Abstract
Plant glucanases and chitinases are defense proteins that participate in pathogenesis; however, very little is known about the glucanase (GLUC) and chitinase (CHIT) gene families in mango. Some mango cultivars are of great economic importance and can be affected by anthracnose, a postharvest disease caused by fungi of the genus Colletotrichum spp. This study identified and characterized 23 putative glucanases and 16 chitinases in the mango genome cv. Tommy Atkins. We used phylogenetic analyses to classify the glucanases into three subclasses (A, B, and C) and the chitinases into four classes (I, II, IV, and V). Information on the salicylic, jasmonic acid, and ethylene pathways was obtained by analyzing the cis-elements of the GLUC and CHIT class I and IV gene promoters. The expression profile of GLUC, CHIT class I, and CHIT class IV genes in mango cv. Ataulfo inoculated with two Colletotrichum spp. revealed different profile expression related to these fungi’s level of virulence. In general, this study provides the basis for the functional validation of these target genes with which the regulatory mechanisms used by glucanases and chitinases as defense proteins in mango can be elucidated.
Keywords: Colletotrichum asianum, Colletotrichum siamense, chitinases, glucanases, mango, Ataulfo, genes
1. Introduction
Mango (Mangifera indica L.) belongs to the Anacardiaceae family and is the most predominant tropical fruit produced worldwide [1,2]. However, the quality of mango pre- and postharvest can be affected by diseases, such as anthracnose, which is caused by at least seven cryptic species of the Colletotrichum gloeosporioides complex (C. siamense, C. asianum, C. tropicale, C. alienum, C. fructicola, C. chrysophilum, and C. queenslandicum) in Mexico [1,3,4]. C. asianum and C. siamense have the highest pathogenicity among mango cultivars. They have been reported in different parts of the world and classified as having high virulence [1,2,3,4,5,6,7,8,9]. In places with high relative humidity, the incidence of this disease can reach up to 100% in fruits [1,4].
Anthracnose affects terminal branches, inflorescences, leaves, and growing fruits [4,9]. This fungus can occur in latent form when the fruits are small, resuming its infection once physiological maturity is reached [10,11]. The infective cycle begins with establishing the conidium on the fruit’s surface; subsequently, the melanized appressorium penetrating the cuticle is formed, producing a latent subcuticular hypha. This hypha develops until the fruit matures and causes black, irregular, sunken lesions or spots that can cover a large part of the fruit [4,10]. Consequently, during postharvest storage, poor-quality fruits are generated, leading to high losses in the production and marketing of mango [2,10]. The varied responses of mango cultivars to this disease in different parts of the world have prevented plant resistance to this fungus and reduced the effectiveness of antifungal treatments [9]. This makes it necessary to search for effective alternatives to combat these phytopathogens by understanding the signaling and plant defense mechanisms. The differences generated during the development of symptoms in mango cultivars are associated with the relationship between plant immunity and pathogen recognition.
During the plant–pathogen interaction, the plant cell has two interaction mechanisms: pathogen-associated molecular pattern (PAMP)-triggered immunity (PTI) and effector-triggered immunity (ETI) [12]. PTI uses receptors that recognize PAMPs, and ETI recognizes effectors through resistance proteins [12,13]. Hormonal pathways are also activated by regulating and changing the concentration or sensitivity of salicylic acid (SA), jasmonic acid (JA), and ethylene (ET) [14]. These generate high expression in genes encoding defense proteins, such as those related to pathogenesis (PRs) [15]. PRs, such as glucanases (PR2) and chitinases (PR3, PR4, PR8, PR11), prevent infection and limit the activity of pathogenic agents [16,17,18].
The classification of β-glucanases and chitinases is based on their sequence of amino acids and common catalytic domains, as well as their distribution within the glycoside hydrolase (GH) families according to the carbohydrate-active enzymes (CAZy) database [19]. Depending on the type of glycoside bond they hydrolyze, there are β-1,4-glucanases, β-1,3-glucanases, and β-1,3-1,4-glucanases [20]. β-1,4-glucanases are divided into three subclasses (A, B, and C) of the GH9 family [20]. β-1,3-glucanases are classified into five groups (I, II, III, IV, and V) that are distinguished by being from the GH17 family [20]. Finally, β-1,3-1,4-glucanases have varied sequences and are shaped similar to the group V of the β-1,3-glucanases; they are also from the GH17 family [20]. Chitinases are classified by the similarity of the amino acid sequences and their catalytic domains, which is why they are divided into five classes (I–V) [18]. Classes III and V belong to the GH18 family, and classes I, II, and IV belong to the GH19 family [18].
Fungal cell walls are primarily made up of β-glucans and chitin, which are degraded by enzymes, such as β-glucanases and plant chitinases, as a defense mechanism against the pathogen [17,21,22]. To date, no studies have reported mango β-glucanases and chitinases involved in the response to the biotic stress caused by Colletotrichum spp.; only the expression of genes that respond to biotic stress stimulation has been analyzed. In mango fruits infected with C. gloeosporioides, the resistance induced by β-aminobutyric acid has been analyzed, and an increase in the activity of chitinase and glucanase enzymes has been demonstrated 2 days post-induction. Additionally, based on quantitative proteomics, 181 proteins differentially regulated by the fungus have been obtained, of which 40 increased in response to C. gloeosporioides [23]. Hong et al. [24] conducted a transcriptomic analysis in which they analyzed the peel of mango cv. Zill infected with C. gloeosporioides. One of their important findings was the overexpression of three genes encoding possible PRs such as protein FATTY ACID EXPORT 1, chloroplastic-like (comp22382_c0_seq1; GenBank: GBCV01028555), a mannan endo-1,4-beta-mannosidase 2-like (comp23077_c0_seq1; GenBank: GBCV01029236); and comp24098_c0_seq1 (GenBank: not found). However, these PR genes do not include glucanases or chitinases.
Although several mango genomes are available, we chose to work with the Tommy Atkins genome (https://mangobase.org, accessed on 4 January 2023), which has a helpful platform with annotations. The objectives of this study were to identify and characterize M. indica glucanases and chitinases, analyze phylogenetic relationships with orthologous proteins induced by fungi, and analyze the cis-elements of the promoter regions of these genes. In addition, we aimed to evaluate the gene expression profile of one glucanase and two chitinases in mango cv. Ataulfo infected with anthracnose (C. siamense and C. asianum) and to elucidate gene products that are part of the defense mechanism of the mango against Colletotrichum spp.
2. Results and Discussion
2.1. Classification and Characterization of Glucanases from M. indica
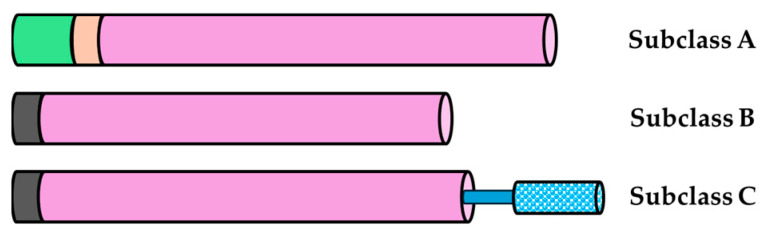
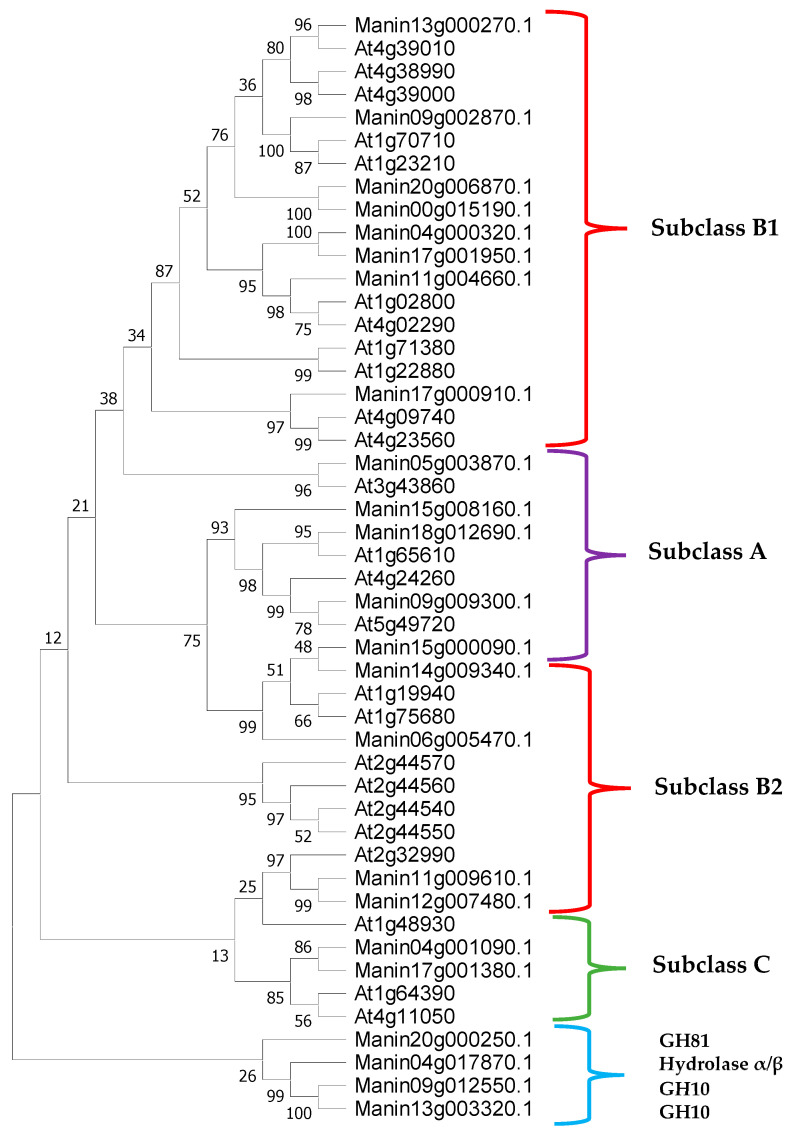
A total of 23 putative glucanases were identified from the genome of mango cv. Tommy Atkins [25], and by locating and predicting the conserved domains and sites, 19 were identified as β-1,4-glucanases. Through alignments and phylogenetic analyses, these were classified into subclasses according to the criteria described above for β-1,4-glucanases of the GH9 family of Arabidopsis (the model plant for the standardized nomenclature of β-1,4-glucanases) (Figure 1) [26,27]. Until now, no β-1,3-1,4-glucanases have been identified in Arabidopsis. In total, 5 β-1,4-glucanases of subclass A, 12 of subclass B (8 of class B1 and 4 of class B2), and 2 of subclass C were found in M. indica (Figure 2 and Table 1) [20,26,27,28]. Of the 23 glucanases, 4 presented different catalytic domains; there were 2 from the GH10 family, 1 from the GH81 family, and 1 from the α/β hydrolase. The length of the M. indica glucanases ranged from 426 to 1095 amino acids, of which 22 varied in size from 426 to 686 amino acids, and 4 contained more than 891 amino acids (Manin18g012690, Manin20g000250, Manin20g006870, and Manin13g003320). These long-sequence glucanases were observed to contain repeated catalytic domains.
