Abstract
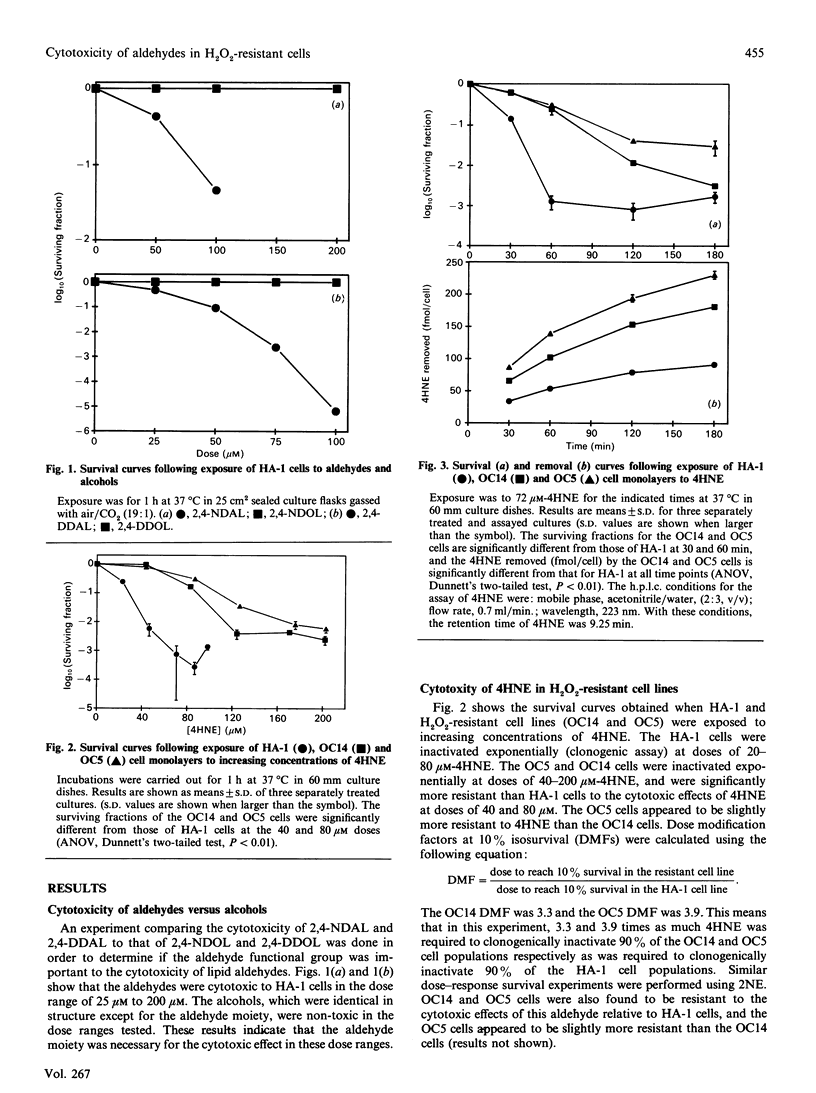
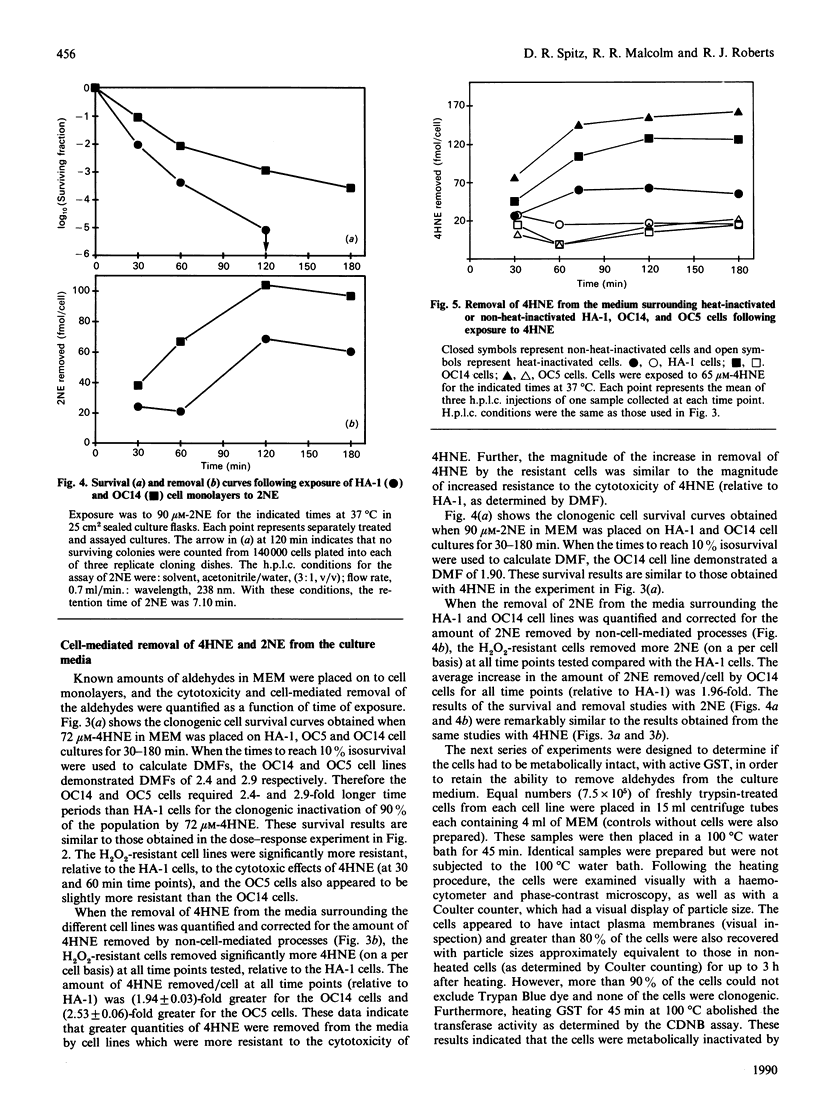
Toxic aldehydes, such as 4-hydroxy-2-nonenal (4HNE) and 2-nonenal (2NE), formed during lipid peroxidation have been isolated and implicated in the cytotoxic effects of oxidative stress. We have investigated the cytotoxicity and metabolism of 4HNE and 2NE in control (HA-1) cells and in two H2O2-resistant Chinese hamster fibroblast cell lines. The H2O2-resistant cells were found to be significantly more resistant than HA-1 cells to the cytotoxicity of 4HNE, as determined by clonogenic cell survival (dose-modifying factors at 10% isosurvival of 2.0-3.0). The H2O2-resistant cells demonstrated a significant 2-3-fold increase in the amount of 4HNE removed (mol/cell) from culture media containing 72 microM-4HNE when compared with HA-1 cells. The enhanced ability of H2O2-resistant cells to metabolize 4HNE was abolished by heating the cells at 100 degrees C for 45 min. Similar results were obtained with 2NE. Total glutathione and glutathione transferase activity, believed to be involved in cellular detoxification of 4HNE, were found to be significantly increased (2-3-fold) in the resistant cells when compared with the HA-1 cells. These results show that cell lines adapted and/or selected in a highly peroxidative environment are also resistant to the cytotoxicity of aldehydes formed during lipid peroxidation. This resistance appears to be related to increased cellular metabolism of these aldehydes, possibly through the glutathione transferase system. These findings suggest that the formation of aldehydes due to lipid peroxidation may contribute significantly to the mechanisms of oxidant-induced injury and the selective pressure exerted by H2O2-mediated cytotoxicity in culture.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benedetti A., Barbieri L., Ferrali M., Casini A. F., Fulceri R., Comporti M. Inhibition of protein synthesis by carbonyl compounds (4-hydroxyalkenals) originating from the peroxidation of liver microsomal lipids. Chem Biol Interact. 1981 Jun;35(3):331–340. doi: 10.1016/0009-2797(81)90008-9. [DOI] [PubMed] [Google Scholar]
- Benedetti A., Casini A. F., Ferrali M., Comporti M. Effects of diffusible products of peroxidation of rat liver microsomal lipids. Biochem J. 1979 May 15;180(2):303–312. doi: 10.1042/bj1800303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti A., Casini A. F., Ferrali M. Red cell lysis coupled to the peroxidation of liver microsomal lipids. Compartmentalization of the hemolytic system. Res Commun Chem Pathol Pharmacol. 1977 Jul;17(3):519–528. [PubMed] [Google Scholar]
