Abstract
Background:
Pig-tailed macaques (PTMs) are commonly used as preclinical models to assess antiretroviral drugs for HIV prevention research. Drug toxicities and disease pathologies are often preceded by changes in blood hematology. To better assess the safety profile of pharmaceuticals, we defined normal ranges of hematological values in PTMs using an Isolation Forest (iForest) algorithm.
Methods:
Eighteen female PTMs were evaluated. Blood was collected 1–24 times per animal for a total of 159 samples. Complete blood counts were performed, and iForest was used to analyze the hematology data to detect outliers.
Results:
Median, IQR, and ranges were calculated for 13 hematology parameters. From all samples, 22 outliers were detected. These outliers were excluded from the reference index.
Conclusions:
Using iForest, we defined a normal range for hematology parameters in female PTMs. This reference index can be a valuable tool for future studies evaluating drug toxicities in PTMs.
Keywords: complete blood counts, drug toxicities, non-human primates
1 |. INTRODUCTION
Macaque models are critical for evaluating pre-exposure prophylaxis (PrEP) modalities for preventing human immunodeficiency virus (HIV) infections. These models have provided proof-of-concept research for all currently approved PrEP regimens in humans, including daily oral emtricitabine (FTC)/tenofovir disoproxil fumarate, FTC/tenofovir alafenamide, and long-acting cabotegravir for PrEP.1–3 The translatability of macaque models for studying HIV pathogenesis and prevention is in part due to the close phylogeny and physiology shared between macaques and humans. In addition, simian immunodeficiency virus (SIV) and simian/human immunodeficiency virus (SHIV) infections closely mimic HIV infection and pathogenesis, including the depletion of systemic and mucosal CD4+ T cells, chronic immune activation, and progression to simian acquired immunodeficiency syndrome.4–8
Pig-tailed macaques (PTMs) (Macaca nemestrina) are the preferred species for modeling vaginal HIV infection due to shared features with the human female reproductive tract, and unlike rhesus macaques (Macaca mulatta), which are seasonal breeders, female PTMs experience a lunar menstrual cycle.9,10 Hormonal fluctuations associated with the menstrual cycle have been shown to impact SIV/SHIV susceptibility, which should be carefully considered when evaluating the efficacy of prevention products.11,12 In addition, the PTM model has been refined to better mimic high-risk sexual transmission using repeated low-dose vaginal SHIV exposures and co-infections with other sexually transmitted infections.13–15 The combination of extensive historical data and continued refinements make the PTM model extremely valuable for assessing the PrEP efficacy of novel HIV prevention products.16,17
Safety and efficacy evaluations are priorities in preclinical studies, and as long-acting PrEP products become more prevalent, these studies can require lengthy assessments. Blood hematology is a common gauge of safety, particularly as it relates to drug toxicities and disease pathologies, which are often preceded by changes in complete blood counts (CBCs). As such, animal research facilities monitor the CBCs of animals to appraise health and aid illness diagnosis. Previous studies have reported baseline blood hematology values for rhesus macaques, Japanese macaques (Macaca fuscata), cynomolgus monkeys (Macaca fascicularis), and northern PTMs (Macaca leonina).18–22 However, published hematology information for southern PTMs (Macaca nemestrina) is limited to wild-caught or infant animals.23,24 One recent study published values for a single adult PTM, but this analysis was limited to white blood cells (WBC) and did not include subsets such as lymphocytes (LY), monocytes (MO), or granulocytes (GR).25
Thus, we aimed to characterize the hematology of female PTMs, as these animals are important for preclinical HIV research, and defining baseline ranges for healthy animals is imperative for understanding study outcomes. To create a reference index of hematology parameters, we evaluated a large sample set consisting of 159 CBCs from 18 female PTMs. We identified statistical outliers in our data set using the Isolation Forest (iForest) model, which is an unsupervised algorithm that builds an ensemble of decision trees (iTrees) that repeatedly segment data using randomly selected features (i.e. hematology parameters) and split values.26 The assumption is that outliers will be isolated in fewer segmentations compared to normal data points. Using the iForest data science method, we have established a reference index of blood hematology parameters in female PTMs. This reference will be an asset for any preclinical research studies involving PTMs, specifically for those evaluating drug toxicities and other disease pathologies.
