Abstract
Background: Autologous fat grafting (AFG) has emerged as a useful technique in breast reconstruction. Utilizing a patient’s own fat from areas like the abdomen or thighs, AFG serves various reconstruction needs. Nevertheless, the oncological safety of AFG in breast cancer patients has become a contentious issue. Concerns about its influence on cancer recurrence and detention have led to significant clinical debate and the need for thorough investigation. Methods: To determine the impact of autologous fat grafting (AFG) on loco-regional recurrence (LRR) in breast cancer survivors undergoing reconstruction, a comprehensive search of databases including PubMed, Medline, Web of Science, and Cochrane libraries was conducted from November 2023 through March 2024. This search adhered to the PRISMA guidelines and aimed to identify all the relevant studies on AFG in the context of breast reconstruction post cancer treatment. A meta-analysis was performed. Results: Out of the studies reviewed, 40 met the inclusion criteria, with a total patient cohort of 14,078. The analysis revealed that AFG had no significant association with increased rates of LRR. Conclusions: According to the available literature, AFG is a safe reconstructive option for breast cancer patients and does not increase the risk of loco-regional recurrence. Nevertheless, further well-structured long-term prospective studies are required, since heterogeneity of available studies is high and requires standardization.
Keywords: autologous fat grafting, breast cancer, recurrence, lipofilling, LRR, breast reconstruction
1. Introduction
Breast cancer constitutes a prominent global health issue, impacting a significant number of women worldwide and presenting complex challenges for both patients and healthcare professionals. It ranks as the most commonly diagnosed cancer in a majority of nations (154 out of 185) and is the foremost cause of cancer-related deaths in over 100 countries [1]. The incidence of local–regional recurrences (LRR) following breast cancer surgery plays a critical role in mortality and disease-free survival (DFS), which, in turn, serves as a reliable surrogate marker for overall survival [2].
Over recent decades, the surgical management of breast cancer has shifted from more radical procedures to those conserving breast tissue. Efforts by oncologists and plastic surgeons are increasingly focused not only on enhancing oncological treatments but also on advancing reconstructive techniques to address contour defects and restore volume, aiming to improve outcomes and patient quality of life [3,4]. The necessity for demolitive surgical approaches, such as mastectomy or breast-conserving surgery (BCS), can significantly alter a patient’s physical appearance and self-perception, thereby impacting their quality of life [5]. This alteration often leads individuals to pursue reconstructive surgery as a means to reclaim their sense of femininity and integrity post treatment.
In recent years, autologous fat grafting (AFG), has gained increasing attention as a valuable adjunctive technique in breast reconstruction following demolitive surgery. This approach involves the transplantation of a patient’s own adipose tissue harvested from donor sites, such as the abdomen or thighs, to address different necessities of the patient. AFG has several indications in breast reconstructive surgery as an ancillary procedure to address asymmetry corrections following BCS [6], contour irregularities of the reconstructed breast [7], thinning of the subcutaneous tissue to prevent expander/implant exposure before [8] or after RT [9,10], and as the sole procedure for reconstruction of a small-sized breast [11]. For the latter, AFG offers several advantages over traditional implant-based or flap reconstruction methods, including its ability to achieve more natural-looking results, enhance breast symmetry, have low incidence of revision surgeries and minimize donor-site morbidity. Nevertheless, oil cysts, fat necrosis and macrocalcifications may occur, mainly following high-volume transfers [12].
Despite its growing popularity and perceived benefits, concerns regarding the oncologic safety of AFG in breast cancer patients have emerged as a topic of considerable debate and scrutiny within the medical community [13,14,15]. Questions regarding its potential impact on cancer detection [16,17], promotion of tumor growth [18], and facilitation of local recurrence [19,20,21,22] have raised important clinical considerations and prompted calls for rigorous evaluation.
The existing literature on the oncologic safety of breast AFG is characterized by a heterogeneous array of studies with varying methodologies, patient populations, and outcomes, resulting in conflicting findings and inconclusive evidence. The recent increase in research on this topic calls for an updated analysis that combines the latest studies with past evidence. By synthesizing and critically appraising the existing literature, this review aims to provide a comprehensive overview of the safety profile of breast AFG in the context of oncologic surgery.
2. Materials and Methods
We performed a systematic review of the literature in accordance with PRISMA guidelines, and was not registered in any systematic review registry. We searched for publications on PubMed, Embase, Web of Science (including Science Citation Index and Conference Proceedings Citation Index), and Cochrane Library databases to identify all publications regarding autologous fat grafting after breast cancer surgery. For all the libraries, the following search term strategy was used: (autologous fat grafting [MeSH] AND breast [MeSH]). As there are several different terms describing autologous fat grafting, and to maintain a systematic approach, available synonyms were also used as search terms. The used Mesh-terms were the following: autologous fat transfer, lipofilling, adipose fat transfer, lipotransfer, adipose tissue, breast cancer, fat grafting, and cancer recurrence. All citations were screened through their titles and abstracts, duplicates were removed, and then full-text manuscripts were assessed according to the following inclusion criteria: only human-based topics and manuscripts written in English were to be taken into consideration. Case reports and case series with less than 15 patients, letters, review, book chapters, or a Jadad modified scale score <2 were used as exclusion criteria for this review.
The review search started on November 2023 and ended in March 2024 and was conducted by L.P. and D.A. The two reviewers independently reviewed the titles and abstracts yielded by this comprehensive search and subsequently selected articles based on the predetermined inclusion and exclusion criteria. Disagreements were resolved through consensus-based discussion with a third reviewer (F.L.T.).
The following data were extracted from the manuscripts included in the final tally: study period, number of patients, mean age, type of surgery before autologous fat grafting, incidence of invasive carcinomas, carcinoma in situ (CIS), radiotherapy (RT) before autologous fat grafting, mean time between surgery and autologous fat grafting, mean follow-up period after autologous fat grafting, and number of patients with local recurrence. The endpoints of this study were to analyze the correlation between AFG and LRR rates and to analyze the factors implicated in a higher incidence of LRR, such as percentage of invasive carcinoma, percentage of RT and follow-up. The level of evidence for included studies was evaluated using the Oxford Centre for Evidence-Based Medicine (OCEBM) [23] and the Oxford quality scoring system (Jadad Score) [24], which are instruments to evaluate the quality of observational and randomized studies.
The meta-analysis was performed using the MetaXL 5.3 software, and the meta-regression was performed using SPSS Statistics v28.0 (IBM, Armonk, NY, USA). The meta-analysis was conducted in distinct phases. First, we assessed the LRR in studies that compared groups of patients undergoing AFG with those who did not receive AFG. Following this comparative analysis, the second phase examined LRR in single-arm studies, comparing with the overall LRR prevalence. Lastly, separate meta-analyses were performed comprising only matched and unmatched studies. A meta-regression analysis was subsequently performed to investigate the impact of the percentage of invasive carcinomas, the proportion of patients receiving radiotherapy, and the follow-up duration on LRR rates.