Figure 1.
Schematic representation of the structure of β-1,4-glucanases of the GH9 family of plants. The subclasses are composed of the cytosolic domain (green), transmembrane domain (pink), signal sequence (dark gray), GH9 catalytic domain (purple), linker region (thick blue line), and CBM49 carbohydrate-binding module (blue dots) (adapted from Urbanowicz et al. [27]).
Figure 2.
Phylogenetic tree of M. indica glucanases identified from the glycosylhydrolase family are GH9 (red/purple/green), GH10, GH81, and α/β hydrolase (blue). The subclasses of β-1,4-glucanases are represented as follows: purple = subclass A, red = subclass B, green = subclass C, and blue = glucanases from other families. The phylogenetic tree shows that the M. indica glucanases belong to A. thaliana subclasses. The GH9 family comprises subclasses A, B1, B2, and C, whereas the other GH families are grouped in the smaller clade. The percentage of trees in which the associated taxa clustered is shown next to the branches.
Table 1.
Characterization of glucanases from Mangifera indica.
| Sequence ID Genome * (Transcriptome) ** |
Description | Size (aa) | Glycosyl Hydrolase Domain |
Catalytic Domain |
Subclass | Cytosolic Domain |
Transmembrane Domain | Signal Peptide | Carbohydrate Binding Module (CBM49) |
|---|---|---|---|---|---|---|---|---|---|
| Manin05g003870.1 (c35418_g1_i1) |
Endoglucanase 16, hydrolase activity, carbohydrate metabolic process. | 487 | GH9 | GH9: 34–478 | A | NO | NO | 1–28 | NO |
| Manin09g009300.1 (c19892_g1_i1) |
Endoglucanase 25, hydrolase activity, carbohydrate metabolic process. | 621 | GH9 | GH9: 111–585 | A | Cytosolic domain CT: 1–72 | 73–95 | NO | NO |
| Manin15g000090.1 (c21105_g1_i1) |
Endoglucanase 10, hydrolase activity, carbohydrate metabolic process. | 524 | GH9 | GH9: 55–509 | A | Cytosolic domain CT: 1–11 | 12–31 | NO | NO |
| Manin15g008160.1 (c19892_g1_i1) |
Endoglucanase 25, hydrolase activity, carbohydrate metabolic process. | 608 | GH9 | GH9: 120–575 | A | Cytosolic domain CT: 1–82 | 83–104 | NO | NO |
| Manin18g012690.1 (c35418_g1_i1) |
Endoglucanase 12, hydrolase activity, carbohydrate metabolic process. | 891 | GH9 | GH9: 153–625, 633–690, 697–872 | A | Cytosolic domain CT: 1–117 | 118–137 | NO | NO |
| Manin04g000320.1 (c19965_g1_i2) |
Endoglucanase, hydrolase activity, carbohydrate metabolic process. | 686 | GH9 | GH9: 262–676 | B1 | NO | NO | NO | NO |
| Manin09g002870.1 (c10916_g1_i2) |
Endoglucanase 8, cellulase activity, starch, and sucrose metabolic process. | 499 | GH9 | GH9: 39–492 | B1 | NO | NO | 1–30 | NO |
| Manin11g004660.1 (c35418_g1_i1) |
Endoglucanase 17, hydrolase activity, carbohydrate metabolic process. | 476 | GH9 | GH9: 43–275, 280–466 | B1 | NO | NO | 1–24 | NO |
| Manin13g000270.1 (c19965_g1_i1) |
Endoglucanase 24, hydrolase activity, carbohydrate metabolic process. | 503 | GH9 | GH9: 37–493 | B1 | NO | NO | 1–34 | NO |
| Manin17g000910.1 (c10916_g1_i2) |
Endoglucanase-like hydrolase activity, metabolic process of hydrolyzed carbohydrates of O-glycosyl compounds. | 497 | GH9 | GH9: 35–481 | B1 | NO | NO | 1–22 | NO |
| Manin17g001950.1 (c19965_g1_i1) |
Endoglucanase CX, hydrolase activity, carbohydrate metabolic process. | 501 | GH9 | GH9: 35–489 | B1 | NO | NO | NO | NO |
| Manin20g006870.1 (c12199_g1_i1) |
Endoglucanase 8, hydrolase activity, carbohydrate metabolic process. | 922 | GH9 | GH9: 24–443, 471–915 | B1 | NO | NO | 1–20 | NO |
| Manin00g015190.1 (c12199_g1_i1) |
Endoglucanase 4, hydrolase activity, carbohydrate metabolic process. | 480 | GH9 | GH9: 24–471 | B1 | NO | NO | 1–21 | NO |
| Manin06g005470.1 (c12199_g1_i1) |
Endoglucanase 2, hydrolase activity, carbohydrate metabolic process. | 539 | GH9 | GH9: 68–526 | B2 | NO | NO | NO | NO |
| Manin11g009610.1 (c10916_g1_i1) |
Endoglucanase 11, hydrolase activity, carbohydrate metabolic process. | 525 | GH9 | GH9: 46–505 | B2 | NO | NO | 1–43 | NO |
| Manin12g007480.1 (c10916_g1_i1) |
Endoglucanase 11, hydrolase activity, carbohydrate metabolic process. | 539 | GH9 | GH9: 60–519 | B2 | NO | NO | 1–36 | NO |
| Manin14g009340.1 (c21105_g1_i1) |
Endoglucanase 2, integral component of membrane cellulase activity, starch, and sucrose metabolic process. | 426 | GH9 | GH9: 2–411 | B2 | NO | NO | NO | NO |
| Manin04g001090.1 (c35418_g1_i1) |
Endoglucanase 6, hydrolase activity, carbohydrate metabolic process. | 627 | GH9 | GH9: 30–489 | C | NO | NO | 1–26 | 535–615 |
| Manin17g001380.1 (c35418_g1_i1) |
Glucanase family 2, hydrolase activity, carbohydrate-binding carbohydrate metabolic process. | 618 | GH9 | GH9: 29–488 | C | NO | NO | 1–25 | 526–606 |
| Manin09g012550.1 (c22667_g2_i1) |
Xylanase-like exoglucanase, hydrolase activity, carbohydrate metabolic process. | 597 | GH10 | GH10: 47–595, 243–540 They hydrolyze glycosidic bonds between two or more carbohydrates | - | - | 32–52 | 1–22 | NO |
| Manin13g003320.1 (c22667_g2_i1) |
Xylanase-like exoglucanase, hydrolase activity, carbohydrate metabolic process. | 1095 | GH10 | GH10: 542–1071, They hydrolyze glycosidic bonds between two or more carbohydrates | - | - | - | Greater Facilitating Superfamily (MFS): 12–533 | Galactose binding domain: 595–680 |
| Manin04g017870.1 (c13218_g1_i1) |
Endo-1,3-1,4-β-D-glucanase, hydrolase activity. | 446 | Hydrolase α/β | α/β hydrolase: 53–234, 235–444 | - | NO | NO | NO | NO |
| Manin20g000250.1 (c24163_g1_i1) |
Probable endo-1,3(4)-β-glucanase ARB_01444, endo-1,3-β-glucanase glucan activity, C-3 substituted reducing group. | 894 | GH81 | Endo-β-glucanase: 60–704, 758–839 GH81: N: 89–363, 777–840; C: 369–704 |
- | NO | NO | 1–59 | NO |
Mango cv. Tommy Atkins genome * (PRJNA450143) and mango cv. Ataulfo transcriptome ** (PRJNA286253). Not found: -.
We identified two glucanases with GH10 family domains that showed xylanase activity. Only two glucanases could hydrolyze endo-β-1,3-1,4 bonds: Manin04g017870, which contained two catalytic domains with endo-α/β hydrolase activity, and Manin20g000250, which had two endo-β-glucanase catalytic domains and two GH81 family domains at its N and C termini. The GH81 family, which has endo-β-1,3-glucanase activity, has a defense function against pathogens [19,20]. Cytosolic and transmembrane domains are contained only by the β-1,4-glucanases of subclass A, except for Manin05g003870, which, instead, presented a signal peptide. The carbohydrate-binding module CBM49 is only contained by the β-1,4-glucanases of subclass C. Meanwhile, the signal peptide sequence was predicted in some glucanases, such as Manin20g006870 and Manin13g003320; however, not all subclass B β-1,4-glucanases have signal peptides at their amino terminus.