2 |. MATERIALS AND METHODS
2.1 |. Humane care guidelines
The authors confirm that the ethical policies of the journal, as noted on the journal’s author guidelines page, have been adhered to and the appropriate ethical review committee approval has been received. This research was conducted under a Centers for Disease Control and Prevention Institutional Animal Care and Use Committee-approved protocol in compliance with the Animal Welfare Act, PHS Policy, and other federal statutes and regulations relating to the use of animals in research. Animals were housed in an AAALAC International accredited facility that adheres to principles stated in the Guide for the Care and Use of Laboratory Animals policy.27
2.2 |. Study animals
Eighteen female PTMs with a median [range] age of 9 [7–17] years and a median [range] weight of 6.72 [5.65–12.05] kg were evaluated. Animals were not undergoing any clinical or protocol-related treatments with antiretrovirals or other drugs except for sedatives required for procedures. Blood was collected between 1 and 24 times per individual animal for a total of 159 samples. All macaques were purpose-bred and confirmed negative against Mycobacterium tuberculosis, Trichuris spp., Shigella, Campylobacter, Salmonella, Yersinia, simian retroviruses (SRVs), SIV, and simian T-lymphotropic viruses by the vendors. The macaques were not screened for herpes B virus.
2.3 |. Sample blood collection
Before blood collections, animals were sedated with Ketamine (10 mg/kg; Dechra Veterinary Products) administered intramuscularly and if needed, were boosted with Telazol (2–6 mg/kg; Zoetis) administered intramuscularly. Approximately 1 cc of femoral venous blood was drawn into BD Vacutainer® EDTA Tubes, using ethylenediamine tetra acetic acid-potassium (EDTA-K2) as the anti-coagulant.
2.4 |. Complete blood count
Whole blood samples were inverted eight times immediately before analysis. CBCs were performed with the Beckman Coulter AcT diff2 Hematology Analyzer in the ‘closed vial whole blood’ mode. Hematology parameters analyzed included WBC, (103/μL), LY, (103/μL), MO, (103/μL), GR, (103/μL), red blood cells (RBC, 106/μL), hemoglobin (HGB, g/dL), hematocrit (HCT, %), mean corpuscular volume (MCV, fL), mean corpuscular hemoglobin (MCH, pg), mean corpuscular hemoglobin concentration (MCHC, g/dL), red cell distribution width (RDW, %), platelets (Plt, 103/μL), and mean platelet volume (MPV, fL).
2.5 |. Statistical analyses
Outliers were detected using the iForest model, implementing the scikit-learn package (v1.0.2) in Python (v3.9.13).28 This algorithm builds an ensemble of decision trees, called iTrees, which consist of nodes and branches. The n_estimators parameter was tuned by identifying the point of convergence (n_estimators = 1000) in plots of the average path lengths against the number of iTrees for randomly selected sets of data points. The max_features parameter was chosen to equal the total number of hematology parameters (max_features = 13). Histograms of anomaly scores were examined to assess the presence of outliers, and the ‘auto’ contamination parameter was chosen. All statistical analyses were conducted in Python (v3.9.13) and R (v4.3.1).
3 |. RESULTS
3.1 |. Complete blood counts
A total of 159 CBCs were analyzed from a cohort of 18 female PTMs. As outlined in Table 1, blood was collected between 1 and 24 times from each animal, with a median [range] of 5 [1–24] collections per individual. Macaques had blood collected weekly for up to 24 weeks, with differing number of collections based on animal allocation for other study needs. Thirteen hematology parameters were analyzed.
TABLE 1.
Sampling frequency and the number of outliers for each animal. One hundred and fifty-nine blood samples were collected from 18 female pig-tailed macaques for hematology analysis. The Isolation Forest algorithm was applied to detect statistical outliers.