3. Results
3.1. Study Selection
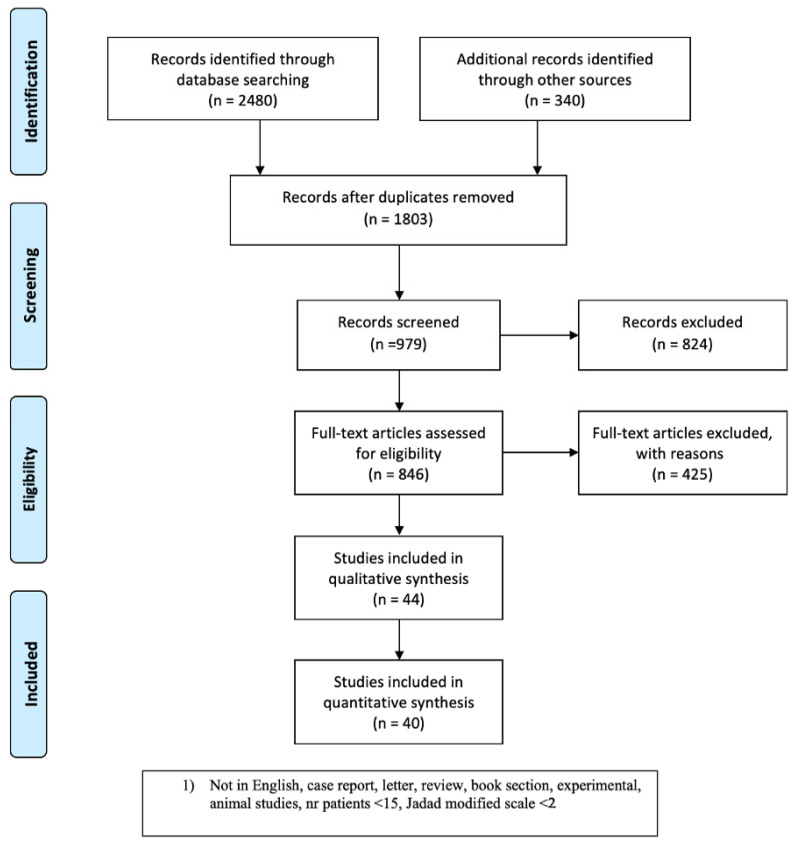
From 2480 starting citations scrutinized in the study period, we identified 1803 articles following the first screening based on the assessment of titles and abstracts. Any citation deemed not relevant to the systematic review endpoints was excluded. After duplicates were excluded, 979 articles were screened and manuscripts not meeting the inclusion criteria or meeting the exclusion criteria were discarded, only leaving 846 articles. After full-text assessment, any manuscript that did not provide clinical data of a patient population undergoing AFG following breast cancer was excluded. Data from 40 manuscripts were included for analysis. A flow chart representation of the search strategy with the included and excluded articles is depicted in Figure 1.
Figure 1.
Flow diagram representation of the search strategy used for the systematic review, in accordance with PRISMA guidelines.
3.2. Analysis of Selected Studies
Seven studies [25,26,27,28,29,30,31] reported a higher rate of LRR in the population of patients who underwent AFG. Seven studies [32,33,34,35,36,37,38] reported a comparable rate of LRR between patients who underwent AFG and patients that did not undergo AFG. Seven studies [39,40,41,42,43,44,45] reported a lower rate of LRR in the population of patients who underwent AFG. Nineteen studies [46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64] reported LRR rates only for patients who underwent AFG.
3.3. Comparative Analysis
Shown in Table 1 is an outline of clinical investigations regarding the potential oncological hazards associated with surgical procedures for breast cancer, related to LRR rate. Among 6459 patients who received mastectomy or breast conservative surgery (MST/BCS) without incorporating AFG, the analysis demonstrated an LRR of 5.3%. This indicates that 342 patients encountered local recurrence. A total of 7619 patients underwent AFG following MST or BCS. Of these, 240 patients experienced a loco-regional recurrence, accounting for 3.15%.
3.4. Meta-Analysis
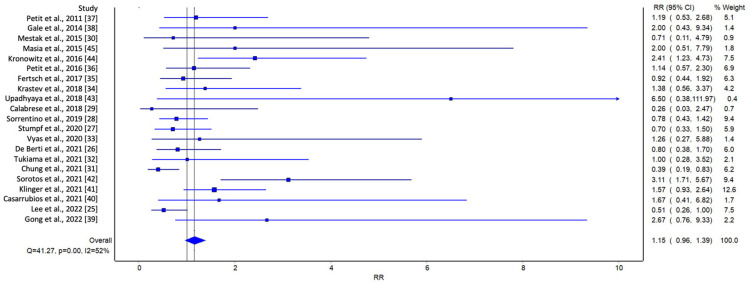
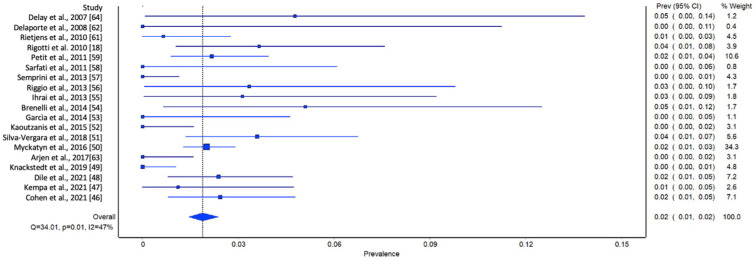
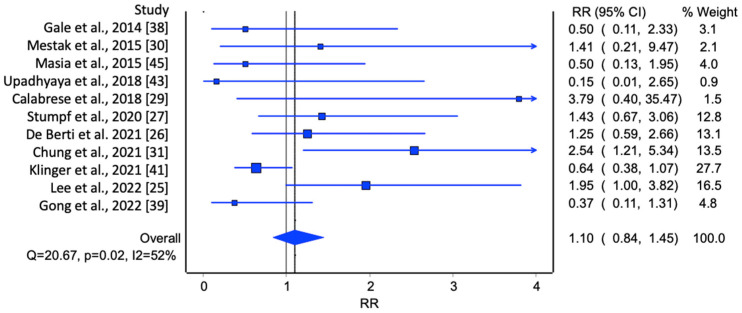
In the initial analysis of comparative studies (Figure 2), a high degree of study heterogeneity was noted. No direct correlation between AFG and LRR was identified. However, even though it was not statistically significant, there was an observable trend favoring AFG. This trend was further corroborated with the cumulative prevalence analysis (Figure 3).
Figure 2.
Meta-analysis evaluating LRR in comparative studies. Overall prevalence of LRR was used to compare each study [25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45].
Figure 3.
Meta-analysis evaluating the prevalence of LRR in single-arm studies. Overall prevalence of LRR was used to compare each study [18,46,47,48,49,50,51,52,53,54,55,56,57,58,59,61,62,63,64].
It was not possible to conduct a comprehensive meta-analysis that examined all the various subgroups extracted from the studies, such as histology, receptor status, timing of AFG, and type of surgery. This was due to the fragmented nature of the data reported and the inconsistent presence of these variables across all the studies examined. Consequently, a meta-regression was performed, identifying two major groups: comparative studies [25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45] and single-arm studies [46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64].
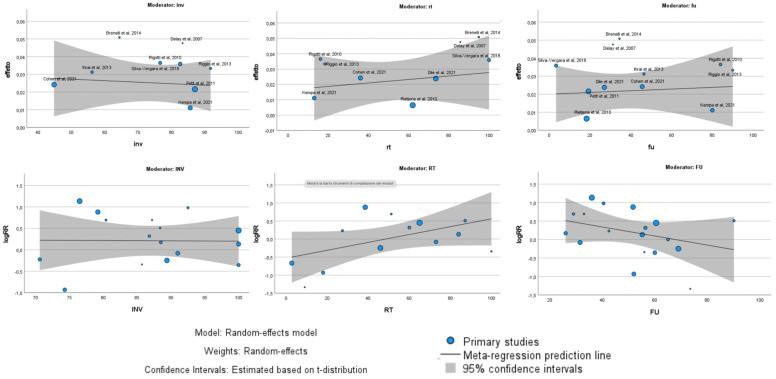
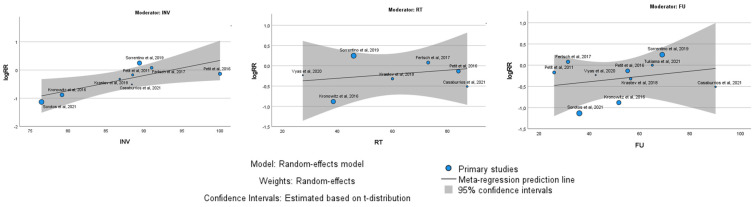
Within these groups, the oncological outcome, specifically LRR, was analyzed by examining possible subgroups, including the percentage of invasive carcinomas, the percentage of patients undergoing radiotherapy, and the follow-up duration (Figure 4). The meta-regression analysis revealed distinct findings between single-arm and comparative studies regarding the factors influencing the oncological outcomes of autologous fat grafting (AFG). In single-arm studies, no significant relationship was found between the outcomes and the percentage of invasive carcinomas (p = 0.74), the percentage of patients receiving radiotherapy (p = 0.54), or the duration of follow-up (p = 0.77). This suggests that in isolated evaluations of AFG, these variables did not substantially impact the effectiveness or results of the procedure. In contrast, comparative studies presented a more nuanced picture. The percentage of invasive cancers remained non-influential on the results (p = 0.79), maintaining consistency with the single-arm studies. However, a trend emerged indicating a potential disadvantage for AFG with longer follow-up durations, although this trend was not statistically significant (p = 0.06). Moreover, a statistically significant relationship was observed with the percentage of patients receiving radiotherapy. Specifically, as the percentage of radiotherapy-treated patients increased, the outcomes for AFG improved significantly (p = 0.009).