- Benedetti A., Comporti M., Esterbauer H. Identification of 4-hydroxynonenal as a cytotoxic product originating from the peroxidation of liver microsomal lipids. Biochim Biophys Acta. 1980 Nov 7;620(2):281–296. doi: 10.1016/0005-2760(80)90209-x. [DOI] [PubMed] [Google Scholar]
- Brambilla G., Sciabà L., Faggin P., Maura A., Marinari U. M., Ferro M., Esterbauer H. Cytotoxicity, DNA fragmentation and sister-chromatid exchange in Chinese hamster ovary cells exposed to the lipid peroxidation product 4-hydroxynonenal and homologous aldehydes. Mutat Res. 1986 Aug-Sep;171(2-3):169–176. doi: 10.1016/0165-1218(86)90051-0. [DOI] [PubMed] [Google Scholar]
- Brawn K., Fridovich I. DNA strand scission by enzymically generated oxygen radicals. Arch Biochem Biophys. 1981 Feb;206(2):414–419. doi: 10.1016/0003-9861(81)90108-9. [DOI] [PubMed] [Google Scholar]
- Cerutti P. A. Prooxidant states and tumor promotion. Science. 1985 Jan 25;227(4685):375–381. doi: 10.1126/science.2981433. [DOI] [PubMed] [Google Scholar]
- Esterbauer H., Cheeseman K. H., Dianzani M. U., Poli G., Slater T. F. Separation and characterization of the aldehydic products of lipid peroxidation stimulated by ADP-Fe2+ in rat liver microsomes. Biochem J. 1982 Oct 15;208(1):129–140. doi: 10.1042/bj2080129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterbauer H., Zollner H., Lang J. Metabolism of the lipid peroxidation product 4-hydroxynonenal by isolated hepatocytes and by liver cytosolic fractions. Biochem J. 1985 Jun 1;228(2):363–373. doi: 10.1042/bj2280363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman B. A., Topolosky M. K., Crapo J. D. Hyperoxia increases oxygen radical production in rat lung homogenates. Arch Biochem Biophys. 1982 Jul;216(2):477–484. doi: 10.1016/0003-9861(82)90236-3. [DOI] [PubMed] [Google Scholar]
- Haberland M. E., Fong D., Cheng L. Malondialdehyde-altered protein occurs in atheroma of Watanabe heritable hyperlipidemic rabbits. Science. 1988 Jul 8;241(4862):215–218. doi: 10.1126/science.2455346. [DOI] [PubMed] [Google Scholar]
- Habig W. H., Pabst M. J., Jakoby W. B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974 Nov 25;249(22):7130–7139. [PubMed] [Google Scholar]
