Abstract
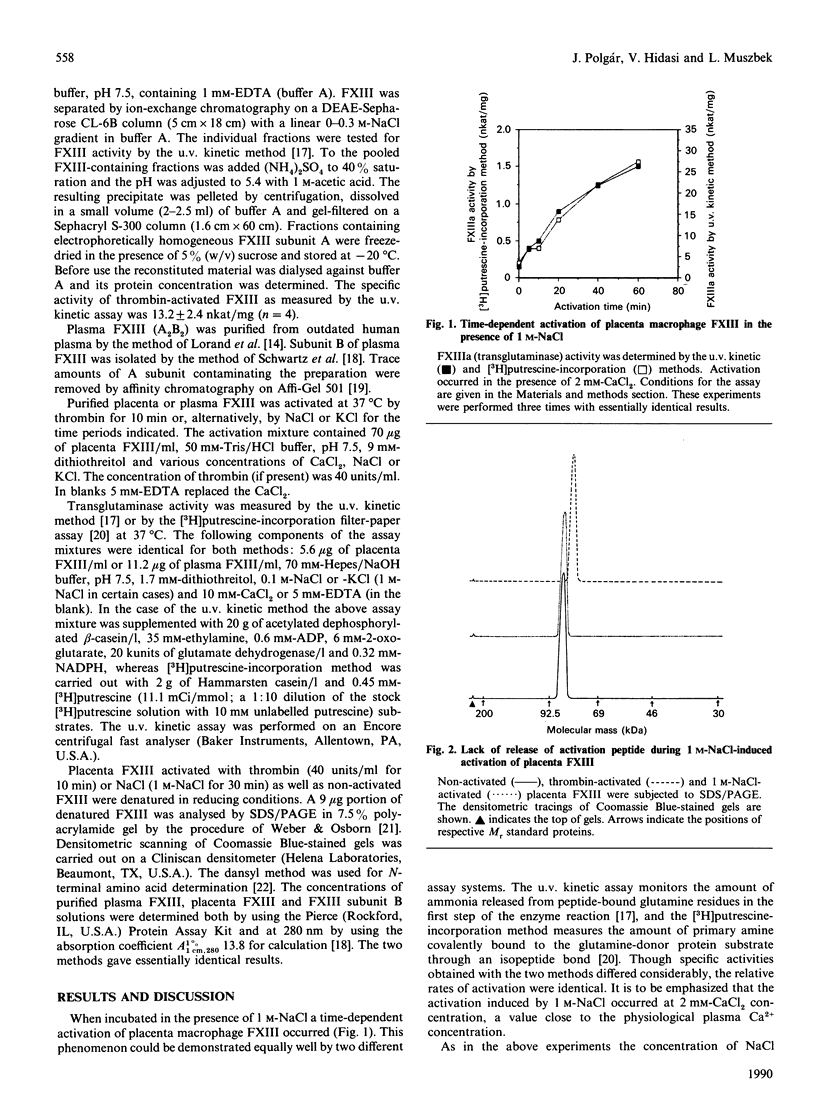
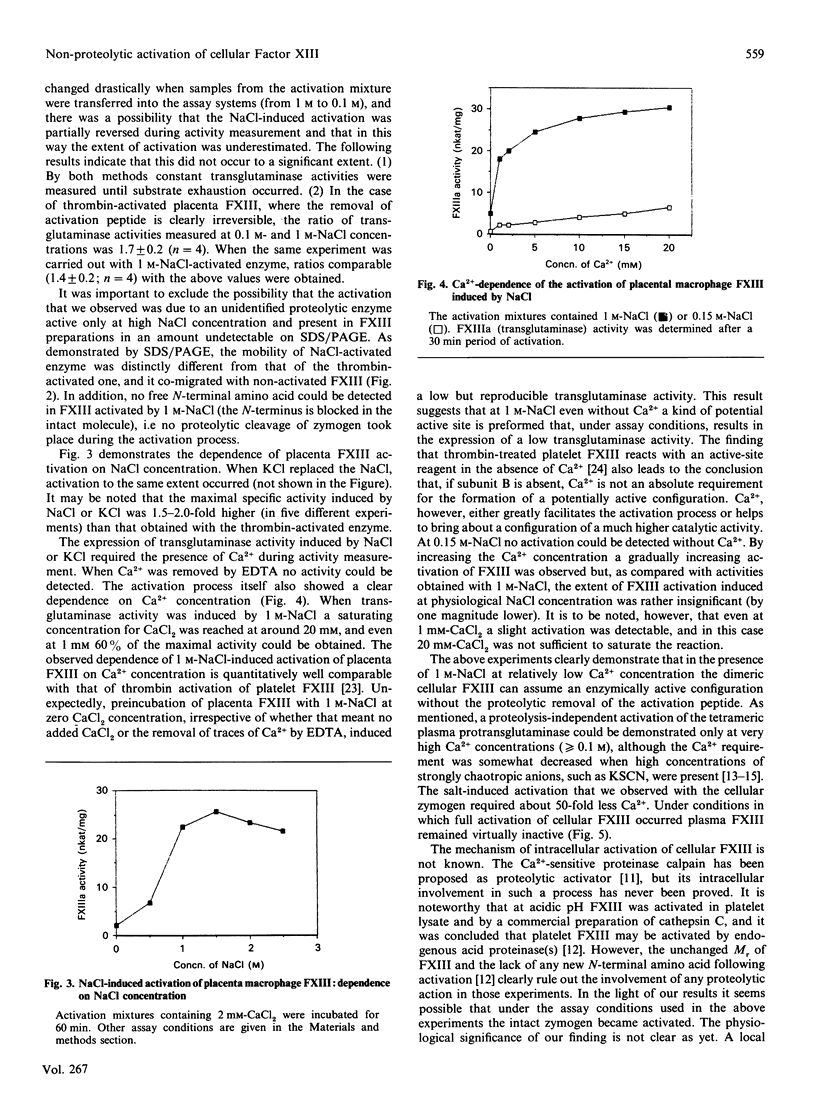
Plasma Factor XIII is a zymogen (plasma protransglutaminase) with the tetrametric structure A2B2, whereas the cellular protransglutaminase, i.e. Factor XIII in the platelet and monocyte/macrophage, consists exclusively of A subunits (A2). It is generally accepted that at Ca2+ concentrations comparable with that in plasma the proteolytic removal of an N-terminal activation peptide is the prerequisite for the Ca2(+)-induced formation of a catalytically active configuration of subunit A. In this study it was demonstrated that at high concentrations NaCl or KCl induced a non-proteolytic activation of cellular (placental macrophage) but not plasma protransglutaminase. The activation depended on time and salt concentration, and Ca2+, in the range 0-20 mM, greatly enhanced the activation process. At 1.25 M-NaCl maximal activation occurred within 60 min in the presence of 2 mM-CaCl2, and even at physiological NaCl concentration a slow progressive activation could be observed in the presence of Ca2+. The specific activity of salt-activated Factor XIII was 1.5-2.0-fold higher than that obtained after thrombin activation. The non-proteolytic activation of cellular protransglutaminase was abolished by the addition of subunit B of plasma Factor XIII in stoichiometric amount, which suggests that (one of) the physiological function(s) of the B subunit in plasma Factor XIII is to prevent the slow spontaneous activation of A subunit that would occur in a plasmatic environment.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adány R., Belkin A., Vasilevskaya T., Muszbek L. Identification of blood coagulation factor XIII in human peritoneal macrophages. Eur J Cell Biol. 1985 Jul;38(1):171–173. [PubMed] [Google Scholar]
- Adány R., Glukhova M. A., Kabakov A. Y., Muszbek L. Characterisation of connective tissue cells containing factor XIII subunit a. J Clin Pathol. 1988 Jan;41(1):49–56. doi: 10.1136/jcp.41.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando Y., Imamura S., Yamagata Y., Kitahara A., Saji H., Murachi T., Kannagi R. Platelet factor XIII is activated by calpain. Biochem Biophys Res Commun. 1987 Apr 14;144(1):484–490. doi: 10.1016/s0006-291x(87)80535-1. [DOI] [PubMed] [Google Scholar]