Figure 4.
Meta-regression analysis with Bubble Plot of single-arm studies (above) and comparative studies (below). Correlations with percentage of invasive carcinomas, percentage of radiotherapy, and follow-up were analyzed.
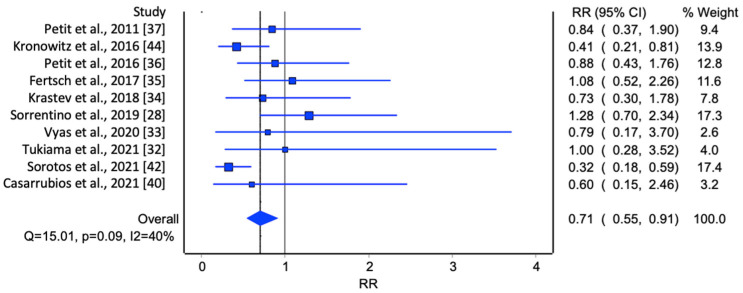
Lastly, two meta-analyses were conducted, one including only unmatched studies (Figure 5) and the other including only matched studies (Figure 6). In the first group, the meta-analysis included unmatched studies, which may introduce more variability and potential confounding factors, whereas the studies included in the second meta-analysis were conducted with patient matching, which means the patient groups were more comparable, potentially reducing bias. For the first meta-analysis, the overall RR was 1.10 (95% CI: 0.84, 1.45). Even though there was an increase in the risk of loco-regional recurrence for patients who underwent breast lipofilling compared to those who did not, this result was not statistically significant. The heterogeneity statistics indicated moderate to substantial variability among the study results, suggesting that the results from these studies are not highly consistent. For the second meta-analysis, the overall RR was 0.71 (95% CI: 0.55, 0.91). This result suggests a 29% reduction in the risk of loco-regional recurrence for patients who underwent breast lipofilling compared to those who did not, and this reduction was statistically significant. The heterogeneity statistics indicated moderate variability among the study results, suggesting that while the studies were not perfectly consistent, they were reasonably comparable.
Figure 5.
Meta-analysis evaluating the prevalence of LRR in unmatched studies [25,26,27,29,30,31,38,39,41,43,45].
Figure 6.
Meta-analysis evaluating the prevalence of LRR in matched studies [28,32,33,34,35,36,37,40,42,44].
The meta-regression analysis (Figure 7) showed a slight positive association with percentage of invasive carcinomas (p = 0.03).
Table 1.
Literature review.
| Author | Year of Publication | Type of Study | Group | Study Period | Patients (n) | Mean Age (y) | Type of Surgery | Invasive Carcinomas (n) | In Situ Carcinomas (n) | RT before AFG (%) | Mean Surgery-AFG (m) | Mean Follow-up (m) | LRR (%) | LRR (n) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Delay et al. [64] | 2007 | Observational study | BCS + AFG | 2002–2007 | 42 | 51 | BCS + AFG | 35 | 3 | 85.70 | 78 | 31.2 | 4.76 | 2 |
| Delaporte et al. [62] | 2008 | Observational study | MST + AFG | 2002–2007 | 15 | 50 | MST + AFG | 9 | 6 | 78.50 | N/A | 27.6 | 0 | 0 |
| Rietjens et al. [61] | 2010 | Observational study | BCS + MST + AFG | 2005–2008 | 155 | 48 | BCS + MST + IBR + ABR + AFG | N/A | N/A | 62 | 50.5 | 18.3 | 0.70 | 1 |
| Rigotti et al. [18] | 2010 | Retrospective cohort study | MST +AFG | 2000–2005 | 137 | 46.5 | MST +AFG | 105 | 31 | 16.10 | 3.2 | 84 | 3.65 | 5 |
| Petit et al. [37] | 2011 | Multicenter retrospective study | BCS + MST + AFG | 2000–2010 | 370 | 52 | BCS + MST +AFG | 87 | 13 | N/A | N/A | 19.2 | 2.16 | 8 |
| Petit et al. [59] | 2011 | Matched cohort study | MST | 1997–2008 | 642 | 46 | MST | 568 | 74 | N/A | N/A | 26 | 3 | 19 |
| MST + AFG | 321 | 45 | MST + AFG | 284 | 37 | N/A | 26 | 26 | 2.50 | 8 | ||||
| Sarfati et al. [58] | 2011 | Prospective study | MST + AFG | 2007–2009 | 28 | 45 | MST + AFG | N/A | N/A | 100 | N/A | 17 | 0 | 0 |
| Semprini et al. [57] | 2013 | Observational study | BCS + AFG | 2006–2012 | 151 | N/A | BCS + AFG | N/A | N/A | N/A | 24 | 45 | 0 | 0 |
| Riggio et al. [56] | 2013 | Observational study | MST + AFG | 2000–2007 | 60 | 49.7 | MST + AFG | 55 | 5 | 18.30 | 55.2 | 90 | 3.30 | 2 |
| Ihrai et al. [55] | 2013 | Retrospective study | MST + AFG | 2004–2009 | 64 | N/A | MST + AFG | 36 | 10 | N/A | N/A | 46.44 | 3.10 | 2 |
| Brenelli et al. [54] | 2014 | Prospective study | BCS + AFG | 2005–2008 | 59 | 50 | BCS + AFG | 38 | 7 | 94.90 | N/A | 34.4 | 5.10 | 3 |
| Gale et al. [38] | 2014 | Clinical study | BCS + MST | 2007–2013 | 422 | 48.2 | BCS + MST | 368 | 54 | N/A | 54 | 34 | 1.90 | 8 |
| BCS + MST + AFG | 211 | 47 | BCS + MST + AFG | 184 | 27 | 108 | 54 | 32 | 0.95 | 2 | ||||
| Garcìa et al. [53] | 2014 | Observational study | BCS + AFG | N/A | 37 | 55 | BCS + AFG | 0 | 37 | N/A | 0 | 1 | 0 | 0 |
| Kaoutzanis et al. [52] | 2015 | Retrospective study | MST + AFG | 2008–2013 | 108 | 48 | MST + AFG | 68 | 40 | 23.30 | 10.8 | 20.2 | 0 | 0 |
| Mestak et al. [30] | 2015 | Prospective study | BCS | 2011–2014 | 45 | 64 | BCS | 41 | 3 | N/A | N/A | 56 | 4.88 | 2 |
| BCS + AFG | 32 | 53 | BCS + AFG | 25 | 4 | 100 | 77 | 56 | 6.25 | 2 | ||||
| Silva-Vergara et al. [51] | 2015 | Retrospective study | BCS + MST + AFG | 2007–2015 | 195 | 52 | BCS + MST + AFG | 161 | 44 | 100 | 4 | 3.3 | 3.58 | 7 |
| Masia et al. [45] | 2015 | Retrospective study case-control | MST + ABR | 1989–2017 | 107 | 49 | MST + ABR | 87 | 16 | N/A | N/A | 29 | 5.60 | 6 |
| MST + ABR + AFG | 107 | 49.19 | MST + ABR + AFG | 85 | 14 | N/A | N/A | 29 | 2.80 | 3 | ||||
| Kronowitz et al. [44] | 2016 | Retrospective cohort study | MST | 2001–2014 | 670 | 46.5 | MST | 548 | 61 | N/A | N/A | 43.8 | 4.10 | 27 |