3.2 |. Outlier detection
Outliers were detected using the iForest algorithm with the following settings: max features = 13, contamination = “auto”, and n_estimators = 1000. In total, 22 outliers from 10 of the 18 animals were detected from the initial 159 sample set (Table 1).
3.3 |. Blood hematology reference index
Each hematology parameter was plotted to visualize the effects of outlier removal on the distribution of the data set (Figure 1). As expected, the removal of outliers reduced the range, but the medians remained similar.
FIGURE 1.
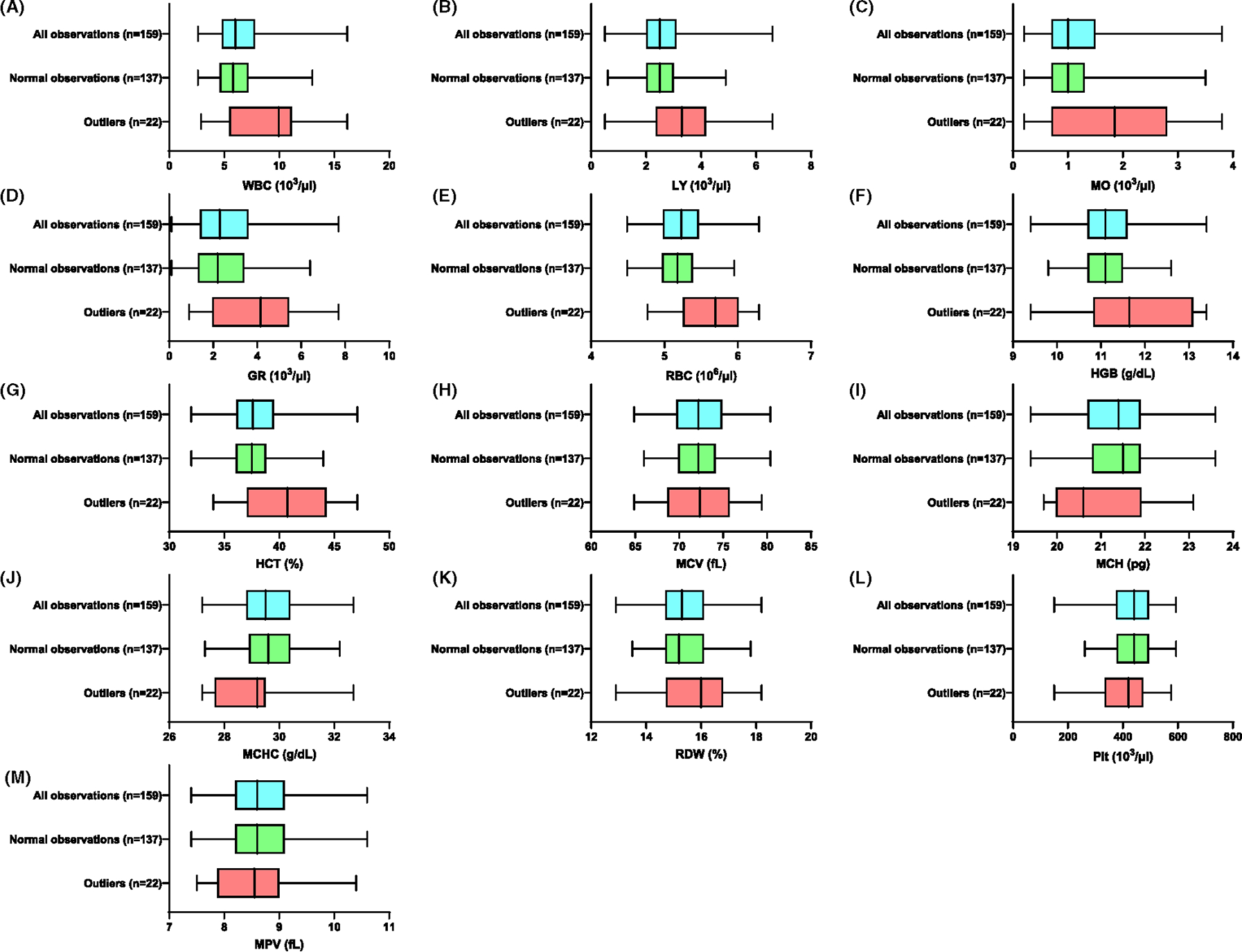
Distribution of hematology parameters. The data distribution of all observations (blue), normal observations (green), and outliers (red) shown for (A) white blood cells (WBC), (B) lymphocytes (LY), (C) monocytes (MO), (D) granulocytes (GR), (E) red blood cells (RBC), (F) hemoglobin (HGB), (G) hematocrit (HCT), (H) mean corpuscular volume (MCV), (I) mean corpuscular hemoglobin (MCH), (J) mean corpuscular hemoglobin concentration (MCHC), (K) red cell distribution width (RDW), (L) platelets (Plt), and (M) mean platelet volume (MPV). The box and whiskers of the plot represent the interquartile range (IQR), minimum, and maximum. The line in the box represents the median.