2.2. Classification and Characterization of Chitinases from M. indica
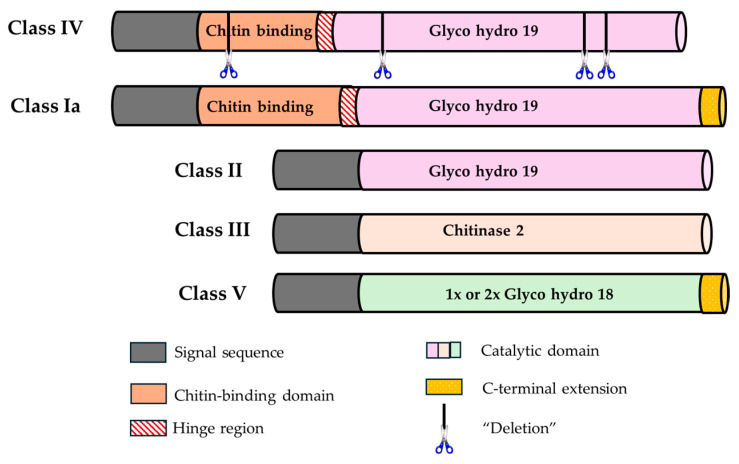
Within the classification of chitinases, classes III and V contain domains from the GH18 family and are present in plants, animals, fungi, and viruses. In contrast, classes I, II, and IV contain domains from the GH19 family that are present only in plants [18,29,30]. Classes I and IV have a conserved chitin-binding domain (CBD) and a GH19 catalytic domain with an N-terminal region rich in cysteine and a C-terminal extension. Class IV chitinases and their sequence do not contain the latter extension; they are found with several deletions. Class II does not have an N-terminal region and lacks a CBD, but it is similar to the amino acid sequence of class I. Class III and V chitinases do not have a CBD and contain a GH18 catalytic domain; those of class V are most similar to fungal and bacterial chitinases and have a C-terminal extension [18,30,31].
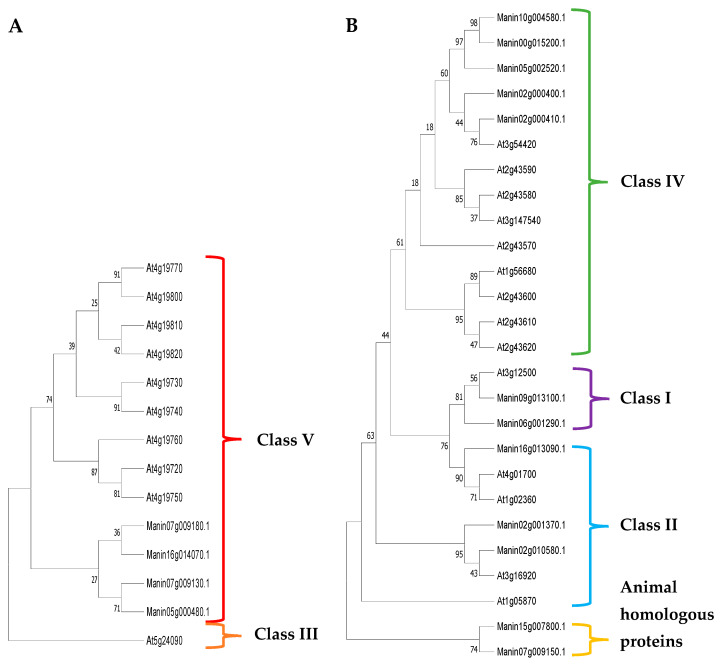
Sixteen putative chitinase sequences were obtained from the genome of mango cv. Tommy Atkins [25]. The GH18 and GH19 subfamilies shared little similarity in their amino acid sequences; thus, their alignment and phylogenetic analyses were performed separately. Four were classified as belonging to the GH18 subfamily and ten as belonging to the GH19 subfamily, according to the classification criteria for plant chitinases proposed by Passarinho and de Vries [30] and Cao et al. [18] in Arabidopsis (Figure 3). Therefore, two class I, three class II, five class IV, and four class V chitinases were identified in M. indica; however, two chitinases showed homology with animal proteins (Figure 4 and Table 2).
Figure 3.
Schematic representation of the structural domains of plant chitinases (adapted from Passarinho and de Vries [30]).
Figure 4.
Phylogenetic trees of M. indica chitinases identified from the glycosylhydrolase family GH18 (A): class III (orange) and class V (red); and GH19 (B): class I (purple), class II (blue), class IV (green), and homologous animal proteins (yellow). The M. indica chitinases (Manin) were found in all but class III chitinases from A. thaliana. The percentage of trees in which the associated taxa clustered is shown next to the branches.
Table 2.
Characterization of chitinases from Mangifera indica.
| Sequence ID Genome * (Transcriptome) ** |
Description | Size (aa) | Glycosyl Hydrolase Domain | Catalytic Domain | Class | Lysozyme Function | Signal Peptide | Chitin-Binding Domain (CBD) Region |
|---|---|---|---|---|---|---|---|---|
| Manin10g004580.1 (c18874_g2_i1) |
PR4 endochitinase, cell wall macromolecule catabolic process. | 718 | GH19 | GH19 with deletions | IV | YES | 1–28 | 29–64, 296–331 |
| Manin16g013090.1 (c26375_g1_i1) |
Chitinase 10, cell wall macromolecule catabolic process. | 265 | GH19 | GH19: 13–265 | II | YES | 1–23 | NO |
| Manin00g015200.1 (c21654_g1_i1) |
Chitinase IV, cell wall macromolecule catabolic process. | 260 | GH19 | GH19 with deletions | IV | YES | 1–28 | 29–64 |
| Manin02g000400.1 (c21682_g1_i1) |
Endochitinase EP3, response to wounds, response to bacteria, hypersensitive response, somatic embryogenesis, cell wall macromolecule catabolic process. | 934 | GH19 | GH19 with deletions | IV | YES | 1–26 | 31–50, 86–105, 316–335, 369–388, 618–637, 657–676, 700–719 |
| Manin02g000410.1 (c21682_g1_i1) |
Endochitinase EP3, cell wall macromolecule catabolic process. | 466 | GH19 | GH19 with deletions | IV | YES | 1–23 | 23–58, 265–300 |
| Manin02g001370.1 (c16890_g1_i1) |
Chitinase 1 response to water stress, salt stress response, lignin biosynthetic process, cell growth, response to metabolic nitrate regulation of salicylic acid. | 320 | GH19 | GH19: 67–293 | II | YES | 1–24 | NO |
| Manin02g010580.1 (c16890_g1_i1) |
Chitinase 2, cell wall macromolecule catabolic process. | 321 | GH19 | GH19: 69–296 | II | YES | 1–23 | NO |
| Manin05g002520.1 (c21654_g1_i1) |
Chitinase tipo 1, cell wall macromolecule catabolic process. | 485 | GH19 | GH19 with deletions | IV | YES | 1–23 | 236–271 |
| Manin06g001290.1 (c25021_g3_i3) |
Pentatricopeptide chitinase containing At2g17670 repeats, cell wall macromolecule catabolic process. | 720 | GH19 | GH19: 89–191, 200–210 | I | YES | 1–23 | 24–65 |
| Manin09g013100.1 (c26375_g1_i1) |
Like endochitinase, cell wall macromolecule catabolic process. | 310 | GH19 | GH19: 70–295, 208–218 | I | YES | 1–20 | 21–62 |
| Manin05g000480.1 (c37799_g1_i1) |
Chitinase 3, 1, protein kinase activity, ATP binding, protein phosphorylation. | 563 | GH18 | GH18: 66–412 Protein kinase: 288–563, Ser/Thr kinase: 462–474 |
V | NO | - | NO |
| Manin16g014070.1 (c24038_g1_i1) |
Chitinase isoform X2, cell wall macromolecule catabolic process. | 869 | GH18 | Chitinase 2: 104–424, GH18: 104–440 | V | YES | 1–36, 37–58 | NO |
| Manin07g009130.1 (c22170_g1_i1) |
Chitinase 3, 1. | 129 | GH18 | GH18: 1–105 | V | NO | - | NO |
| Manin07g009180.1 (c22170_g1_i4) |
Like mammalian acid chitinase, cell wall biogenesis. | 892 | GH18 | Chitinase 2: 544–875, GH18: 544–892 | V | NO | 1–20 | NO |
| Manin15g007800.1 (No transcript) |
Like endochitinase A. | 306 | - | - | - | - | - | - |
| Manin07g009150.1 (c23148_g1_i2) |
Acidic isoform like mammalian chitinase X2. | 160 | - | - | - | - | 1–34 | - |
Mango cv. Tommy Atkins genome * (PRJNA450143) and mango cv. Ataulfo transcriptome ** (PRJNA286253). Not found: -.
The length of the M. indica chitinases varied from 129 to 934 amino acids; the longest 5 contained more than 718 amino acids: Manin10g004580, Manin02g000400, Manin06g001290, Manin16g014070, and Manin07g009180. All class I, II, and IV chitinases were predicted to have a lysozyme function, and three (Manin05g000480, Manin07g009130.1, and Manin07g009180) of the four class V chitinases did not have this function. Two of these chitinases did not contain signal peptides (Manin05g000480 and Manin07g009130), while Manin07g009180 and Manin16g014070 of class V showed this signal sequence. Furthermore, all class I and IV chitinases presented CBDs.
2.3. Glucanases and Chitinases from M. indica with Possible Defense Function against Anthracnose
On average, β-glucan is the main component of fungal cell walls, making up 50–60% of its dry weight, while chitin accounts for between 10 and 20% of the dry weight [22,32,33]. Within the structure of the fungal wall, β-glucans are mainly formed in β-1,3 bonds (65–90%) and, to a lesser extent, in β-1,6 and β-1,3-1,4 bonds [22,32,33]. However, chitin is made up of N-acetyl-D-glucosamine (GlcNAc) with β-1,4 bonds [32,33].
It has been reported that endo-β-1,3-1,4-glucanases degrade Mixed Linked Glucan (MLG), which is present in monocotyledonous plants and cell walls of some fungi and bacteria [20,34,35,36,37,38,39]. However, the characterization of a few β-glucanases in dicotyledons has given us limited information about the signaling function that molecules generated from the hydrolysis of pathogens’ MLG could have [20]. Therefore, it has been suggested that the mixed-linked oligosaccharides of β-1,3-1,4-glucans derived from phytopathogens in dicotyledonous plants, which do not contain MLG, can act as PAMPs [20,35,38].
A previous study indicated that class I and IV chitinases are characterized by the presence of a conserved CBD that recognizes and hydrolyzes the endo-β-1,4 bonds of the GlcNAc subunits of chitin polymers [32]. In tomatoes, Jashni et al. [17] demonstrated that inoculation with Cladosporium fulvum and Fusarium oxysporum induced and positively regulated the expression of chitinase genes with CBD, i.e., class I and IV, and that the elimination of this catalytic domain significantly reduced their chitinase and antifungal activity. In addition, PAMPs, which are effector molecules that activate the plant immune system, are released from the hydrolysis of the fungal cell wall [33,40]. Other authors have reported that the overexpression of plant chitinases can reduce the symptoms of fungal diseases [41,42].