| BCS + MST + AFG | 719 | 47.1 | BCS + MST + AFG | 552 | 108 | 38.50 | 2.63 | 59.6 | 1.60 | 12 | ||||
| Myckatyn et al. [50] | 2016 | Multicenter case cohort study | MST + AFG | 2006–2011 | 1197 | 47 | MST + IBR + ABR | N/A | N/A | N/A | N/A | N/A | 11.00% | 24 |
| Petit et al. [36] | 2016 | Matched case-control study | MST | 2006–2013 | 322 | N/A | MST + BCS | 322 | 0 | 86 | N/A | 52.8 | 5 | 16 |
| MST + AFG | 322 | N/A | MST + BCS + AFG | 322 | 0 | 84 | N/A | 57.6 | 4.30 | 14 | ||||
| Arjen et al. [63] | 2017 | Retrospective cohort study | BCS + AFG | 2008–2016 | 109 | 55 | BCS + AFG | N/A | N/A | 100 | 18 | 26.4 | 0 | 0 |
| Fertsch et al. [35] | 2017 | Matched retrospective cohort study | MST + DIEP | 2009–2013 | 100 | 50.7 | MST + ABR | 91 | 9 | N/A | N/A | 31 | 12 | 12 |
| MST + DIEP + AFG | 100 | 49.6 | MST + ABR + AFG | 91 | 9 | 73 | 40.5 | 32 | 13 | 13 | ||||
| Krastev et al. [34] | 2018 | Matched cohort study | MST + BCS | 2006–2014 | 300 | 49.4 | MST + BCS | 260 | 40 | N/A | N/A | 52.8 | 3.60 | 11 |
| MST + BCS + AFG | 300 | 48.1 | MST + BCS + AFG | 261 | 39 | 60 | N/A | 60 | 2.60 | 8 | ||||
| Upadhyaya et al. [43] | 2018 | Retrospective chart review study | MST | 2011–2016 | 449 | N/A | MST + IBR + ABR | N/A | N/A | N/A | N/A | 26 | 1.70 | 8 |
| MST + AFG | 171 | 50.51 | MST + IBR + ABR + AFG | N/A | N/A | N/A | N/A | 26 | 0 | 0 | ||||
| Calabrese et al. [29] | 2018 | Prospective multi arm single center cohort study | MST | 2008–2011 | 72 | 47.7 | MST | N/A | N/A | N/A | N/A | 72 | 1.60 | 1 |
| MST + AFG | 57 | 50.3 | MST + AFG | N/A | N/A | 9 | 9 | 75 | 4.70 | 3 | ||||
| MST + EAFG | 54 | 48.8 | MST +EAFG | N/A | N/A | 17 | 10 | 84 | 2.40 | 1 | ||||
| Sorrentino et al. [28] | 2019 | Retrospective exact matching study | MST + BCS | 2007–2017 | 597 | 50.7 | MST + BCS | 535 | 62 | N/A | N/A | 63.8 | 5.00% | 30 |
| MST + BCS + AFG | 233 | 49.4 | MST + BCS + AFG | 207 | 26 | 45.90 | 22.9 | 74.1 | 6.40 | 15 | ||||
| Knackstedt et al. [49] | 2019 | Retrospective cohort study | MST + IBR + AFG | 2006–2015 | 166 | 52 | MST + IBR + AFG | 106 | 52 | 20 | N/A | 28 | 0 | 0 |
| Stumpf et al. [27] | 2020 | Matched retrospective cohort study | BCS | 2004–2016 | 255 | 54 | BCS | 255 | 0 | N/A | N/A | 60 | 8.60 | 22 |
| BCS + AFG | 65 | 53 | BCS + AFG | 65 | 0 | N/A | N/A | 60 | 12.30 | 8 | ||||
| Vyas et al. [33] | 2020 | Matched case-control study | MST | 2000–2017 | 69 | N/A | MST | N/A | N/A | N/A | N/A | 42.5 | 8.50 | 6 |
| MST +AFG | 29 | 48.6 | MST + AFG | N/A | N/A | 27.4 | N/A | 42.5 | 8.20 | 2 | ||||
| Dile et al. [48] | 2021 | Retrospective study | MST + BCS + AFG | 2013–2016 | 252 | 50 | MST + BCS + ABR + IBR + AFG | N/A | N/A | 73.50 | 35 | 27 | 2.40 | 6 |
| Kempa et al. [47] | 2021 | Monocentric cohort study | MST + BCS + AFG | 2008–2020 | 90 | 46.1 | MST + BCS + AFG | 77 | 13 | 13 | 57 | 80 | 0.90 | 1 |
| De Berti et al. [26] | 2021 | Retrospective monocentric case-control study | MST | 2007–2017 | 303 | 52 | MST + BCS + ABR + IBR | 202 | 87 | N/A | N/A | N/A | 6.60 | 20 |
| MST + AFG | 109 | 50 | MST + BCS + ABR +IBR + AFG | 89 | 16 | N/A | N/A | N/A | 8.30 | 9 | ||||
| Tukiama et al. [32] | 2021 | Retrospective matched cohort study | MST | 2007–2016 | 126 | N/A | MST + BCS | N/A | N/A | N/A | N/A | 65 | 7.10 | 9 |
| MST + AFG | 42 | N/A | MST + BCS + AFG | N/A | N/A | N/A | N/A | 65 | 6.30% | 3 | ||||
| Chung et al. [31] | 2021 | Retrospective cohort study | MST | 2009–2019 | 272 | 50.4 | MST + BCS + ABR + IBR | 200 | 66 | N/A | N/A | 52 | 6 | 16 |
| MST + AFG | 67 | 50.4 | MST + BCS + ABR + IBR + AFG | 52 | 15 | 18 | N/A | 52 | 15 | 10 | ||||
| Sorotos et al. [42] | 2021 | Retrospective matched case control study | MST | 2005–2017 | 494 | 45 -49 | MST + IBR + ABR | 379 | 115 | N/A | N/A | 36 | 9.60 | 47 |
| MST + AFG | 425 | 45–49 | MST + IBR + ABR + AFG | 324 | 101 | N/A | N/A | 36 | 3 | 13 | ||||
| Klinger et al. [41] | 2021 | Retrospective multicenter study case- control | MST + BCS | 2000–2018 | 923 | 52.9 | MST + BCS | 923 | N/A | N/A | N/A | 58 | 6.10 | 56 |
| MST +BCS + AFG | 466 | 51.4 | MST + BCS + AFG | 466 | N/A | 65 | N/A | 63 | 3.90 | 18 | ||||
| Casarrubios et al. [40] | 2021 | Matched cohort study | MST | 2011–2019 | 125 | 47.2 | MST + BCS | 115 | 10 | N/A | N/A | 85 | 4 | 5 |
| MST + AFG | 125 | 45.6 | MST + BCS + AFG | 106 | 19 | 87.20 | 48.1 | 95.3 | 2.40 | 3 | ||||
| Cohen et al. [46] | 2021 | Retrospective cohort study | MST + AFG | 2010–2015 | 248 | 47.95 | MST + AFG | 111 | 51 | 36 | 13.2 | 45.6 | 2.40 | 6 |
| Lee et al. [25] | 2022 | Retrospective cohort study | MST | 2011–2016 | 126 | 43.9 | MST + IBR | N/A | N/A | N/A | N/A | N/A | 9 | 11 |
| MST + AFG | 141 | 43.9 | MST + IBR + AFG | N/A | N/A | 2.70 | 12 | N/A | 17 | 24 | ||||
| Gong et al. [39] | 2022 | Retrospective cohort study | BCS | 2018 | 40 | 50.8 | BCS | 36 | 4 | N/A | N/A | 40.28 | 10 | 8 |
| BCS + AFG | 40 | 50.2 | BCS + AFG | 38 | 2 | N/A | N/A | 40.58 | 7.50 | 3 |
MST: Mastectomy; BCS: Breast conserving surgery; AFG: Autologous fat grating; EAFG: Enriched autologous fat grafting; IBR: Implant-based reconstruction; ABR: Autologous breast reconstruction.
