stiell I.G., Clement C.M., Brison R.J., et al. A risk scoring system to identify emergency department patients with heart failure at high risk for serious adverse events. Acad Emerg Med. 2013;20(1):17–26. doi: 10.1111/acem.12056. [DOI] [PubMed] [Google Scholar]
- 6.Stiell I.G., Perry J.J., Clement C.M., et al. Prospective and explicit clinical validation of the Ottawa heart failure risk scale, with and without use of quantitative NT-proBNP. Acad Emerg Med. 2017;24(3):316–327. doi: 10.1111/acem.13141. [DOI] [PubMed] [Google Scholar]
- 7.McDonagh T.A., Metra M., Adamo M., et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599–3726. doi: 10.1093/eurheartj/ehab368. [DOI] [PubMed] [Google Scholar]
- 8.Heidenreich P.A., Bozkurt B., Aguilar D., et al. 2022 AHA/ACC/HFSA guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022;79(17):e263–e421. doi: 10.1016/j.jacc.2021.12.012. [DOI] [PubMed] [Google Scholar]
- 9.Michaud A.M., Parker S.I.A., Ganshorn H., et al. Prediction of early adverse events in emergency department patients with acute heart failure: a systematic review. Can J Cardiol. 2018;34(2):168–179. doi: 10.1016/j.cjca.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 10.Thygesen K., Alpert J.S., Jaffe A.S., et al. Third universal definition of myocardial infarction. Circulation. 2012;126(16):2020–2035. doi: 10.1161/CIR.0b013e31826e1058. [DOI] [PubMed] [Google Scholar]
- 11.Thygesen K., Alpert J.S., Jaffe A.S., et al. Fourth universal definition of myocardial infarction (2018) Circulation. 2018;138(20):e618–e651. doi: 10.1161/cir.0000000000000617. [DOI] [PubMed] [Google Scholar]
- 12.Collins G.S., Reitsma J.B., Altman D.G., et al. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BMJ. 2015;350 doi: 10.1136/bmj.g7594. [DOI] [PubMed] [Google Scholar]
- 13.Harrell Jr F (2023). Hmisc: Harrell Miscellaneous. R package version 5.1-2. Accessed May 4, 2024. https://hbiostat.org/R/Hmisc/
- 14.Harrell F.E., Jr. 2nd ed. Springer International Publishing; 2015. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis; p. 582. [Google Scholar]
- 15.Ambler G., Brady A.R., Royston P. Simplifying a prognostic model: a simulation study based on clinical data. Stat Med. 2002;21(24):3803–3822. doi: 10.1002/sim.1422. [DOI] [PubMed] [Google Scholar]
- 16.Collins S.P., Jenkins C.A., Harrell F.E., Jr., et al. Identification of emergency department patients with acute heart failure at low risk for 30-day adverse events: the STRATIFY decision tool. JACC Heart Fail. 2015;3(10):737–747. doi: 10.1016/j.jchf.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia-Gutierrez S., Quintana J.M., Antón-Ladislao A., et al. Creation and validation of the acute heart failure risk score: ahfrs. Intern Emerg Med. 2017;12(8):1197–1206. doi: 10.1007/s11739-016-1541-4. [DOI] [PubMed] [Google Scholar]
- 18.Hsiao J., Motta M., Wyer P. Validating the acute heart failure index for patients presenting to the emergency department with decompensated heart failure. Emerg Med J. 2012;29(12):e5. doi: 10.1136/emermed-2011-200610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee D.S., Stitt A., Austin P.C., et al. Prediction of heart failure mortality in emergent care: a cohort study. Ann Intern Med. 2012;156(11):767–775. doi: 10.7326/0003-4819-156-11-201206050-00003. [DOI] [PubMed] [Google Scholar]
- 20.Greig D., Austin P.C., Zhou L., et al. Ischemic electrocardiographic abnormalities and prognosis in decompensated heart failure. Circ Heart Fail. 2014;7(6):986–993. doi: 10.1161/circheartfailure.114.001460. [DOI] [PubMed] [Google Scholar]
- 21.Gil V., Miró Ò., Schull M.J., et al. Emergency Heart Failure Mortality Risk Grade score performance for 7-day mortality prediction in patients with heart failure attended at the emergency department: validation in a Spanish Cohort. Eur J Emerg Med. 2018;25(3):169–177. doi: 10.1097/mej.0000000000000422. [DOI] [PubMed] [Google Scholar]
- 22.Martín-Sánchez F.J., Gil V., Llorens P., et al. Barthel index-enhanced feedback for effective cardiac treatment (BI-effect) study: contribution of the barthel index to the heart failure risk scoring system model in elderly adults with acute heart failure in the emergency department. J Am Geriatr Soc. 2012;60(3):493–498. doi: 10.1111/j.1532-5415.2011.03845.x. [DOI] [PubMed] [Google Scholar]
- 23.Lee D.S., Lee J.S., Schull M.J., et al. Prospective validation of the emergency heart failure mortality risk grade for acute heart failure. Circulation. 2019;139(9):1146–1156. doi: 10.1161/circulationaha.118.035509. [DOI] [PubMed] [Google Scholar]
- 24.Lee D.S., Straus S.E., Farkouh M.E., et al. Trial of an intervention to improve acute heart failure outcomes. N Engl J Med. 2023;388(13):e46. doi: 10.1056/NEJMoa2211680. [DOI] [PubMed] [Google Scholar]
- 25.Stiell I.G., Clement C.M., Aaron S.D., et al. Clinical characteristics associated with adverse events in patients with exacerbation of chronic obstructive pulmonary disease: a prospective cohort study. CMAJ. 2014;186(6):E193–E204. doi: 10.1503/cmaj.130968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stiell I.G., Perry J.J., Clement C.M., et al. Clinical validation of a risk scale for serious outcomes among patients with chronic obstructive pulmonary disease managed in the emergency department. CMAJ. 2018;190(48):E1406–E1413. doi: 10.1503/cmaj.180232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flegel K., Stanbrook M.B. To keep patients with COPD out of hospital, look beyond the lungs. CMAJ. 2018;190(48):E1402–E1403. doi: 10.1503/cmaj.181462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miró Ò., Rossello X., Gil V., et al. Predicting 30-day mortality for patients with acute heart failure in the emergency department: a cohort study. Ann Intern Med. 2017;167(10):698–705. doi: 10.7326/m16-2726. [DOI] [PubMed] [Google Scholar]
- 29.Miró Ò., Rossello X., Gil V., et al. Analysis of how emergency physicians' decisions to hospitalize or discharge patients with acute heart failure match the clinical risk categories of the MEESSI-AHF scale. Ann Emerg Med. 2019;74(2):204–215. doi: 10.1016/j.annemergmed.2019.03.010. [DOI] [PubMed] [Google Scholar]
- 30.Steyerberg E.W. 2nd ed. Springer Nature; 2019. Clinical Prediction Models: A Practical Approach to Development, Validation, and Updating; p. 558. [Google Scholar]
- 31.Stiell I.G., Mielniczuk L., Clark H.D., et al. Interdepartmental program to improve outcomes for acute heart failure patients seen in the emergency department. CJEM. 2021;23(2):169–179. doi: 10.1007/s43678-020-00047-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.