Age and weight are important factors to consider when allocating animals to research study groups.29 Therefore, we sought to understand if age and weight are determinants of hematological outliers. The median [range] age of animals that had outliers (n = 22) was 16.27 [6.63–17.31] years while the median [range] age of animals that did not have outliers (n = 137) was 8.67 [6.75–17.35] years. For weight, the median [range] of animals that had outliers (n = 22) was 7.19 [6.01–12.05] kg while the median [range] weight of animals that did not have outliers (n = 137) was 6.68 [5.65–10.63] kg (Figure 2).
FIGURE 2.
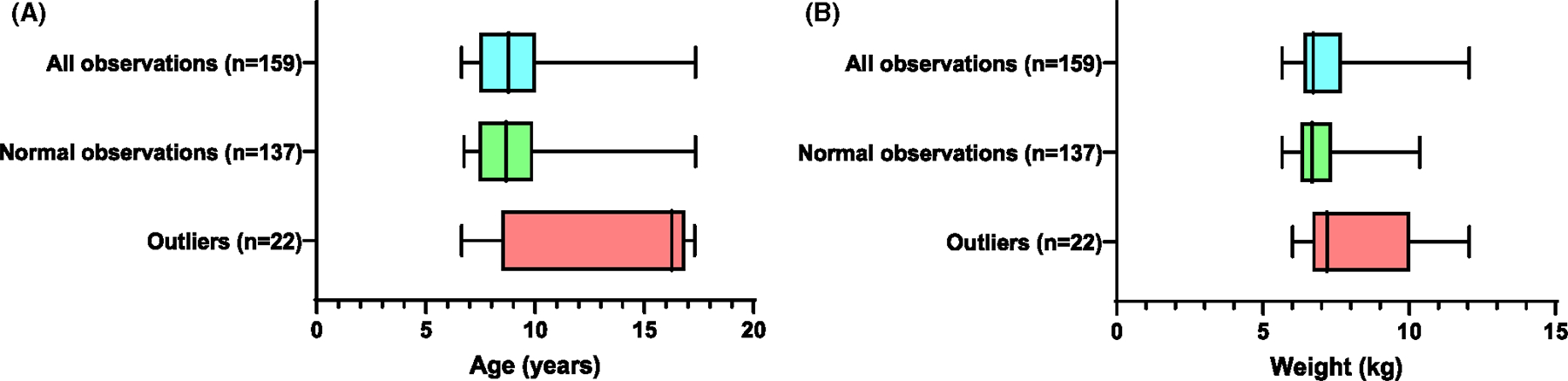
Macaque age and weight distribution. The data distribution of all observations (blue), normal observations (green), and outliers (red) is shown for (A) age and (B) weight. The box and whiskers plots show the interquartile range (IQR) and the minimum and maximum. The line in the box represents the median.
After outliers were excluded from the data set, four PTMs from the initial 18 were completely excluded from further analysis (Table 1: BB125, Z15211, Z14140, and Z15336). A reference index of 13 blood hematology parameters was then created using 137 CBCs from 14 PTMs, with a median [range] of 7 [1–24] collections per animal (Table 2).
TABLE 2.