Figure 7.
Meta-regression analysis with Bubble Plot of studies with patients matching. Correlations with percentage of invasive carcinomas, percentage of radiotherapy, and follow-up were analyzed.
4. Discussion
The oncological safety of AFG in breast reconstruction has been a subject of considerable debate for decades, stemming from diverse findings in cellular, biological, and clinical studies. This debate centers on whether the clinical advantages of AFG surpass its possible hazards. In our review we analyzed a total of 7619 patients who underwent AFG, with a total incidence of LRR of 3.15% and a total of 6459 patients who did not undergo AFG, with an LRR rate of 5.3% (Table 2).
Table 2.
Risk of LRR with or without AFG.
| Patients (No.) | LRR (No) | LRR (%) | |
|---|---|---|---|
| MST/BCS + AFG | 7619 | 240 | 3.15% |
| MST/BCS | 6459 | 342 | 5.30% |
MST: Mastectomy; BCS: Breast conserving surgery; AFG: Autologous fat grating.
A total of 40 articles were included in the evaluation and a meta-analysis was performed, highlighting the importance of this study to possibly overcome previous attempts to evaluate the oncological safety of AFG. In fact, previous reviews were hindered by the small number of articles reviewed.
From our analysis, even though the heterogeneity of the studies was wide, no direct correlation could be found between AFG and an increased risk of LRR.
Although conducting a comprehensive meta-analysis that included all subgroups from the studies was not feasible, a meta-regression analysis was carried out focusing on single-arm studies and comparative studies.
For single-arm studies, factors such as the percentage of invasive carcinomas, percentage of patients receiving radiotherapy, or the duration of follow-up did not influence LRR rates. However, a trend indicating a potential disadvantage for AFG with longer follow-up periods was observed in comparative studies. This trend was not statistically significant. Additionally, a significant statistical relationship was found between the percentage of patients receiving radiotherapy and LRR rates. Specifically, as the percentage of radiotherapy-treated patients increased, the LRR rate for patients who underwent AFG was lower. Lastly, while the meta-analysis results for unmatched studies were statistically non-significant, the one performed with matched studies revealed a reduced LRR rate in the AFG group. This reduction was slightly inferior when considering studies with a higher percentage of invasive carcinoma.
Concerns have been raised regarding the potential oncological risks associated with the use of adipose-derived mesenchymal stem cells (ADMSCs) in autologous fat grafting (AFG), particularly their secreting factors that may interact with primary breast cancer cells [65,66]. It is postulated that these factors may help develop and maintain an inflammatory state, in which tissue regeneration is stimulated, but that, on the other hand, they contribute to the process of tumor genesis and progression [67,68,69,70]. For this reason, concerns about the placement of regenerative tissue in a tumor bed raised doubts about the oncological safety of AFG in this context. Even though the American Society of Plastic Surgeons set up a task force to assess the indications, safety, and efficacy of AFG [71], a low grade of scientific evidence was present, thus failing to provide specific recommendations on the topic.
Nevertheless, after almost 15 years, there is still not scientific evidence to support such a possibility.
Analyzing the results of our study, we believe that the heterogeneity of the included studies, variations in study methodologies, and a paucity of long-term follow-up data are the main issues that should be resolved to obtain high-quality studies. Additionally, the lack of standardized reporting and inconsistent definitions of outcomes across studies pose challenges in synthesizing and interpreting the findings. Some studies indicate that AFG has little to no effect on local recurrence or cancer progression, while other studies suggest a possible risk of tumor recurrence and complications in monitoring for cancer. In our study, conflicting results in terms of the LRR rate were observed. Therefore, in our opinion, the decision to incorporate breast AFG into clinical practice should be made judiciously, considering patient-specific factors, tumor characteristics, and potential oncologic risks. The lack of a clear correlation between AFG and LRR seems to underline the importance of other factors, such as oncological and surgical variables, in breast cancer recurrence.
5. Future Directions
Future research efforts should focus on prospective, multicenter studies with standardized protocols to elucidate the long-term oncologic outcomes of breast AFG and identify patient subgroups that may benefit most from this procedure. We believe that characteristics of the tumor (such as clinical stage, histology, etc.) and genetic factors may play an important role in LRR and must be analyzed in these types of studies. Overall survival (OS) and disease-free survival (DFS) are additional sources of information that should be included. Furthermore, investigations into the underlying mechanisms of tumor interactions with adipose-derived stem cells and the tumor microenvironment are warranted to understand the oncologic implications of breast AFG better and inform evidence-based clinical practice guidelines.
6. Conclusions
Despite the fact that the cautions of the American Society of Plastic Surgeons remain pertinent, the current literature supports that AFG is an oncologically safe procedure, whose routine use appears to be justified. The discrepancy between experimental and in vivo studies may be due to the complexity of oncological processes and the inability to recreate, in vitro, the intricacy of the in vivo microenvironment. Further well-structured long-term prospective studies are required for more solid evidence.
Author Contributions
Conceptualization, F.L.T. and D.R.; methodology, F.L.T. and L.P.; validation, F.L.T., A.P. and D.R.; formal analysis, F.L.T., L.P. and D.A.; investigation, F.L.T., L.P. and D.A.; resources, D.A. and L.P.; data curation, D.A. and L.P.; writing—original draft preparation, F.L.T., L.P. and D.A.; writing—review and editing, L.P.; visualization, D.A.; supervision, F.L.T. and A.P. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Institutional review board approval was not needed for this study.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.DeSantis C.E., Ma J., Gaudet M.M., Newman L.A., Miller K.D., Goding Sauer A., Jemal A., Siegel R.L. Breast cancer statistics, 2019. Cancer J. Clin. 2019;69:438–451. doi: 10.3322/caac.21583. [DOI] [PubMed] [Google Scholar]