There is little information about mango glucanases and chitinases, and, thus far, there are no specific gene expression studies on mango PRs induced by species of the C. gloeosporioides complex. Hong et al. [24] reported the overexpression of two mango peel genes from cv. Zill infected with C. gloeosporioides encoding possible PRs other than glucanases and chitinases (GenBank: GBCV01028555, GBCV01029236). These nucleotide sequences were mapped onto the genome of mango cv. Tommy Atkins, but they were unrelated to glucanases and chitinases PRs (). These genes encode a protein FATTY ACID EXPORT 1, chloroplastic-like, and a mannan endo-1,4-beta-mannosidase 2-like.
Due to the lack of molecular information on mango, we used orthologous sequences of glucanases and chitinases reported in other plant organisms that were induced by biotic stress caused specifically by fungi. For β-1,3-glucanases, we used sequences from various dicotyledonous and monocotyledonous plant species, including Arabidopsis, jujube, potato, wheat, oats, rice, and barley (Table 3). The chitinases that responded to fungal infection were class I and IV in Arabidopsis, blackberry, and white pine. In addition, some class I chitinase genes were overexpressed in tea, lychee, and carrot, decreasing the damage caused by some fungal diseases (Table 3). We partially infer that mango’s endo-β-1,3-1,4-glucanases and class I and IV chitinases hydrolyze β-glucans [20] and chitin [17,32,33], respectively, which are both components of the fungal cell wall.
Table 3.
Fungal-induced glucanases and chitinases associated with plant defense.
| Plant | Glucanase (β-1,3-Glucanase) |
Inducing Pathogen | References |
|---|---|---|---|
| Jujube | AAY25165.1 | Cryptococcus laurentii | Tian et al., [43] |
| A. thaliana | AAM67102.1 | 77% ID with jujube | * Tian et al. [43] |
| NP_001325845.1 (At3g57260) | Botrytis cinérea, Erysiphe cichoracearum, Erysiphe orontii | Doxey et al. [44] | |
| NP_191283.2 (At3g57240) | Erysiphe cichoracearum, Erysiphe orontii | Doxey et al. [44] | |
| Potato | pir||S31196 | 49% ID with jujube | Tian et al. [43] * |
| Wheat | AAY96422.1 | Rhizoctonia sp. | Liu et al. [45] |
| CAA77085.1 | Fusarium graminearum, Alternaria sp., A. glaucus, A. flavus, A. niger, Penicillium sp. | Zhang et al. [46] | |
| AAD28734.1 | Bipolaris sorokiniana | Aggarwal et al. [47] | |
| Oatmeal | AAP33176.1 | 80% ID with wheat | * Liu et al. [45] |
| Rice | AAL35900.1 | 74% ID with wheat | * Liu et al. [45] |
| Barley | AAM75342.1 | 94% ID with wheat | * Liu et al. [45] |
| Tobacco | BAB17320.1 | 41% ID with jujube | * Tian et al. [43] |
| Corn | NP001148461.1 (β-1,3-1,4-glucanase) |
--------- | Perrot et al. [20] |
| Plant |
Chitinase
(Classes I y IV) |
Inducing Pathogen | References |
| Blackberry |
EXB44469.1 class I EXB55191.1 class IV EXB55192.1 class IV |
Botrytis cinerea | Xin et al. [40] |
| A. thaliana |
NP_566426.2 class I AAP88360.1 class IV |
Botrytis cinerea | Xin et al. [40] |
| AAA32769.1 class I | Sasaki et al. [21] | ||
| NP_191010.1 class IV | Liu et al. [48] | ||
| White Pine | AAS83984.1 class IV | Cronartium ribicola, rust fungus | Liu et al. [48] |
| Tea (potato gene overexpression) | AAF25602.1 class I | Phytophthora infestans (blight) in Camellia sinensis (Tea) | Singh et al. [49] |
| Kumar et al. [50] | |||
| Litchi (rice gene overexpression) | CAA38249.1 class I | Phomopsis sp. in litchi | Das et al. [42] |
| Carrot (barley gene overexpression) | AAA18586.1 class I | Alternaria radicicola and Botrytis cinerea in carrot | Jayaraj and Punja, [41] |
% ID: identity percentage. Jujube (* Tian et al. [43]) and wheat (* Liu et al. [45]) share significant identity with β-1,3-glucanase identified in plants like A. thaliana, potato, tobacco, oats, rice, and barley, suggesting a possible defense response against fungi such as Cryptococcus laurentii and Rhizoctonia sp.
Only two glucanases that hydrolyze endo-β-1,3-1,4 bonds and two class I and five class IV chitinases were identified. Two phylogenetic analyses were carried out on these mango proteins—one with the two endo-β-1,3-1,4-glucanases and another with the seven chitinases—using their respective orthologous sequences from Table 3 (from the phylogenetic analyses). The glucanase Manin20g000250, the class I chitinase Manin09g013100, and class IV chitinase Manin05g002520 were chosen based on their greatest phylogenetic closeness with these orthologous glucanase and chitinase. Moreover, their relative expression was evaluated in mango cv. Ataulfo inoculated with Colletotrichum spp.
2.4. Prediction of cis Elements Acting in the Promoter Region of Endo-β-1,3-1,4-Glucanase and Class I and IV Chitinases Coding Genes from M. indica
1500 bp upstream of the ATG from the coding sequence (promoter regions) were chosen to identify cis-elements important for gene expression regulation of the three genes of interest. Those cis elements are responsible for interaction with transcription factors related to the defense response to biotic stress and the hormonal response. These are related to the presence of elicitor response elements in Colletotrichum spp. [51]. The response of hormones such as ethylene, salicylic, and jasmonic acid defines the pathways by which some pathogens, based on their lifestyle, carry out signal transduction during disease development [8,51]. Response factors to salicylic acid are related to immunity against biotrophic and hemibiotrophic pathogens, and response factors to ethylene and jasmonic acid are related to defense against necrotrophic pathogens [8].
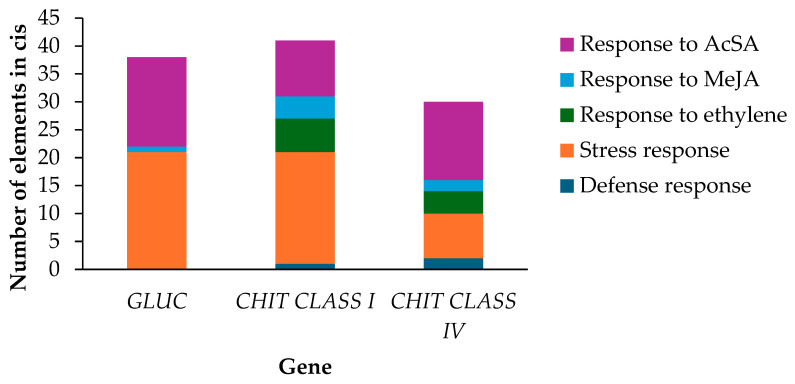
Concerning the signaling pathway, transcription factors target the cis elements of the promoter regions to regulate gene expression at the transcriptional levels. In this study, cis elements were found in the three genes, glucanase (GLUC) and chitinase (CHIT) class I and IV of M. indica that respond to jasmonic acid and salicylic acid, as well as in response to stress (Figure 5). However, compared with the chitinase genes, the GLUC gene did not contain elements in response to ethylene or those in response to plant defense. Furthermore, the GLUC gene had the highest number of cis elements in response to salicylic acid (Figure 5), followed by the CHIT class IV gene. Response elements to ethylene and salicylic acid were found in the CHIT genes and marked by different signaling pathways, indicating that they can act on the lifestyle of hemibiotrophic Colletotrichum spp.
Figure 5.
Frequency of appearance of cis elements in three sequences that correspond to the genes GLUC, CHIT class I, and CHIT class IV of M. indica. The vertical axis indicates the number of cis-regulatory elements, and the horizontal axis shows the genes.