- BULUK K. Nieznane działanie krwinek płytkowych. Pol Tyg Lek (Wars) 1955 Feb 7;10(6):191–191. [PubMed] [Google Scholar]
- Cooke R. D. Calcium-induced dissociation of human plasma factor XIII and the appearance of catalytic activity. Biochem J. 1974 Sep;141(3):683–691. doi: 10.1042/bj1410683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R. D., Holbrook J. J. Calcium and the assays of human plasma clotting factor XIII. Biochem J. 1974 Jul;141(1):71–78. doi: 10.1042/bj1410071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Credo R. B., Curtis C. G., Lorand L. Ca2+-related regulatory function of fibrinogen. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4234–4237. doi: 10.1073/pnas.75.9.4234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folk J. E., Chung S. I. Molecular and catalytic properties of transglutaminases. Adv Enzymol Relat Areas Mol Biol. 1973;38:109–191. doi: 10.1002/9780470122839.ch3. [DOI] [PubMed] [Google Scholar]
- Halkier T., Magnusson S. Contact activation of blood coagulation is inhibited by plasma factor XIII b-chain. Thromb Res. 1988 Aug 1;51(3):313–324. doi: 10.1016/0049-3848(88)90108-9. [DOI] [PubMed] [Google Scholar]
- Henriksson P., Becker S., Lynch G., McDonagh J. Identification of intracellular factor XIII in human monocytes and macrophages. J Clin Invest. 1985 Aug;76(2):528–534. doi: 10.1172/JCI112002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorand L. Activation of blood coagulation factor XIII. Ann N Y Acad Sci. 1986;485:144–158. doi: 10.1111/j.1749-6632.1986.tb34577.x. [DOI] [PubMed] [Google Scholar]
- Lorand L., Campbell-Wilkes L. K., Cooperstein L. A filter paper assay for transamidating enzymes using radioactive amine substrates. Anal Biochem. 1972 Dec;50(2):623–631. doi: 10.1016/0003-2697(72)90074-7. [DOI] [PubMed] [Google Scholar]
- Lorand L., Credo R. B., Janus T. J. Factor XIII (fibrin-stabilizing factor). Methods Enzymol. 1981;80(Pt 100):333–341. doi: 10.1016/s0076-6879(81)80029-8. [DOI] [PubMed] [Google Scholar]