- 2.Buyse M., Saad E.D., Burzykowski T., Regan M.M., Sweeney C.S. Surrogacy beyond prognosis: The importance of “trial-level” surrogacy. Oncologist. 2022;27:266–271. doi: 10.1093/oncolo/oyac006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Black D.M., Mittendorf E.A. Landmark trials affecting the surgical management of invasive breast cancer. Surg. Clin. N. Am. 2013;93:501–518. doi: 10.1016/j.suc.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calabrese C., Casella D., Di Taranto G., Marcasciano M., Kothari A., Sordi S., Barellini L., Torto F.L., Tarallo M., Perra A., et al. Oncoplastic conservative surgery for breast cancer: Long-term outcomes of our first ten years experience. Eur. Rev. Med. Pharmacol. Sci. 2018;22:7333–7342. doi: 10.26355/eurrev_201811_16270. [DOI] [PubMed] [Google Scholar]
- 5.Hanson S.E., Lei X., Roubaud M.S., DeSnyder S.M., Caudle A.S., Shaitelman S.F., Hoffman K.E., Smith G.L., Jagsi R., Peterson S.K., et al. Long-term Quality of Life in Patients with Breast Cancer after Breast Conservation vs. Mastectomy and Reconstruction. JAMA Surg. 2022;157:e220631. doi: 10.1001/jamasurg.2022.0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biazus J.V., Falcão C.C., Parizotto A.C., Stumpf C.C., Cavalheiro J.A.C., Schuh F., Cericatto R., Zucatto E., Melo M.P. Immediate Reconstruction with Autologous fat Transfer Following Breast-Conserving Surgery. Breast J. 2015;21:268–275. doi: 10.1111/tbj.12397. [DOI] [PubMed] [Google Scholar]
- 7.Torto F.L., Marcasciano M., Kaciulyte J., Redi U., Barellini L., De Luca A., Perra A., Frattaroli J.M., Cavalieri E., Di Taranto G., et al. Prepectoral breast reconstruction with TiLoop® Bra Pocket: A single center prospective study. Eur. Rev. Med. Pharmacol. Sci. 2020;24:991–999. doi: 10.26355/eurrev_202002_20149. [DOI] [PubMed] [Google Scholar]
- 8.Vaia N., Torto F.L., Marcasciano M., Casella D., Cacace C., De Masi C., Ricci F., Ribuffo D. From the “Fat Capsule” to the “Fat Belt”: Limiting Protective Lipofilling on Irradiated Expanders for Breast Reconstruction to Selective Key Areas. Aesthetic Plast. Surg. 2018;42:986–994. doi: 10.1007/s00266-018-1120-3. [DOI] [PubMed] [Google Scholar]
- 9.Ribuffo D., Atzeni M., Guerra M., Bucher S., Politi C., Deidda M., Atzori F., Dessi M., Madeddu C., Lay G. Treatment of irradiated expanders: Protective lipofilling allows immediate prosthetic breast reconstruction in the setting of postoperative radiotherapy. Aesthetic Plast. Surg. 2013;37:1146–1152. doi: 10.1007/s00266-013-0221-2. [DOI] [PubMed] [Google Scholar]
- 10.Lo Torto F., Parisi P., Casella D., Di Taranto G., Cigna E., Ribuffo D. Impact of Evolving Radiation Therapy Techniques on Implant-Based Breast Reconstruction. Plast. Reconstr. Surg. 2018;141:182e–183e. doi: 10.1097/PRS.0000000000003972. [DOI] [PubMed] [Google Scholar]
- 11.Zhang X., Cai L., Yin B., Han X., Li F. Total breast reconstruction using large-volume condensed and viable fat grafting after mastectomy. J. Plast. Reconstr. Aesthetic Surg. 2021;74:966–973. doi: 10.1016/j.bjps.2020.10.109. [DOI] [PubMed] [Google Scholar]
- 12.Doren E.L., Parikh R.P., Laronga C., Hiro M.E., Sun W., Lee M.C., Smith P.D., Fulp W.J. Sequelae of fat grafting postmastectomy: An algorithm for management of fat necrosis. Eplasty. 2012;12:e53. [PMC free article] [PubMed] [Google Scholar]
- 13.Choi J., Cha Y.J., Koo J.S. Adipocyte biology in breast cancer: From silent bystander to active facilitator. Prog. Lipid Res. 2018;69:11–20. doi: 10.1016/j.plipres.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Tan J., Buache E., Chenard M.P., Dali-Youcef N., Rio M.C. Adipocyte is a non-trivial, dynamic partner of breast cancer cells. Int. J. Dev. Biol. 2011;55:851–859. doi: 10.1387/ijdb.113365jt. [DOI] [PubMed] [Google Scholar]
- 15.Report on autologous fat transplantation. ASPRS Ad-Hoc committee on new procedures, September 30, 1987. Plast. Aesthetic Nurs. 1987;7:140–141. [PubMed] [Google Scholar]
- 16.Juhl A.A., Redsted S., Engberg Damsgaard T. Autologous fat grafting after breast conserving surgery: Breast imaging changes and patient-reported outcome. J. Plast. Reconstr. Aesthetic Surg. 2018;71:1570–1576. doi: 10.1016/j.bjps.2018.08.012. [DOI] [PubMed] [Google Scholar]
- 17.Lohsiriwat V., Curigliano G., Rietjens M., Goldhirsch A., Petit J.Y. Autologous fat transplantation in patients with breast cancer: “silencing” or “fueling” cancer recurrence? Breast. 2011;20:351–357. doi: 10.1016/j.breast.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 18.Rigotti G., Marchi A., Galiè M., Baroni G., Benati D., Krampera M., Pasini A., Sbarbati A. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: A healing process mediated by adipose-derived adult stem cells. Plast. Reconstr. Surg. 2007;119:1409–1422. doi: 10.1097/01.prs.0000256047.47909.71. [DOI] [PubMed] [Google Scholar]
- 19.Iyengar P., Combs T.P., Shah S.J., Gouon-Evans V., Pollard J.W., Albanese C., Flanagan L., Tenniswood M.P., Guha C., Lisanti M.P., et al. Adipocyte-secreted factors synergistically promote mammary tumorigenesis through induction of anti-apoptotic transcriptional programs and proto-oncogene stabilization. Oncogene. 2003;22:6408–6423. doi: 10.1038/sj.onc.1206737. [DOI] [PubMed] [Google Scholar]
- 20.Manabe Y., Toda S., Miyazaki K., Sugihara H. Mature adipocytes, but not preadipocytes, promote the growth of breast carcinoma cells in collagen gel matrix culture through cancer-stromal cell interactions. J. Pathol. 2003;201:221–228. doi: 10.1002/path.1430. [DOI] [PubMed] [Google Scholar]
- 21.Salgado A.J., Reis R.L., Sousa N.J., Gimble J.M. Adipose tissue derived stem cells secretome: Soluble factors and their roles in regenerative medicine. Curr. Stem Cell Res. Ther. 2010;5:103–110. doi: 10.2174/157488810791268564. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y., Daquinag A., Traktuev D.O., Amaya-Manzanares F., Simmons P.J., March K.L., Pasqualini R., Arap W., Kolonin M.G. White adipose tissue cells are recruited by experimental tumors and promote cancer progression in mouse models. Cancer Res. 2009;69:5259–5266. doi: 10.1158/0008-5472.CAN-08-3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Howick J., Chalmers I., Glasziou P., Greenhalgh T., Heneghan C., Liberati A., Moschetti I., Phillips B., Thornton H. OCEBM Levels of Evidence—Centre for Evidence-Based Medicine (CEBM). University of Oxford. 2011. [(accessed on 26 May 2024)]. Available online: https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence.