Reference values of blood hematology parameters in female pig-tailed macaques. Median [range] listed for the 13 blood hematology parameters. Outliers defined by iForest were excluded.
| Parameter (abbreviation, units) | Median [range] (n = 137) |
|---|---|
| White blood cells (WBC, 103/μL) | 5.80 [2.60–13.00] |
| Lymphocytes (LY, 103/μL) | 2.50 [0.60–4.90] |
| Monocytes (MO, 103/μL) | 1.00 [0.20–3.50] |
| Granulocytes (GR, 103/μL) | 2.20 [0.10–6.40] |
| Red blood cells (RBC, 106/μL) | 5.18 [4.49–5.95] |
| Hemoglobin (HGB, g/dL) | 11.10 [9.80–12.60] |
| Hematocrit (HCT, %) | 37.50 [32.00–44.00] |
| Mean corpuscular volume (MCV, fL) | 72.20 [66.00–80.40] |
| Mean corpuscular hemoglobin (MCH, pg) | 21.50 [19.40–23.60] |
| Mean corpuscular hemoglobin concentration (MCHC, g/dL) | 29.60 [27.30–32.20] |
| Red cell distribution width (RDW, %) | 15.20 [13.50–17.80] |
| Platelets (Plt, 103/μL) | 441.00 [261.00–593.00] |
| Mean platelet volume (MPV, fL) | 8.60 [7.40–10.60] |
4 |. DISCUSSION
Here, we report a reference index of blood hematology parameters in female PTMs, an important animal model for HIV research. These values provide an understanding of natural and expected variations in PTM hematology and can serve as a baseline for future studies evaluating drug safety and toxicities. The importance of these reference values was recently highlighted when clinical trials with the anti-HIV drug islatravir (ISL) were discontinued by the FDA after observing lymphopenia in the participants.30 Before its clinical hold, ISL was an attractive candidate for long-acting PrEP due to its high antiviral potency, long half-life, and predicted efficacy in macaque models.31,32 Unfortunately, the effect of ISL on LY was not fully investigated during early preclinical development. By providing an index of PTM reference values, our study may help to understand if the PTM model could detect lymphocyte toxicity due to ISL or other novel anti-HIV drugs prior to clinical advancement.
Hematology reference values have been reported for rhesus macaques (Macaca mulatta),18,19 Japanese macaques (Macaca fuscata),20 and cynomolgus macaques (Macaca fascicularis).21 Comparisons of their CBC values indicate many similarities among species and captivity status. However, there are some differences, such as WBCs, which were 2-fold greater in rhesus, Japanese, and cynomolgus macaques compared to PTMs. LYs and GRs were also 2-fold greater in rhesus and Japanese macaques than in PTMs. Interestingly, MO values were similar across all the studies. However, these variations may also have resulted from differences in the hematology analyzer used or other factors such as sedation and captivity status, making direct comparisons across studies difficult. Despite these factors, it is evident that the hematology of each species may have distinct and unpredictable characteristics, confirming the need for a reference index specific to PTMs.
For nonhuman primate research, the Animal Research: Reporting of In Vivo Experiments guidelines are followed when reporting study groups and results. During preclinical assessments of PrEP and other HIV treatments, these study groups are often randomized according to factors such as animal sex, age, and weight to control for innate differences in drug safety, pharmacokinetics, and efficacy.33–35 Thus, we sought to determine if age and/or weight impacted the likelihood an animal would have an outlier value in their hematology. The median age and weight of the outliers were higher than those of non-outliers, indicating that the majority of outliers were among the oldest and heaviest animals in our cohort, although their IQRs had some overlap with normal observations. This data reinforces the importance of age and weight-matched randomization for macaque research. Aging animals also have reduced regularity of hormonal cycles and low progesterone levels.36,37 We did not assess the menstrual cycle, but future studies evaluating the hematology in cycling female macaques could be beneficial to understand the effect of age and cycle phase on SHIV susceptibility.