2.5. Gene Expression Profile of GLUC and CHIT class I and IV in Mango Fruits cv. Ataulfo in Response to C. siamense and C. asianum
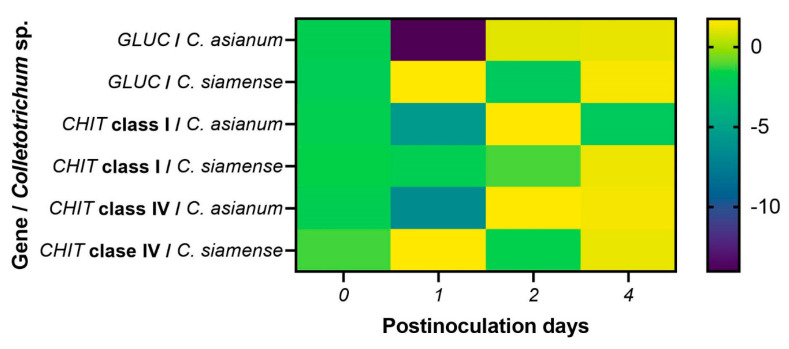
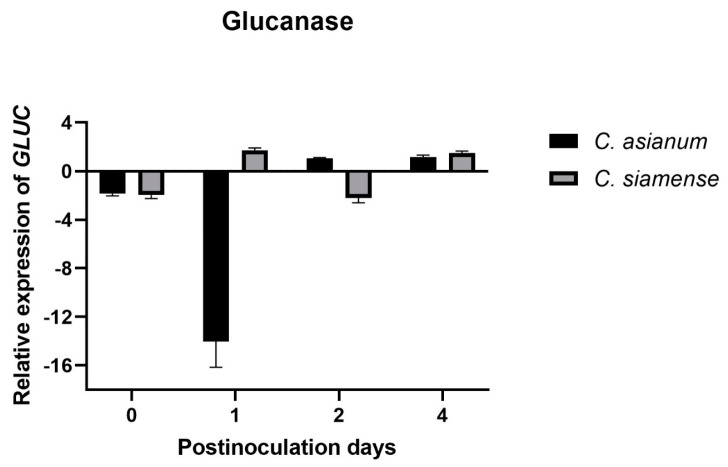
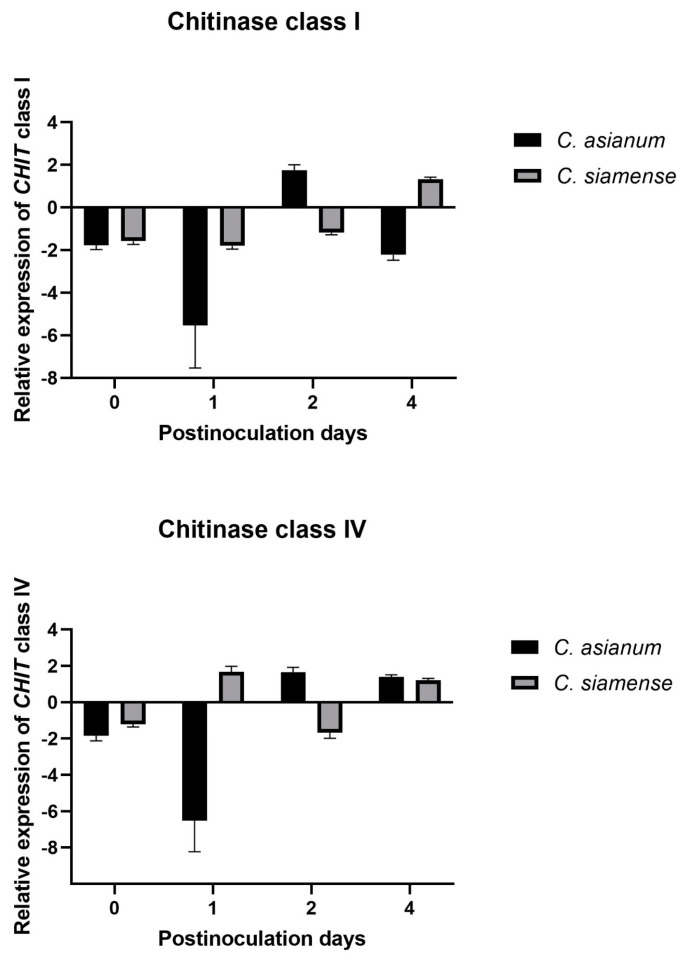
Relative gene expression analysis showed that the GLUC gene was expressed on day 1 in fruit inoculated with C. siamense, while it was expressed 2 days post-inoculation (dpi) in those inoculated with C. asianum. In addition, the response to both pathogens was maintained at 4 dpi. We observed an early induction in the expression of the CHIT class I gene in fruits inoculated with C. asianum compared to those inoculated with C. siamense, in which expression occurred at 4 dpi. The CHIT class IV gene was expressed early on day 1 in fruits inoculated with C. siamense and on day 2 in fruits inoculated with C. asianum (Figure 6). The pathogenicity and virulence of fungi can vary due to the genetic factors of the hosts and the environmental conditions in which they are found [2,6].
Figure 6.
Expression profile of the glucanase gene (GLUC I) and chitinases class I (CHIT class I) and IV (CHIT class IV) of M. indica under biotic stress induced by C. asianum and C. siamense. Uninoculated mango fruits were used as internal control.
In a previous work reported by Jiménez-Maldonado et al. [2], the authors found that C. siamense was more virulent than C. asianum in Ataulfo mango fruits during postharvest, with mycelial growths of 33 and 28 mm, respectively, after 10 days of fruit storage at 28 °C. It was also reported that the highest peak of respiratory activity occurred at 2 dpi, which was accelerated by the presence of these two Colletotrichum spp. This correlates with GLUC and CHIT class IV expression from day 2 onwards, with C. asianum inoculation. In contrast, in mango fruit inoculated with C. siamense, their expression was triggered at 1 dpi because it was more virulent than C. asianum. An increase in ethylene and CO2 has been reported to coincide with fruit ripening and induces germination and the formation of the appressorium of Colletotrichum spp., but this only occurs in climacteric fruits [52,53,54]. The asymptomatic phase of anthracnose (a biotrophic fungal infection) occurs 48 h after inoculation, during which the species acquires nutrients from the infected host before necrosis; 72 to 96 h after the initial penetration of Colletotrichum spp., necrotrophic secondary hyphae develop that degrade the plant cell wall [55]. Therefore, the necrotrophic development of Colletotrichum spp. occurs at 3–4 dpi, which induces constant gene expression at 4 dpi in fruit subjected to both pathogens (Figure 6).
The primary hyphae of Colletotrichum spp., which have a hemibiotrophic mode of infection, are formed during its biotrophic phase. Subsequently, during its necrotrophic phase, secondary hyphae are formed [11]. Oliveira-Garcia and Deising [56] reported that β-glucan is necessary to stiffen the cell wall in fast-growing appressoria and the necrotrophic hyphae of Colletotrichum graminicola in maize. However, its synthesis is downregulated during biotrophic development. Colletotrichum siamense is more virulent and acts quickly to penetrate its host. It may be synthesizing β-glucans for the formation of an appressorium, explaining why the expression of glucanase in fruit subjected to C. siamense was positively induced at 1 dpi and again at 4 dpi (Figure 7). In contrast, the C. asianum isolate, which was less virulent, evaded the immunity caused by the downwardly synthesized β-glucan, and its GLUC expression was therefore induced at 2 dpi (Figure 7).
Figure 7.
Expression of GLUC and CHIT class I and IV of M. indica induced by C. asianum and C. siamense. Uninoculated mango fruits were used as internal control. The days of analysis were 0, 1, 2, and 4 days post-inoculation (dpi) for RNA extraction and the assay by real-time quantitative reverse transcription polymerase chain reaction. Gene expression was normalized to the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene [57] using the 2−∆∆Ct method [58]. Data are shown as mean ± SD, analyzed by one-way ANOVA/Tukey test.
Furthermore, in rice, fungi such as Magnaporthe oryzae, Cochliobolus miyabeanus, and Rhizoctonia solani mask chitin and β-1,3-glucan (only in M. oryzae) in the fungal cell wall with α-1,3-glucan, preventing their enzymatic digestion by plant chitinases and glucanases and, thus, delaying the release of PAMPs [9,59]. α-1,3-glucan synthase genes are present in the genomic sequences of fungal pathogens, such as C. graminicola, Myco-sphaerella graminicola, Puccinia graminis, Sclerotinia sclerotiorum, and Botrytis cinerea [59]. This is also a strategy that Colletotrichum spp. may use to be detected early or late.
CHIT class IV was induced at the earliest dpi (1 and 2). However, CHIT class I was induced on the same day (2 dpi) when CHIT class IV was expressed in mango fruit inoculated with C. asianum; this is compared to the fruit inoculated with C. siamense, in which the expression of the CHIT class I gene was induced at 4 dpi (Figure 7). Some studies on rice plants provide information on the targets of chitinases, and these report that, in the early stage of pathogenesis, class IV chitinases release elicitor molecules from the fungal cell wall, and GlcNAc oligosaccharides activate the defense system. Subsequently, class I chitinases degrade the newly synthesized chitin chain of the fungus, inhibiting its growth [21,60]. The results in mango inoculated with C. siamense coincide with those reported in tomato inoculated with F. oxysporum, in which class IV chitinases were highly induced within 4 dpi and class I chitinases were positively induced only at 8 dpi [17]. We can also propose that C. siamense evades the mango immune system by delaying the expression of CHIT class I, causing larger necrotic spots to appear in the fruit [2].
Subnanomolar concentrations of GlcNAc oligosaccharides are sufficient to induce defense responses [61]. However, some studies have indicated that Colletotrichum spp. can convert exposed chitin to its nonacetylated chitosan derivative, preventing plant recognition of chitin fragments or PAMPs. This may occur in fruit inoculated with C. siamense compared to C. asianum, which is immediately recognized by class IV and I chitinases at 2 dpi [56,62,63]. Chitosan is a poor substrate for chitinase, and its fragments are less active as elicitors than chitin. Therefore, chitin deacetylation by C. siamense may interfere with its recognition by host chitinases [56,62,63].
Karunanayake et al. [64] reported that chitinases in latex and mango peel can rapidly degrade the conidial wall of C. gloeosporioides. An increase in the activity of chitinase and glucanase enzymes has also been reported from 2 to 8 dpi in mango fruits infected with anthracnose [23]. The genes that encode PRs have shown an expression on a basal level that increases drastically during a fungal infection. By expressing these genes, better adaptation to pathogen stress and greater plant performance have been observed [64,65]. Therefore, the overexpression of PRs genes, such as GLUCs and CHITs, improves plant resistance to diseases caused by fungi [24,42,66,67,68].
The results of this research can help develop effective control strategies to improve postharvest mango quality and avoid losses caused by these phytopathogens. We have provided important information about the classification of glucanases and chitinases to elucidate some of their functions in mango.
3. Materials and Methods
3.1. Classification of Glucanases and Chitinases from M. indica
3.1.1. Identification of Glucanases and Chitinases
The genome (https://mangobase.org) of mango cv. Tommy Atkins from project PRJNA450143 [25] was blasted for sequences coding for glucanases and chitinases. The transcriptome of mango cv. Ataulfo (PRJNA286253) and orthologous sequences of Arabidopsis were used to obtain these gene families sequences from mango.
The sequences of 23 glucanases and 16 chitinases were obtained from the genome of mango cv. Tommy Atkins [25] and used to search for their homologous and orthologous sequences in the NCBI database. Subsequently, PSI-BLAST (http://www.ncbi.nlm.nih.gov/BLAST, accessed on 4 January 2023) was performed to compare their amino acid sequences through alignments, and their regions of similarity were located using NCBI’s conserved domain (CD) search tool. Functional analyses of the proteins and predictions of conserved domains and sites were performed using the online EMBL-EBI (http://www.ebi.ac.uk/interpro/ (accessed on 4 January 2023)—European Molecular Biology Laboratory, 2023) and Scan Prosite programs (https://prosite.expasy.org/scanprosite/ (accessed on 4 January 2023)—ExPASy, 2023). We used the standardized Arabidopsis nomenclature of 25 GH9 family amino acid sequences for the glucanases [27] and 24 GH18 and GH19 family sequences for the chitinases [18,30]. As additional information, the names of the corresponding transcripts of mango cv Ataulfo (PRJNA286253) were mapped to the genomic proteins and are indicated in parentheses (Table 2 and Table 4).