- 24.Jadad A.R., Moore R.A., Carroll D., Jenkinson C., Reynolds DJ M., Gavaghan D.J., McQuay H.J. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control. Clin. Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 25.Lee K.-T., Kim J.H., Jeon B.-J., Pyon J.K., Mun G.-H., Lee S.K., Yu J., Kim S.W., Lee J.E., Ryu J.M., et al. Association of Fat Graft with Breast Cancer Recurrence in Implant-Based Reconstruction: Does the Timing Matter? Ann. Surg. Oncol. 2023;30:1087–1097. doi: 10.1245/s10434-022-12389-0. [DOI] [PubMed] [Google Scholar]
- 26.De Berti M., Goupille C., Doucet M., Arbion F., Vilde A., Body G., Ouldamer L. Oncological Safety of Autologous Fat Grafting in Breast Reconstruction after Mastectomy for cancer: A case-control study. J. Gynecol. Obstet. Hum. Reprod. 2022;51:102257. doi: 10.1016/j.jogoh.2021.102257. [DOI] [PubMed] [Google Scholar]
- 27.Stumpf C.C., Zucatto E., Cavalheiro J.A.C., de Melo M.P., Cericato R., Damin A.P.S., Biazús J.V. Oncologic safety of immediate autologous fat grafting for reconstruction in breast-conserving surgery. Breast Cancer Res. Treat. 2020;180:301–309. doi: 10.1007/s10549-020-05554-0. [DOI] [PubMed] [Google Scholar]
- 28.Sorrentino L., Regolo L., Scoccia E., Petrolo G., Bossi D., Albasini S., Caruso A., Vanna R., Morasso C., Mazzucchelli S., et al. Autologous fat transfer after breast cancer surgery: An exact-matching study on the long-term oncological safety. Eur. J. Surg. Oncol. 2019;45:1827–1834. doi: 10.1016/j.ejso.2019.05.013. [DOI] [PubMed] [Google Scholar]
- 29.Calabrese C., Kothari A., Badylak S., Di Taranto G., Marcasciano M., Sordi S., Barellini L., Torto F.L., Tarallo M., Gaggelli I., et al. Oncological safety of stromal vascular fraction enriched fat grafting in two-stage breast reconstruction after nipple sparing mastectomy: Long-term results of a prospective study. Eur. Rev. Med. Pharmacol. Sci. 2018;22:4768–4777. doi: 10.26355/eurrev_201808_15610. [DOI] [PubMed] [Google Scholar]
- 30.Mestak O., Hromadkova V., Fajfrova M., Molitor M., Mestak J. Evaluation of Oncological Safety of Fat Grafting After Breast-Conserving Therapy: A Prospective Study. Ann. Surg. Oncol. 2016;23:776–781. doi: 10.1245/s10434-015-4908-2. [DOI] [PubMed] [Google Scholar]
- 31.Chung J.H., Kim K.J., Jung S.P., Park S.H., Yoon E.S. Analysis of oncological safety of autologous fat grafting after immediate breast reconstruction. Gland. Surg. 2021;10:584–594. doi: 10.21037/gs-20-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tukiama R., Vieira R.A.C., Facina G., da Cunha Leal P., Zucca-Matthes G. Oncologic Safety of Autologous Fat Grafting after Breast Cancer Surgical Treatment: A Matched Cohort Study. Plast. Reconstr. Surg. 2021;148:11–20. doi: 10.1097/PRS.0000000000008037. [DOI] [PubMed] [Google Scholar]
- 33.Vyas K.S.M., DeCoster R.C., Burns J.C., Rodgers L.T.B., Shrout M.A., Mercer J.P.B., Coquillard C., Dugan A.J., Baratta M.D., Rinker B.D.M., et al. Autologous Fat Grafting Does Not Increase Risk of Oncologic Recurrence in the Reconstructed Breast. Ann. Plast. Surg. 2020;84((Suppl. S6)):S405–S410. doi: 10.1097/SAP.0000000000002285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krastev T., van Turnhout A., Vriens E., Smits L., van der Hulst R. Long-term Follow-up of Autologous Fat Transfer vs Conventional Breast Reconstruction and Association with Cancer Relapse in Patients with Breast Cancer. JAMA Surg. 2019;154:56–63. doi: 10.1001/jamasurg.2018.3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fertsch S., Hagouan M., Munder B., Schulz T., Abu-Ghazaleh A., Schaberick J., Stambera P., Aldeeri M., Andree C., Thamm O.C. Increased risk of recurrence associated with certain risk factors in breast cancer patients after DIEP-flap reconstruction and lipofilling-a matched cohort study with 200 patients. Gland Surg. 2017;6:315–323. doi: 10.21037/gs.2017.03.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petit J.Y., Maisonneuve P., Rotmensz N., Bertolini F., Rietjens M. Fat Grafting after Invasive Breast Cancer: A Matched Case-Control Study. Plast. Reconstr. Surg. 2017;139:1292–1296. doi: 10.1097/PRS.0000000000003339. [DOI] [PubMed] [Google Scholar]
- 37.Petit J.Y., Botteri E., Lohsiriwat V., Rietjens M., De Lorenzi F., Garusi C., Rossetto F., Martella S., Manconi A., Bertolini F., et al. Locoregional recurrence risk after lipofilling in breast cancer patients. Ann. Oncol. 2012;23:582–588. doi: 10.1093/annonc/mdr158. [DOI] [PubMed] [Google Scholar]
- 38.Gale K.L., Rakha E.A., Ball G., Tan V.K., McCulley S.J., Macmillan R.D. A case-controlled study of the oncologic safety of fat grafting. Plast. Reconstr. Surg. 2015;135:1263–1275. doi: 10.1097/PRS.0000000000001151. [DOI] [PubMed] [Google Scholar]
- 39.Gong F.-X., Zhou X., Niu Z.-H., Mao Y., Wang Y.-M., Lv M., Gao X.-Q., Liu W.-J., Wang H.-B. Effects of Breast-Conserving Surgery Combined with Immediate Autologous Fat Grafting on Oncologic Safety, Satisfaction and Psychology in Patients with Breast Cancer: A Retrospective Cohort Study. Cancer Manag. Res. 2022;14:1113–1124. doi: 10.2147/CMAR.S353370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Casarrubios J.M., Francés M., Fuertes V., Singer M., Navarro C., Duque O.G., Fernández-Palacios J. Oncological outcomes of lipofilling in breast reconstruction: A matched cohort study with 250 patients. Gland Surg. 2021;10:914–923. doi: 10.21037/gs-20-775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klinger M., Losurdo A., Lisa A.V.E., Morenghi E., Vinci V., Corsi F., Albasini S., Leonardi M.C., Jereczek-Fossa B.A., Veronesi P., et al. Safety of autologous fat grafting in breast cancer: A multicenter Italian study among 17 senonetwork breast units autologous fat grafting safety: A multicenter Italian retrospective study. Breast Cancer Res. Treat. 2022;191:355–363. doi: 10.1007/s10549-021-06444-9. [DOI] [PubMed] [Google Scholar]
- 42.Sorotos M., Paolini G., D’orsi G., Firmani G., Timmermans F.W., di Pompeo F.S. Oncologic Outcome of 1000 Postmastectomy Breast Reconstructions with Fat Transfer: A Single-Center, Matched Case-Control Study. Plast. Reconstr. Surg. 2022;150:4S–12S. doi: 10.1097/PRS.0000000000009494. [DOI] [PubMed] [Google Scholar]
- 43.Upadhyaya S.N., Bernard S.L., Grobmyer S.R., Yanda C., Tu C., Valente S.A. Outcomes of Autologous Fat Grafting in Mastectomy Patients Following Breast Reconstruction. Ann. Surg. Oncol. 2018;25:3052–3056. doi: 10.1245/s10434-018-6597-0. [DOI] [PubMed] [Google Scholar]
- 44.Kronowitz S.J., Mandujano C.C., Liu J., Kuerer H.M., Smith B., Garvey P., Jagsi R., Hsu L., Hanson S., Valero V. Lipofilling of the Breast Does Not Increase the Risk of Recurrence of Breast Cancer: A Matched Controlled Study. Plast. Reconstr. Surg. 2016;137:385–393. doi: 10.1097/01.prs.0000475741.32563.50. [DOI] [PubMed] [Google Scholar]
- 45.Masia J., Bordoni D., Pons G., Liuzza C., Castagnetti F., Falco G. Oncological safety of breast cancer patients undergoing free-flap reconstruction and lipofilling. Eur. J. Surg. Oncol. 2015;41:612–616. doi: 10.1016/j.ejso.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 46.Cohen O., Lam G., Karp N., Choi M. Determining the Oncologic Safety of Autologous Fat Grafting as a Reconstructive Modality: An Institutional Review of Breast Cancer Recurrence Rates and Surgical Outcomes. Plast. Reconstr. Surg. 2017;140:382e–392e. doi: 10.1097/PRS.0000000000003576. [DOI] [PubMed] [Google Scholar]