To create a comprehensive reference index from this large data set, we employed the iForest algorithm to detect outliers in place of classic statistical methods. We considered several other outlier detection algorithms, including Mahalanobis distance and one-class Support Vector Machine (SVM). The Mahalanobis distance method can identify outliers in multivariate normal data sets.38 However, 10 of the hematology features were not normally distributed, and thus, parametric methods such as Mahalanobis distance were not appropriate. One-class SVM is a non-parametric test that is appropriate for multivariate data. However, the iForest method was selected over the one-class SVM due to faster run times and reduced sensitivity to outliers, which enabled the model to be trained on contaminated data (data containing both normal and outlier data points). Additionally, the iForest method has several adjustable model parameters including contamination, max features, and n_estimators.
In this study, we analyzed 159 CBCs from 18 female PTMs, with a median of five blood collections per animal. One limitation of this data set is that each animal had different sampling frequencies, which may create a bias towards animals that were sampled more often. However, longitudinal sampling is also imperative in understanding how factors such as weight gain/loss, and aging can alter intra-animal variability. Additionally, other factors, such as infection, may induce immune responses that can cause significant changes in hematology. In this study, all animals were confirmed to be free of Mycobacterium tuberculosis, Trichuris, Shigella, Campylobacter, Salmonella, Yersinia, and SRVs through initial and routine health screenings. However, macaques were not screened for ubiquitous pathogens such as Herpes B virus or other active infections that have the potential to alter CBCs.39 Sedative medications and the frequency of sedation can also affect hematology. Previous studies have observed decreases in LY, HGB, HCT, and other parameters in rhesus macaques following sedations.40 All blood samples in this study were collected from anesthetized animals, providing consistency within the study samples tested. However, the effects of sedation should be considered when comparing these results to other studies that do not sedate animals for blood collection. Our results were obtained from the analysis of whole blood collected in K2 EDTA BD Vacutainer® EDTA Tubes using the Beckman Coulter AcT diff2 Hematology Analyzer.41 Other analyzers and alternative blood diluents may yield varied results. Lastly, another potential limitation of our study was the use of the iForest which has a tunable contamination parameter. This function requires a prior estimation of the percentage of outliers in the data. Due to the paucity of historical data on which to base these assumptions, the automatic estimation value was instead chosen for outlier analysis.
In conclusion, we defined a reference index of 13 hematology parameters from 137 CBCs from 14 female PTMs, using an iForest algorithm to detect and remove outliers from the total data set. After removing outliers, we characterized the expected values and ranges for each hematology parameter. This established reference index will be useful for monitoring the health of PTMs and will provide important baseline data for future studies that utilize this animal model for preclinical research.
ACKNOWLEDGMENTS
This project was supported in part by an appointment to the Research Participation Program at the Centers for Disease Control and Prevention administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and the Centers for Disease Control and Prevention. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
FUNDING INFORMATION
This research was supported by CDC intramural funds.
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors declared no conflict of interest with respect to the research, authorship, or publication of this article.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon request.
REFERENCES
- 1.Subbarao S, Otten RA, Ramos A, et al. Chemoprophylaxis with tenofovir disoproxil fumarate provided partial protection against infection with simian human immunodeficiency virus in macaques given multiple virus challenges. J Infect Dis. 2006;194(7):904–911. [DOI] [PubMed] [Google Scholar]