- Lorand L., Losowsky M. S., Miloszewski K. J. Human factor XIII: fibrin-stabilizing factor. Prog Hemost Thromb. 1980;5:245–290. [PubMed] [Google Scholar]
- Lynch G. W., Pfueller S. L. Thrombin-independent activation of platelet factor XIII by endogenous platelet acid protease. Thromb Haemost. 1988 Jun 16;59(3):372–377. [PubMed] [Google Scholar]
- Mary A., Achyuthan K. E., Greenberg C. S. The binding of divalent metal ions to platelet factor XIII modulates its proteolysis by trypsin and thrombin. Arch Biochem Biophys. 1988 Feb 15;261(1):112–121. doi: 10.1016/0003-9861(88)90110-5. [DOI] [PubMed] [Google Scholar]
- Mary A., Achyuthan K. E., Greenberg C. S. b-chains prevent the proteolytic inactivation of the a-chains of plasma factor XIII. Biochim Biophys Acta. 1988 Sep 8;966(3):328–335. doi: 10.1016/0304-4165(88)90082-7. [DOI] [PubMed] [Google Scholar]
- Mcdonagh J., Waggoner W. G., Hamilton E. G., Hindenbach B., Mcdonagh R. P. Affinity chromatography of human plasma and platelet factor XIII on organomercurial agarose. Biochim Biophys Acta. 1976 Oct 28;446(2):345–357. doi: 10.1016/0005-2795(76)90002-7. [DOI] [PubMed] [Google Scholar]
- Muszbek L., Adány R., Kávai M., Boda Z., Lopaciuk S. Monocytes of patients congenitally deficient in plasma factor XIII lack factor XIII subunit a antigen and transglutaminase activity. Thromb Haemost. 1988 Apr 8;59(2):231–235. [PubMed] [Google Scholar]
- Muszbek L., Adány R., Szegedi G., Polgár J., Kávai M. Factor XIII of blood coagulation in human monocytes. Thromb Res. 1985 Feb 1;37(3):401–410. doi: 10.1016/0049-3848(85)90069-6. [DOI] [PubMed] [Google Scholar]
- Muszbek L., Polgár J., Fésüs L. Kinetic determination of blood coagulation Factor XIII in plasma. Clin Chem. 1985 Jan;31(1):35–40. [PubMed] [Google Scholar]
- Schwartz M. L., Pizzo S. V., Hill R. L., McKee P. A. Human Factor XIII from plasma and platelets. Molecular weights, subunit structures, proteolytic activation, and cross-linking of fibrinogen and fibrin. J Biol Chem. 1973 Feb 25;248(4):1395–1407. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]