- 47.Kempa S., Brix E., Heine N., Hösl V., Strauss C., Eigenberger A., Brébant V., Seitz S., Prantl L. Autologous fat grafting for breast reconstruction after breast cancer: A 12-year experience. Arch. Gynecol. Obstet. 2022;305:921–927. doi: 10.1007/s00404-021-06241-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dile P., Hannebicque K., Renaudeau C., Bogart É., Ceugnart L., Regis C., Boulanger L., Chauvet M.-P. Palpable Nodules After Autologous Fat Grafting in Breast Cancer Patients: Incidence and Impact on Follow-up. Aesthetic Plast. Surg. 2023;47:503–511. doi: 10.1007/s00266-021-02530-x. [DOI] [PubMed] [Google Scholar]
- 49.Knackstedt R.W., Gatherwright J., Ataya D., Duraes E.F.R., Schwarz G.S. Fat Grafting and the Palpable Breast Mass in Implant-Based Breast Reconstruction: Incidence and Implications. Plast. Reconstr. Surg. 2019;144:265–275. doi: 10.1097/PRS.0000000000005790. [DOI] [PubMed] [Google Scholar]
- 50.Myckatyn T.M., Wagner I.J., Mehrara B.J., Crosby M.A., Park J.E., Qaqish B.F.M., Moore D.T., Busch E.L., Silva A.K., Kaur S., et al. Cancer Risk after Fat Transfer: A Multicenter Case-Cohort Study. Plast. Reconstr. Surg. 2017;139:11–18. doi: 10.1097/PRS.0000000000002838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Silva-Vergara C., Fontdevila J., Descarrega J., Burdio F., Yoon T.S., Grande L. Oncological outcomes of lipofilling breast reconstruction: 195 consecutive cases and literature review. J. Plast. Reconstr. Aesthetic Surg. 2016;69:475–481. doi: 10.1016/j.bjps.2015.12.029. [DOI] [PubMed] [Google Scholar]
- 52.Kaoutzanis C., Xin M., Ballard T.N., Welch K.B., Momoh A.O., Kozlow J.H., Brown D.L., Cederna P.S., Wilkins E.G. Autologous Fat Grafting After Breast Reconstruction in Postmastectomy Patients: Complications, Biopsy Rates, and Locoregional Cancer Recurrence Rates. Ann. Plast. Surg. 2016;76:270–275. doi: 10.1097/SAP.0000000000000561. [DOI] [PubMed] [Google Scholar]
- 53.Moltó García R., González Alonso V., Villaverde Doménech M.E. Fat grafting in immediate breast reconstruction. Avoiding breast sequelae. Breast Cancer. 2016;23:134–140. doi: 10.1007/s12282-014-0541-3. [DOI] [PubMed] [Google Scholar]
- 54.Brenelli F., Rietjens M., De Lorenzi F., Pinto-Neto A., Rossetto F., Martella S., Rodrigues J.R., Barbalho D. Oncological safety of autologous fat grafting after breast conservative treatment: A prospective evaluation. Breast J. 2014;20:159–165. doi: 10.1111/tbj.12225. [DOI] [PubMed] [Google Scholar]
- 55.Ihrai T., Georgiou C., Machiavello J.-C., Chignon-Sicard B., Figl A., Raoust I., Bourgeon Y., Fouche Y., Flipo B. Autologous fat grafting and breast cancer recurrences: Retrospective analysis of a series of 100 procedures in 64 patients. J. Plast. Surg. Hand Surg. 2013;47:273–275. doi: 10.3109/2000656X.2012.759583. [DOI] [PubMed] [Google Scholar]
- 56.Riggio E., Bordoni D., Nava M.B. Oncologic surveillance of breast cancer patients after lipofilling. Aesthetic Plast. Surg. 2013;37:728–735. doi: 10.1007/s00266-013-0166-5. [DOI] [PubMed] [Google Scholar]
- 57.Semprini G., Cattin F., Vaienti L., Brizzolari M., Cedolini C., Parodi P.C. Oncoplastic surgery and cancer relapses: Cosmetic and oncological results in 489 patients. Breast. 2013;22:946–951. doi: 10.1016/j.breast.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 58.Sarfati I., Ihrai T., Kaufman G., Nos C., Clough K.B. Adipose-tissue grafting to the post-mastectomy irradiated chest wall: Preparing the ground for implant reconstruction. J. Plast. Reconstr. Aesthetic Surg. 2011;64:1161–1166. doi: 10.1016/j.bjps.2011.03.031. [DOI] [PubMed] [Google Scholar]
- 59.Petit J.Y., Lohsiriwat V., Clough K.B., Sarfati I., Ihrai T., Rietjens M., Veronesi P., Rossetto F., Scevola A., Delay E. The oncologic outcome and immediate surgical complications of lipofilling in breast cancer patients: A multicenter study-Milan-Paris-Lyon experience of 646 lipofilling procedures. Plast. Reconstr. Surg. 2011;128:341–346. doi: 10.1097/PRS.0b013e31821e713c. Erratum in Plast. Reconstr. Surg. 2011, 128, 1317. [DOI] [PubMed] [Google Scholar]
- 60.Rigotti G., Marchi A., Stringhini P., Baroni G., Galiè M., Molino A.M., Mercanti A., Micciolo R., Sbarbati A. Determining the oncological risk of autologous lipoaspirate grafting for post-mastectomy breast reconstruction. Aesthetic Plast. Surg. 2010;34:475–480. doi: 10.1007/s00266-010-9481-2. [DOI] [PubMed] [Google Scholar]
- 61.Rietjens M., De Lorenzi F., Rossetto F., Brenelli F., Manconi A., Martella S., Intra M., Venturino M., Lohsiriwat V., Ahmed Y., et al. Safety of fat grafting in secondary breast reconstruction after cancer. J. Plast. Reconstr. Aesthetic Surg. 2011;64:477–483. doi: 10.1016/j.bjps.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 62.Delaporte T., Delay E., Toussoun G., Delbaere M., Sinna R. Reconstruction mammaire par transfert graisseux exclusif: À propos de 15 cas consécutifs [Breast volume reconstruction by lipomodeling technique: About 15 consecutive cases] Ann. Chir. Plast. Esthét. 2009;54:303–316. doi: 10.1016/j.anplas.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 63.van Turnhout A.A., Fuchs S., Lisabeth-Broné K., Vriens-Nieuwenhuis E.J.C., van der Sluis W.B. Surgical Outcome and Cosmetic Results of Autologous Fat Grafting After Breast Conserving Surgery and Radiotherapy for Breast Cancer: A Retrospective Cohort Study of 222 Fat Grafting Sessions in 109 Patients. Aesthetic Plast. Surg. 2017;41:1334–1341. doi: 10.1007/s00266-017-0946-4. [DOI] [PubMed] [Google Scholar]
- 64.Delay E., Gosset J., Toussoun G., Delaporte T., Delbaere M. Efficacité du lipomodelage pour la correction des séquelles du traitement conservateur du cancer du sein [Efficacy of lipomodelling for the management of sequelae of breast cancer conservative treatment] Ann. Chir. Plast. Esthét. 2008;53:153–168. doi: 10.1016/j.anplas.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 65.Pearl R.A., Leedham S.J., Pacifico M.D. The safety of autologous fat transfer in breast cancer: Lessons from stem cell biology. J. Plast. Reconstr. Aesthetic Surg. 2012;65:283–288. doi: 10.1016/j.bjps.2011.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Perrot P., Rousseau J., Bouffaut A.-L., Rédini F., Cassagnau E., Deschaseaux F., Heymann M.-F., Heymann D., Duteille F., Trichet V., et al. Safety concern between autologous fat graft, mesenchymal stem cell and osteosarcoma recurrence. PLoS ONE. 2010;5:e10999. doi: 10.1371/journal.pone.0010999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Karnoub A.E., Weinberg R.A. Chemokine networks and breast cancer metastasis. Breast Dis. 2006;26:75–85. doi: 10.3233/BD-2007-26107. [DOI] [PubMed] [Google Scholar]
- 68.Yu J.L., Rak J.W. Host microenvironment in breast cancer development: Inflammatory and immune cells in tumour angiogenesis and arteriogenesis. Breast Cancer Res. 2003;5:83–88. doi: 10.1186/bcr573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Müller A., Homey B., Soto H., Ge N., Catron D., Buchanan M.E., McClanahan T., Murphy E., Yuan W., Wagner S.N., et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 70.Dvorak H.F. Tumors: Wounds that do not heal. Similarities between tumor stroma generation and wound healing. N. Engl. J. Med. 1986;315:1650.e9. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 71.Fat Transfer/Fat Graft and Fat Injection ASPS Guiding Principles. [(accessed on 26 May 2024)]. Available online: www.plasticsurgery.org.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.