- 2.Bekerman E, Cox S, Babusis D, et al. Two-dose emtricitabine/tenofovir alafenamide plus bictegravir prophylaxis protects macaques against SHIV infection. J Antimicrob Chemother. 2021;76(3):692–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrews CD, Yueh YL, Spreen WR, et al. A long-acting integrase inhibitor protects female macaques from repeated high-dose intravaginal SHIV challenge. Sci Transl Med. 2015;7(270):270ra4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson PR, Hirsch VM. SIV infection of macaques as a model for AIDS pathogenesis. Int Rev Immunol. 1992;8(1):55–63. [DOI] [PubMed] [Google Scholar]
- 5.Hatziioannou T, Evans DT. Animal models for HIV/AIDS research. Nat Rev Microbiol. 2012;10(12):852–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Veazey RS, DeMaria M, Chalifoux LV, et al. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280(5362):427–431. [DOI] [PubMed] [Google Scholar]
- 7.Spira AI, Marx PA, Patterson BK, et al. Cellular targets of infection and route of viral dissemination after an intravaginal inoculation of simian immunodeficiency virus into rhesus macaques. J Exp Med. 1996;183(1):215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mansfield KG, Lerch NW, Gardner MB, Lackner AA. Origins of simian immunodeficiency virus infection in macaques at the New England Regional Primate Research Center. J Med Primatol. 1995;24(3):116–122. [DOI] [PubMed] [Google Scholar]
- 9.Patton DL, Sweeney YT, Paul KJ. A summary of preclinical topical microbicide rectal safety and efficacy evaluations in a pigtailed macaque model. Sex Transm Dis. 2009;36(6):350–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blakley GB, Beamer TW, Dukelow WR. Characteristics of the menstrual cycle in nonhuman primates. IV. Timed mating in Macaca nemestrina. Lab Anim. 1981;15(4):351–353. [DOI] [PubMed] [Google Scholar]
- 11.Kersh EN, Henning T, Vishwanathan SA, et al. SHIV susceptibility changes during the menstrual cycle of pigtail macaques. J Med Primatol. 2014;43(5):310–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vishwanathan SA, Guenthner PC, Lin CY, et al. High susceptibility to repeated, low-dose, vaginal SHIV exposure late in the luteal phase of the menstrual cycle of pigtail macaques. J Acquir Immune Defic Syndr. 2011;57(4):261–264. [DOI] [PubMed] [Google Scholar]
- 13.Kim CN, Adams DR, Bashirian S, Butera S, Folks TM, Otten RA. Repetitive exposures with simian/human immunodeficiency viruses: strategy to study HIV pre-clinical interventions in non-human primates. J Med Primatol. 2006;35(4–5):210–216. [DOI] [PubMed] [Google Scholar]
- 14.Vishwanathan SA, Aubert RD, Morris MR, et al. A macaque model for rectal lymphogranuloma Venereum and non-lymphogranuloma Venereum Chlamydia trachomatis: impact on rectal simian/human immunodeficiency virus acquisition. Sex Transm Dis. 2017;44(9):551–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vishwanathan SA, Zhao C, Luthra R, et al. Sexually transmitted infections and depot medroxyprogesterone acetate do not impact protection from simian HIV acquisition by long-acting cabotegravir in macaques. AIDS. 2022;36(2):169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parikh UM, Dobard C, Sharma S, et al. Complete protection from repeated vaginal simian-human immunodeficiency virus exposures in macaques by a topical gel containing tenofovir alone or with emtricitabine. J Virol. 2009;83(20):10358–10365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dobard CW, Peet MM, Nishiura K, et al. Single dose topical inserts containing tenofovir alafenamide fumarate and elvitegravir provide pre- and post-exposure protection against vaginal SHIV infection in macaques. EBioMedicine. 2022;86:104361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Y, Qin S, Ding Y, et al. Reference values of clinical chemistry and hematology parameters in rhesus monkeys (Macaca mulatta). Xenotransplantation. 2009;16(6):496–501. [DOI] [PubMed] [Google Scholar]
- 19.Shah NA, Bhatt LK, Patel RJ, et al. Hematological and biochemical reference intervals of wild-caught and inhouse adult Indian rhesus macaques (Macaca mulatta). Lab Anim Res. 2022;38(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miyabe-Nishiwaki T, MacIntosh AJJ, Kaneko A, et al. Hematological and blood chemistry values in captive Japanese macaques (Macaca fuscata fuscata). J Med Primatol. 2019;48(6):338–350. [DOI] [PubMed] [Google Scholar]