Table 4.
Primers were used for relative expression analysis of mango glucanases and chitinases.
| Primers | |
|---|---|
| Manin20g000250.1 ß-1,3-1,4-Glucanasa |
F: 5′CAGGATTTCACCCGAGAGAATAG3′ |
| R: 5′TCAGGAGGAGCAAACCAAAG3′ | |
| Manin05g002520.1 Quitinasa class IV |
F: 5′GCTCCCAACTTGTGTTGCAG3′ |
| R: 5′CCCCTTACACCCCAATCCAC3′ | |
| Manin09g013100.1 Quitinasa class I |
F: 5′CCTCCAAGAGCTTCTACAGTTAC3′ |
| R: 5′CATGGGAAGTTTGGGCTAAGA3′ |
F: forward, R: reverse.
3.1.2. Sequence Alignment and Phylogenetic Analysis
Phylogenetic and molecular evolutionary analyses of mango cv. Tommy Atkins chitinases and glucanases were performed to classify them into GH families and classes or subclasses. Since the three families have different sequences and catalytic domains, phylogenetic analyses were performed separately for GH9, GH18, and GH19. MUSCLE and ClustalW were used for multiple alignments of the full lengths of the amino acid sequences [69,70], and MEGA-X 2021 was used to construct phylogenetic trees [50]. Bootstrap consensus trees for the heuristic search and their evolutionary history were inferred using the neighbor-joining (NJ) method [71], and evolutionary distances were calculated using the model based on the Jones–Taylor–Thornton (JTT) matrix with 1000 replicates start.
3.2. Glucanases and Chitinases from M. indica with Possible Defense Functions
The analysis for the selection of mango glucanases and chitinases induced by C. siamense and C. asianum was carried out using orthologous sequences reported in response to the defense against fungi. For glucanases, sequences that hydrolyze endo-β-1,3 bonds with a genomic background in Arabidopsis thaliana, Ziziphus jujuba, Solanum tuberosum, Nicotiana tabacum, Triticum aestivum, Avena sativa, Oryza sativa, and Hordeum vulgare were used. To select chitinases, sequences containing a chitin-binding domain (CBD) were searched in A. thaliana, Morus notabilis, Pinus monticola, S. tuberosum, O. sativa, and H. vulgare. Due to the classification of glucanases and chitinases, only the amino acid sequences of the glucanases Manin04g017870 and Manin20g000250 and class I chitinases Manin06g001290 and Manin09g013100 were used; of the class IV chitinases, we used Manin10g004580, Manin00g015200, Manin02g000400, Manin02g000410, and Manin05g002520. These glucanases and chitinases from mango cv. Tommy Atkins and their respective orthologous sequences were compared and aligned using MUSCLE and ClustalW with default settings [69,70]. The NJ method was used for the bootstrap consensus trees [71], and evolutionary distances were calculated using the JTT model, with a bootstrap value of 1000 repetitions in MEGA-X 2021 [50].
3.3. Prediction of cis-Acting Elements in Promoter Regions of the Glucanase and Chitinases Genes from M. indica
The CiiiDER tool [72] and the JASPAR 2022 open-access database of transcription factor binding profiles [73] were used to predict the cis-acting elements in the promoter regions (1500 bp upstream of the start codon) of the GLUC and the CHIT class I and IV of M. indica (selected from the genome of mango cv. Tommy Atkins).
3.4. Gene Expression Analysis of Glucanases and Chitinases in Mango cv. Ataulfo Infected with Anthracnose
3.4.1. Vegetal Material
Mango cv. Ataulfo fruits, uniform in size and lacking visual damage, were selected from a commercial orchard in Los Mochis, Sinaloa, Mexico (DD 25.9300010, -109.0957430), based on their color, state number 2, to ensure equivalent physiological maturity. The fruits were disinfested with 1% sodium hypochlorite for 2 min, rinsed, and sprayed with 70% ethanol [4].
3.4.2. Inoculation and Storage of Mango cv. Ataulfo Fruits
Isolates of C. siamense (UACH 334) and C. asianum (UACH 310) were provided by Tovar-Pedraza et al. [4]. The fungal colonies were incubated at 28 °C for 10 days on potato dextrose agar culture medium (PDA, BD Bioxon, CDMX, Mexico). Wounds were made on the surface of the epicarp of the mango cv. Ataulfo fruits, which were disinfested before, and mycelial discs of 5 mm diameter were emplaced [4,74]. A batch of mango cv. Ataulfo was inoculated with C. siamense and another with C. asianum, and control fruits without inoculation and without visible damage were included. All fruits were stored at 28 °C with a relative humidity of 85–90% for 4 days.
3.4.3. RNA Extraction, cDNA Synthesis, and Relative Expression of Glucanases and Chitinases Genes
The expression levels of the genes involved in the defense mechanism in mango cv. Ataulfo were quantified by qRT-PCR. Two 5 cm diameter mango peel sections were cut for 3 biological replicates per treatment (2 inoculated and 1 noninoculated) at 0, 1, 2, and 4 dpi. Sections were frozen, sprayed with liquid nitrogen, and stored at −80 °C for analysis. Total RNA was extracted from mango epicarp (peel) tissue following the method described by López-Gómez and Gómez-Lim [75] with modifications from Dautt-Castro et al. [57]. RNA was treated with the Turbo DNA-free kit (Invitrogen, Carlsbad, CA, USA) to remove contaminating genomic DNA. cDNA was synthesized by reverse transcription using the SuperScript III First Strand kit (Invitrogen, CA, USA) following the manufacturer’s manual.
Quantitative PCR was carried out using the iTaq Universal SYBR Green Supermix kit (Bio-Rad, Hercules, CA, USA). All samples were amplified in triplicate PCR reactions that included 100 ng of template cDNA. Specific primers to amplify the glucanase and chitinases genes and the internal reference normalizing gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) [57], are shown in Table 4. PCR products were amplified on a QIAxpert-QUIAGEN system. The conditions for amplification were 1 cycle of 95 °C for 10 min and 40 cycles of 95 °C for 15 s and 60 °C for 1 min. The specificity of the PCR product was confirmed by constructing a melting curve after amplification by raising the temperature to 60 °C for 1 min and then gradually to 95 °C for 15 s. Relative gene expression was calculated using the 2−∆∆Ct [58].
3.4.4. Statistical Analysis
For gene expression analysis, standard deviations were calculated from three biological replicates with three technical replicates. The results were analyzed using one-way analysis of variance (ANOVA) with a significance level of 5% and a Tukey test for differences in means; the NCSS 2024 version 24.0.2 statistical package was used.
4. Conclusions
Our study provides important information about the classification of glucanases and chitinases in M. indica. The genome sequence of mango cv. Tommy Atkins (M. indica) (PRJNA450143) identified 23 glucanases and 16 chitinases. In total, 19 glucanases were classified as β-1,4-glucanases of the GH9 family, while 4 could not be classified because they possessed different catalytic domains. Of the classified glucanases, five were subclass A, twelve were subclass B, and two were subclass C. A total of four chitinases were classified in the GH18 subfamily and ten in the GH19 subfamily. Overall, two class I, three class II, five class IV, and four class V chitinases were identified. Two β-1,3-1,4-glucanases and two class I and five class IV chitinases were identified in mango and were studied for their expression analysis in response to Colletotrichum spp.
We identified and demonstrated the different expression profile of the GLUC and QUIT class I and IV genes of mango cv. Ataulfo in response to infection induced by C. siamense and C. asianum, species of the C. gloeosporioides complex. Both the stimulation of plant’s innate immunity through the release of PAMPs from the fungal cell wall and the expression of plant glucanases and chitinases could potentially be used against diseases, such as anthracnose in mango, to create resistant cultivars through elicitors or genetic modifications.
Acknowledgments
The authors thank the technical support of QFB Jesús Héctor Carrillo Yáñez of the Molecular Biology and Functional Genomics laboratory of CIAD, AC. María I. Jiménez-Maldonado received a doctoral scholarship from CONAHCYT.