- Ishikawa T., Esterbauer H., Sies H. Role of cardiac glutathione transferase and of the glutathione S-conjugate export system in biotransformation of 4-hydroxynonenal in the heart. J Biol Chem. 1986 Feb 5;261(4):1576–1581. [PubMed] [Google Scholar]
- Jamieson D. Oxygen toxicity and reactive oxygen metabolites in mammals. Free Radic Biol Med. 1989;7(1):87–108. doi: 10.1016/0891-5849(89)90103-2. [DOI] [PubMed] [Google Scholar]
- Jensson H., Guthenberg C., Alin P., Mannervik B. Rat glutathione transferase 8-8, an enzyme efficiently detoxifying 4-hydroxyalk-2-enals. FEBS Lett. 1986 Jul 28;203(2):207–209. doi: 10.1016/0014-5793(86)80743-8. [DOI] [PubMed] [Google Scholar]
- Kaneko T., Honda S., Nakano S., Matsuo M. Lethal effects of a linoleic acid hydroperoxide and its autoxidation products, unsaturated aliphatic aldehydes, on human diploid fibroblasts. Chem Biol Interact. 1987;63(2):127–137. doi: 10.1016/0009-2797(87)90093-7. [DOI] [PubMed] [Google Scholar]
- Kaneko T., Kaji K., Matsuo M. Cytotoxicities of a linoleic acid hydroperoxide and its related aliphatic aldehydes toward cultured human umbilical vein endothelial cells. Chem Biol Interact. 1988;67(3-4):295–304. doi: 10.1016/0009-2797(88)90065-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McCord J. M., Day E. D., Jr Superoxide-dependent production of hydroxyl radical catalyzed by iron-EDTA complex. FEBS Lett. 1978 Feb 1;86(1):139–142. doi: 10.1016/0014-5793(78)80116-1. [DOI] [PubMed] [Google Scholar]
- Mello Filho A. C., Meneghini R. In vivo formation of single-strand breaks in DNA by hydrogen peroxide is mediated by the Haber-Weiss reaction. Biochim Biophys Acta. 1984 Feb 24;781(1-2):56–63. doi: 10.1016/0167-4781(84)90123-4. [DOI] [PubMed] [Google Scholar]
- Myers C. E., McGuire W. P., Liss R. H., Ifrim I., Grotzinger K., Young R. C. Adriamycin: the role of lipid peroxidation in cardiac toxicity and tumor response. Science. 1977 Jul 8;197(4299):165–167. doi: 10.1126/science.877547. [DOI] [PubMed] [Google Scholar]
- Palinski W., Rosenfeld M. E., Ylä-Herttuala S., Gurtner G. C., Socher S. S., Butler S. W., Parthasarathy S., Carew T. E., Steinberg D., Witztum J. L. Low density lipoprotein undergoes oxidative modification in vivo. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1372–1376. doi: 10.1073/pnas.86.4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poli G., Dianzani M. U., Cheeseman K. H., Slater T. F., Lang J., Esterbauer H. Separation and characterization of the aldehydic products of lipid peroxidation stimulated by carbon tetrachloride or ADP-iron in isolated rat hepatocytes and rat liver microsomal suspensions. Biochem J. 1985 Apr 15;227(2):629–638. doi: 10.1042/bj2270629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poot M., Verkerk A., Koster J. F., Esterbauer H., Jongkind J. F. Reversible inhibition of DNA and protein synthesis by cumene hydroperoxide and 4-hydroxy-nonenal. Mech Ageing Dev. 1988 Apr;43(1):1–9. doi: 10.1016/0047-6374(88)90093-0. [DOI] [PubMed] [Google Scholar]
- Puppo A., Halliwell B. Formation of hydroxyl radicals from hydrogen peroxide in the presence of iron. Is haemoglobin a biological Fenton reagent? Biochem J. 1988 Jan 1;249(1):185–190. doi: 10.1042/bj2490185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin R., Farber J. L. Mechanisms of the killing of cultured hepatocytes by hydrogen peroxide. Arch Biochem Biophys. 1984 Feb 1;228(2):450–459. doi: 10.1016/0003-9861(84)90010-9. [DOI] [PubMed] [Google Scholar]
- Simons P. C., Vander Jagt D. L. Purification of glutathione S-transferases from human liver by glutathione-affinity chromatography. Anal Biochem. 1977 Oct;82(2):334–341. doi: 10.1016/0003-2697(77)90169-5. [DOI] [PubMed] [Google Scholar]
- Spitz D. R., Li G. C., McCormick M. L., Sun Y., Oberley L. W. Stable H2O2-resistant variants of Chinese hamster fibroblasts demonstrate increases in catalase activity. Radiat Res. 1988 Apr;114(1):114–124. [PubMed] [Google Scholar]
- Spitz D. R., Mackey M. A., Li G. C., Elwell J. H., McCormick M. L., Oberley L. W. Relationship between changes in ploidy and stable cellular resistance to hydrogen peroxide. J Cell Physiol. 1989 Jun;139(3):592–598. doi: 10.1002/jcp.1041390320. [DOI] [PubMed] [Google Scholar]
- Steinberg D., Parthasarathy S., Carew T. E., Khoo J. C., Witztum J. L. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989 Apr 6;320(14):915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- Winkler P., Lindner W., Esterbauer H., Schauenstein E., Schaur R. J., Khoschsorur G. A. Detection of 4-hydroxynonenal as a product of lipid peroxidation in native Ehrlich ascites tumor cells. Biochim Biophys Acta. 1984 Dec 6;796(3):232–237. doi: 10.1016/0005-2760(84)90122-x. [DOI] [PubMed] [Google Scholar]
- Yang S. J., Hahn G. M., Bagshaw M. A. Chromosome aberrations induced by thymidine. Exp Cell Res. 1966 Apr;42(1):130–135. doi: 10.1016/0014-4827(66)90326-0. [DOI] [PubMed] [Google Scholar]