- 21.Schuurman HJ, Smith HT. Reference values for clinical chemistry and clinical hematology parameters in cynomolgus monkeys. Xenotransplantation. 2005;12(1):72–75. [DOI] [PubMed] [Google Scholar]
- 22.Pang W, Lu LB, Wang Y, et al. Measurement and analysis of hematology and blood chemistry parameters in northern pig-tailed macaques (Macaca leonina). Dongwuxue Yanjiu. 2013;34(2):89–96. [DOI] [PubMed] [Google Scholar]
- 23.Rahlmann DF, Pace N, Barnstein NJ. Hematology of the pig-tailed monkey, Macaca nemestrina. Folia Primatol. 1967;5(4):280–284. [Google Scholar]
- 24.Rainwater E. Hematology and serum chemistry reference values for pigtailed macaque (Macaca nemestrina) infants. In: Sackett GP, Ruppentahal GC, Elias K, eds. Nursery Rearing of Nonhuman Primates in the 21st Century. Springer New York; 2006:583–591. [Google Scholar]
- 25.Syahbani N, Supiyani A, Rosmanah L. Evaluation of the haematology profile and blood chemistry of Macaca Nemestrina (Linnaeus, 1766) at primate research center IPB University. Indonesian J Primatol. 2023;2(1):1–12. [Google Scholar]
- 26.Liu FT, Kai MT, Zhou Z. Isolation-based anomaly detection. ACM Trans Knowl Discov Data. 2012;6:1. [Google Scholar]
- 27.National Research Council (US). Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals. 8th ed. National Academies Press (US); 2011. [PubMed] [Google Scholar]
- 28.Sklearn.Ensemble.IsolationForest Scikit-Learn. 2023. Accessed August 29, 2023. https://scikit-learn.org/stable/modules/generated/sklearn.ensemble.IsolationForest.html [Google Scholar]
- 29.Percie du Sert N, Ahluwalia A, Alam S, et al. Reporting animal research: explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol. 2020;18(7):e3000411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Merck and Co., Inc. Merck Announces Clinical Holds on Studies Evaluating Islatravir for the Treatment and Prevention of HIV-1 Infection 2021. Accessed January 12, 2023. https://www.merck.com/news/merck-announces-clinical-holds-on-studies-evaluating-islatravir-for-the-treatment-and-prevention-of-hiv-1-infection/
- 31.Markowitz M, Gettie A, St Bernard L, et al. Once-weekly oral dosing of MK-8591 protects male rhesus macaques from intrarectal challenge with SHIV109CP3. J Infect Dis. 2020;221(9):1398–1406. [DOI] [PubMed] [Google Scholar]
- 32.Barrett SE, Teller RS, Forster SP, et al. Extended-duration MK-8591-eluting implant as a candidate for HIV treatment and prevention. Antimicrob Agents Chemother. 2018;62(10):e01058–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taves DR. Minimization: a new method of assigning patients to treatment and control groups. Clin Pharmacol Ther. 1974;15(5):443–453. [DOI] [PubMed] [Google Scholar]
- 34.Saint-Mont U Randomization does not help much, comparability does. PLoS One. 2015;10(7):e0132102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laajala TD, Jumppanen M, Huhtaniemi R, et al. Optimized design and analysis of preclinical intervention studies in vivo. Sci Rep. 2016;6:30723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hedayat KM, Lapraz J. Regulation of the menstrual cycles. The Theory of Endobiogeny. Academic Press; 2019:69–87. [Google Scholar]
- 37.Greendale GA, Lee NP, Arriola ER. The menopause. Lancet. 1999;353(9152):571–580. [DOI] [PubMed] [Google Scholar]
- 38.Ghorbani H Mahalanobis distance and its application for detecting multivariate outliers. Facta Universitatis Series Mathematics and Informatics. 2019;34(3):583–595. [Google Scholar]
- 39.Ohta E Pathologic characteristics of infectious diseases in macaque monkeys used in biomedical and toxicologic studies. J Toxicol Pathol. 2023;36(2):95–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bennett JS, Gossett KA, McCarthy MP, Simpson ED. Effects of ketamine hydrochloride on serum biochemical and hematologic variables in rhesus monkeys (Macaca mulatta). Vet Clin Pathol. 1992;21(1):15–18. [DOI] [PubMed] [Google Scholar]
- 41.Beckman Coulter Inc. Coulter®AC•T diff 2™ Analyzer 2010. Accessed December 13, 2023. https://www.beckmancoulter.com/download/file/wsr-32905/4237515BA?type=pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon request.