Author Contributions
Conceptualization, M.I.J.-M., M.A.I.-O. and J.L.-F.; methodology, M.I.J.-M., M.A.I.-O., J.L.-F. and J.M.T.-P.; validation, M.I.J.-M., M.A.I.-O., J.L.-F., M.D.M.-R. and J.M.T.-P.; investigation, M.I.J.-M. and M.A.I.-O.; resources, M.A.I.-O., J.L.-F., M.D.M.-R. and J.M.T.-P.; data curation, M.I.J.-M., M.A.I.-O. and J.L.-F.; writing—original draft preparation, M.I.J.-M.; writing—review and editing, M.I.J.-M., M.A.I.-O., J.L.-F., M.D.M.-R. and J.M.T.-P.; visualization, M.I.J.-M., M.A.I.-O., J.L.-F., M.D.M.-R. and J.M.T.-P.; supervision, M.I.J.-M., M.A.I.-O. and J.L.-F.; project administration, M.I.J.-M.; funding acquisition, M.D.M.-R. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This work was supported by the CONACYT-FORDECYT, project # 292474 (2017).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Mora-Aguilera J.A., Ríos-López E.G., Yáñez-Zúñiga M., Rebollar-Alviter A., Nava-Díaz C., Leyva-Mir S.G., Sandoval-Islas J.S., Tovar-Pedraza J.M. Sensitivity to MBC fungicides and prochloraz of Colletotrichum gloeosporioides species complex isolates from mango orchards in Mexico. J. Plant Dis. Protect. 2021;128:481–491. doi: 10.1007/s41348-020-00412-z. [DOI] [Google Scholar]
- 2.Jiménez-Maldonado M.I., Tovar-Pedraza J.M., León-Félix J., Muy-Rangel M.D., Islas-Osuna M.A. Respuesta fisiológica y calidad de mango cv Ataulfo infectado por Colletotrichum spp. Rev. Mex. Cienc. Agríc. 2022;13:1103–1115. doi: 10.29312/remexca.v13i6.3028. [DOI] [Google Scholar]
- 3.Fuentes-Aragón D., Guarnaccia V., Rebollar-Alviter A., Juárez-Vázquez S.B., Aguirre-Rayo F., Silva-Rojas H.V. Multilocus identification and thiophanate-methyl sensitivity of Colletotrichum gloeosporioides species complex associated with fruit with symptoms and symptomless leaves of mango. Plant Pathol. 2020;69:1125–1138. doi: 10.1111/ppa.13195. [DOI] [Google Scholar]
- 4.Tovar-Pedraza J.M., Mora-Aguilera J.A., Nava-Diaz C., Lima N.B., Michereff S.J., Sandoval-Islas J.S., Câmara M.P.S., Téliz-Ortiz D., Leyva-Mir S.G. Distribution and pathogenicity of Colletotrichum species associated with mango anthracnose in Mexico. Plant Dis. 2020;104:137–146. doi: 10.1094/PDIS-01-19-0178-RE. [DOI] [PubMed] [Google Scholar]
- 5.Lima N.B., de A. Batista M.V., De Morais M.A., Barbosa M.A., Michereff S.J., Hyde K.D., Câmara M.P. Five Colletotrichum species are responsible for mango anthracnose in northeastern Brazil. Fungal Divers. 2013;61:75–88. doi: 10.1007/s13225-013-0237-6. [DOI] [Google Scholar]
- 6.Sharma G., Kumar N., Weir B.S., Hyde K.D., Shenoy B.D. The ApMat marker can resolve Colletotrichum species: A case study with Mangifera indica. Fungal Divers. 2013;61:117–138. doi: 10.1007/s13225-013-0247-4. [DOI] [Google Scholar]
- 7.Pardo-De la Hoz C.J., Calderón C., Rincón A.M., Cárdenas M., Danies G., López-Kleine L., Jiménez P. Species from the Colletotrichum acutatum, Colletotrichum boninense and Colletotrichum gloeosporioides species complexes associated with tree tomato and mango crops in Colombia. Plant Pathol. 2016;65:227–237. doi: 10.1111/ppa.12410. [DOI] [Google Scholar]
- 8.Liu L.P., Shu J., Zhang L., Hu R., Chen C.Q., Yang L.N., Hsiang T. First report of post-harvest anthracnose on mango (Mangifera indica) caused by Colletotrichum siamense in China. Plant Dis. 2017;101:833. doi: 10.1094/PDIS-08-16-1130-PDN. [DOI] [Google Scholar]
- 9.Dofuor A.K., Quartey N.K.A., Osabutey A.F., Antwi-Agyakwa A.K., Asante K., Boateng B.O., Ablormeti F.K., Lutuf H., Osei-Owusu J., Osei J.H.N., et al. Mango anthracnose disease: The current situation and direction for future research. Front. Microbiol. 2023;14:1168203. doi: 10.3389/fmicb.2023.1168203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ploetz R.C. Antracnosis en Mango: Manejo de la Enfermedad más Importante pre y Postcosecha. Universidad de la Florida, TREC-Homestead Departamento de Fitopatología; Homestead, FL, USA: 2008. pp. 1–11. [Google Scholar]
- 11.Paudel A., Poudel P., Yogi M. Insights on the mango anthracnose and its management. J. Plant Pathol. Res. 2022;4:81–90. doi: 10.36959/394/629. [DOI] [Google Scholar]
- 12.Jones J.D., Dangl J.L. The plant immune system. Nature. 2006;444:323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- 13.Asai T., Tena G., Plotnikova J., Willmann M.R., Chiu W.L., Gomez-Gomez L., Boller T., Ausubel F.M., Sheen J. MAP kinase signalling cascade in Arabidopsis innate immunity. Nature. 2002;415:977–983. doi: 10.1038/415977a. [DOI] [PubMed] [Google Scholar]
- 14.Bari R., Jones J.D. Role of plant hormones in plant defence responses. Plant Mol. Biol. 2009;69:473–488. doi: 10.1007/s11103-008-9435-0. [DOI] [PubMed] [Google Scholar]
- 15.Trouvelot S., Héloir M.C., Poinssot B., Gauthier A., Paris F., Guillier C., Combier M., Trdá L., Daire X., Adrian M. Carbohydrates in plant immunity and plant protection: Roles and potential application as foliar sprays. Front. Plant Sci. 2014;5:592. doi: 10.3389/fpls.2014.00592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vidhyasekaran P. Fungal Pathogenesis in Plants and Crops: Molecular Biology and Host Defense Mechanisms. 2nd ed. CRC Press; Boca Raton, FL, USA: 2007. Induction and evasion of pathogenesis-related proteins; pp. 345–409. [DOI] [Google Scholar]
- 17.Jashni M.K., Dols I.H., Iida Y., Boeren S., Beenen H.G., Mehrabi R., Collemare J., de Wit P.J. Synergistic action of a metalloprotease and a serine protease from Fusarium oxysporum f. sp. lycopersici cleaves chitin-binding tomato chitinases, reduces their antifungal activity, and enhances fungal virulence. Mol. Plant Microbe Interact. 2015;28:996–1008. doi: 10.1094/MPMI-04-15-0074-R. [DOI] [PubMed] [Google Scholar]
- 18.Cao S., Wang Y., Li Z., Shi W., Gao F., Zhou Y., Zhang G., Feng J. Genome-wide identification and expression analyses of the chitinases under cold and osmotic stress in Ammopiptanthus nanus. Genes. 2019;10:472. doi: 10.3390/genes10060472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cantarel B.L., Coutinho P.M., Rancurel C., Bernard T., Lombard V., Henrissat B. The Carbohydrate-Active EnZymes database (CAZy): An expert resource for glycogenomics. Nucleic Acids Res. 2009;37((Suppl. S1)):233–238. doi: 10.1093/nar/gkn663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perrot T., Pauly M., Ramírez V. Emerging roles of β-glucanases in plant development and adaptative responses. Plants. 2022;11:1119. doi: 10.3390/plants11091119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sasaki C., Vårum K.M., Itoh Y., Tamoi M., Fukamizo T. Rice chitinases: Sugar recognition specificities of the individual subsites. Glycobiology. 2006;16:1242–1250. doi: 10.1093/glycob/cwl043. [DOI] [PubMed] [Google Scholar]
- 22.Wawra S., Fesel P., Widmer H., Timm M., Seibel J., Leson L., Kesseler L., Nostadt R., Hilbert M., Langen G., et al. The fungal-specific β-glucan-binding lectin FGB1 alters cell-wall composition and suppresses glucan-triggered immunity in plants. Nat. Commun. 2016;7:13188. doi: 10.1038/ncomms13188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li T., Fan P., Yun Z., Jiang G., Zhang Z., Jiang Y. β-aminobutyric acid priming acquisition and defense response of mango fruit to Colletotrichum gloeosporioides infection based on quantitative proteomics. Cells. 2019;8:1029. doi: 10.3390/cells8091029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hong K., Gong D., Zhang L., Hu H., Jia Z., Gu H., Song K. Transcriptome characterization and expression profiles of the related defense genes in postharvest mango fruit against Colletotrichum gloeosporioides. Gene. 2016;576:275–283. doi: 10.1016/j.gene.2015.10.041. [DOI] [PubMed] [Google Scholar]
- 25.Bally I.S., Bombarely A., Chambers A.H., Cohen Y., Dillon N.L., Innes D.J., Islas-Osuna M.A., Kuhn D.N., Mueller L.A., Ophir R., et al. Mango Genome Consortium, The ‘Tommy Atkins’ mango genome reveals candidate genes for fruit quality. BMC Plant Biol. 2021;21:1–18. doi: 10.1186/s12870-021-02858-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Libertini E., Li Y., McQueen-Mason S.J. Phylogenetic analysis of the plant endo-β-1,4-glucanase gene family. J. Mol. Evol. 2004;58:506–515. doi: 10.1007/s00239-003-2571-x. [DOI] [PubMed] [Google Scholar]
- 27.Urbanowicz B.R., Bennett A.B., Del Campillo E., Catalá C., Hayashi T., Henrissat B., Höfte H., McQueen-Mason S.J., Patterson S.E., Shoseyov O., et al. Structural organization and a standardized nomenclature for plant endo-1,4-β-glucanases (cellulases) of glycosyl hydrolase family 9. Plant Physiol. 2007;144:1693–1696. doi: 10.1104/pp.107.102574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Du Q., Wang L., Yang X., Gong C., Zhang D. Populus endo-β-1,4-glucanases gene family: Genomic organization, phylogenetic analysis, expression profiles and association mapping. Planta. 2015;241:1417–1434. doi: 10.1007/s00425-015-2271-y. [DOI] [PubMed] [Google Scholar]
- 29.Kasprzewska A.N.N.A. Plant chitinases-regulation and function. Cell. Mol. Biol. Lett. 2003;8:809–824. [PubMed] [Google Scholar]
- 30.Passarinho P.A., de Vries S.C. Arabidopsis chitinases: A genomic survey. Arab. Book. 2002;1:23. doi: 10.1199/tab.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grover A. Plant chitinases: Genetic diversity and physiological roles. Crit. Rev. Plant Sci. 2012;31:57–73. doi: 10.1080/07352689.2011.616043. [DOI] [Google Scholar]
- 32.Pontón J. La pared celular de los hongos y el mecanismo de acción de la anidulafungina. Rev. Iberoamer. Micol. 2008;25:78–82. doi: 10.1016/S1130-1406(08)70024-X. [DOI] [PubMed] [Google Scholar]
- 33.Zipfel C. Pattern-recognition receptors in plant innate immunity. Curr. Opin. Immunol. 2008;20:10–16. doi: 10.1016/j.coi.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 34.Ao J., Free S.J. Genetic and biochemical characterization of the GH72 family of cell wall transglycosylases in Neurospora crassa. Fungal Genet. Biol. 2017;101:46–54. doi: 10.1016/j.fgb.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barghahn S., Arnal G., Jain N., Petutschnig E., Brumer H., Lipka V. Mixed linkage β-1,3/1,4-glucan oligosaccharides induce defense responses in Hordeum vulgare and Arabidopsis thaliana. Front. Plant Sci. 2021;12:682439. doi: 10.3389/fpls.2021.682439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pettolino F., Sasaki I., Turbic A., Wilson S.M., Bacic A., Hrmova M., Fincher G.B. Hyphal cell walls from the plant pathogen Rhynchosporium secalis contain (1,3/1,6)-β-d-glucans, galacto-and rhamnomannans, (1,3; 1,4)-β-d-glucans and chitin. FEBS J. 2009;276:3698–3709. doi: 10.1111/j.1742-4658.2009.07086.x. [DOI] [PubMed] [Google Scholar]
- 37.Pérez-Mendoza D., Rodríguez-Carvajal M.Á., Romero-Jiménez L., Farias G.D.A., Lloret J., Gallegos M.T., Sanjuán J. Novel mixed-linkage β-glucan activated by c-di-GMP in Sinorhizobium meliloti. Proc. Natl. Acad. Sci. USA. 2015;112:E757–E765. doi: 10.1073/pnas.1421748112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rebaque D., Del Hierro I., López G., Bacete L., Vilaplana F., Dallabernardina P., Pfrengle F., Jordá L., Sánchez-Vallet A., Pérez R., et al. Cell wall-derived mixed-linked β-1,3/1,4-glucans trigger immune responses and disease resistance in plants. Plant J. 2021;106:601–615. doi: 10.1111/tpj.15185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Samar D., Kieler J.B., Klutts J.S. Identification and deletion of Tft1, a predicted glycosyltransferase necessary for cell wall β-1,3; 1,4-glucan synthesis in Aspergillus fumigatus. PLoS ONE. 2015;10:117336. doi: 10.1371/journal.pone.0117336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xin Y., Wang D., Han S., Li S., Gong N., Fan Y., Ji X. Characterization of the chitinase gene family in mulberry (Morus notabilis) and MnChi18 involved in resistance to Botrytis cinerea. Genes. 2022;13:98. doi: 10.3390/genes13010098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jayaraj J., Punja Z.K. Combined expression of chitinase and lipid transfer protein genes in transgenic carrot plants enhances resistance to foliar fungal pathogens. Plant Cell Rep. 2007;26:1539–1546. doi: 10.1007/s00299-007-0368-x. [DOI] [PubMed] [Google Scholar]
- 42.Das D.K., Rahman A. Expression of a rice chitinase gene enhances antifungal response in transgenic Litchi (cv. Bedana) Am. J. Plant Sci. 2018;9:2256–2275. doi: 10.4236/ajps.2018.911163. [DOI] [Google Scholar]
- 43.Tian S.P., Yao H.J., Deng X., Xu X.B., Qin G.Z., Chan Z.L. Characterization and expression of β-1,3-glucanase genes in jujube fruit induced by the microbial biocontrol agent Cryptococcus laurentii. Phytopathology. 2007;97:260–268. doi: 10.1094/PHYTO-97-3-0260. [DOI] [PubMed] [Google Scholar]
- 44.Doxey A.C., Yaish M.W., Moffatt B.A., Griffith M., McConkey B.J. Functional divergence in the Arabidopsis β-1,3-glucanase gene family inferred by phylogenetic reconstruction of expression states. Mol. Biol. Evol. 2007;24:1045–1055. doi: 10.1093/molbev/msm024. [DOI] [PubMed] [Google Scholar]
- 45.Liu B., Lu Y., Xin Z., Zhang Z. Identification and antifungal assay of a wheat β-1,3-glucanase. Biotechnol. Lett. 2009;31:1005–1010. doi: 10.1007/s10529-009-9958-8. [DOI] [PubMed] [Google Scholar]
- 46.Zhang S.B., Zhang W.J., Zhai H.C., Lv Y.Y., Cai J.P., Jia F., Wang J.-S., Hu Y.S. Expression of a wheat β-1,3-glucanase in Pichia pastoris and its inhibitory effect on fungi commonly associated with wheat kernel. Protein Expr. Purif. 2019;154:134–139. doi: 10.1016/j.pep.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 47.Aggarwal R., Purwar S., Kharbikar L., Gupta S. Induction of a wheat β-1,3-glucanase gene during the defense response to Bipolaris sorokiniana. Acta Phytopathol. Entomol. Hungarica. 2011;46:39–47. doi: 10.1556/APhyt.46.2011.1.5. [DOI] [Google Scholar]
- 48.Liu J.J., Ekramoddoullah A.K., Zamani A. A class IV chitinase is up-regulated by fungal infection and abiotic stresses and associated with slow-canker-growth resistance to Cronartium ribicola in western white pine (Pinus monticola) Phytopathology. 2005;95:284–291. doi: 10.1094/PHYTO-95-0284. [DOI] [PubMed] [Google Scholar]
- 49.Singh H.R., Deka M., Das S. Enhanced resistance to blister blight in transgenic tea (Camellia sinensis [L.] O. Kuntze) by overexpression of class I chitinase gene from potato (Solanum tuberosum) Funct. Integr. Genomics. 2015;15:461–480. doi: 10.1007/s10142-015-0436-1. [DOI] [PubMed] [Google Scholar]
- 50.Kumar S., Stecher G., Li M., Knyaz C., Tamura K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zheng T., Zhang K., Sadeghnezhad E., Jiu S., Zhu X., Dong T., Liu Z., Guan L., Jia H., Fang J. Chitinase family genes in grape differentially expressed in a manner specific to fruit species in response to Botrytis cinerea. Mol. Biol. Rep. 2020;47:7349–7363. doi: 10.1007/s11033-020-05791-y. [DOI] [PubMed] [Google Scholar]
- 52.Flaishman M.A., Kolattukudy P.E. Timing of fungal invasion using host’s ripening hormone as a signal. Proc. Natl. Acad. Sci. USA. 1994;91:6579–6583. doi: 10.1073/pnas.91.14.6579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.de Bellaire L.D.L., Chillet M., Mourichon X. Elaboration of an early quantification method of quiescent infections of Colletotrichum musae on bananas. Plant Dis. 2000;84:128–133. doi: 10.1094/PDIS.2000.84.2.128. [DOI] [PubMed] [Google Scholar]
- 54.Dautt-Castro M., López-Virgen A.G., Ochoa-Leyva A., Contreras-Vergara C.A., Sortillón-Sortillón A.P., Martínez-Téllez M.A., González-Aguilar G.A., Casas-Flores J.S., Sañudo-Barajas A., Kuhn D.N., et al. Genome-wide identification of mango (Mangifera indica L.) polygalacturonases: Expression analysis of family members and total enzyme activity during fruit ripening. Front. Plant Sci. 2019;10:969. doi: 10.3389/fpls.2019.00969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rodríguez-López E.S., González-Prieto J.M., Mayek-Pérez N. La infección de Colletotrichum gloeosporioides (Penz.) Penz. y Sacc. en aguacatero (Persea americana Mill.): Aspectos bioquímicos y genéticos. Rev. Mex. Fitopatol. 2009;27:53–63. [Google Scholar]
- 56.Oliveira-Garcia E., Deising H.B. Infection structure–specific expression of β-1,3-glucan synthase is essential for pathogenicity of Colletotrichum graminicola and evasion of β-glucan–triggered immunity in maize. Plant Cell. 2013;25:2356–2378. doi: 10.1105/tpc.112.103499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dautt-Castro M., Ochoa-Leyva A., Contreras-Vergara C.A., Pacheco-Sanchez M.A., Casas-Flores S., Sanchez-Flores A., Kuhn D.N., Islas-Osuna M.A. Mango (Mangifera indica L.) cv. Kent fruit mesocarp de novo transcriptome assembly identifies gene families important for ripening. Front. Plant Sci. 2015;6:62. doi: 10.3389/fpls.2015.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schmittgen T.D., Livak K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 59.Fujikawa T., Sakaguchi A., Nishizawa Y., Kouzai Y., Minami E., Yano S., Koga H., Meshi T., Nishimura M. Surface α-1,3-glucan facilitates fungal stealth infection by interfering with innate immunity in plants. PLoS Pathog. 2012;8:1002882. doi: 10.1371/journal.ppat.1002882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang J., Tian N., Huang X., Chen L.Y., Schläppi M., Xu Z.Q. The tall fescue turf grass class I chitinase gene FaChit1 is activated by fungal elicitors, dehydration, ethylene, and mechanical wounding. Plant Mol. Biol. Rep. 2009;27:305–314. doi: 10.1007/s11105-008-0086-8. [DOI] [Google Scholar]
- 61.Felix G., Regenass M., Boller T. Specific perception of subnanomolar concentrations of chitin fragments by tomato cells: Induction of extracellular alkalinization, changes in protein phosphorylation, and establishment of a refractory state. Plant J. 1993;4:307–316. doi: 10.1046/j.1365-313X.1993.04020307.x. [DOI] [Google Scholar]
- 62.El Gueddari N.E., Rauchhaus U., Moerschbacher B.M., Deising H.B. Developmentally regulated conversion of surface-exposed chitin to chitosan in cell walls of plant pathogenic fungi. New Phytol. 2002;156:103–112. doi: 10.1046/j.1469-8137.2002.00487.x. [DOI] [Google Scholar]
- 63.de Jonge R., Peter van Esse H., Kombrink A., Shinya T., Desaki Y., Bours R., van der Krol S., Shibuya N., Joosten M.H.A.J., Thomma B.P.H.J. Conserved fungal LysM effector Ecp6 prevents chitin-triggered immunity in plants. Science. 2010;329:953–955. doi: 10.1126/science.1190859. [DOI] [PubMed] [Google Scholar]
- 64.Karunanayake L.C., Adikaram N., Kumarihamy B.M., Bandara B.R., Abayasekara C. Role of antifungal gallotannins, resorcinols and chitinases in the constitutive defence of immature mango (Mangifera indica L.) against Colletotrichum gloeosporioides. J. Phytopathol. 2011;159:657–664. doi: 10.1111/j.1439-0434.2011.01818.x. [DOI] [Google Scholar]
- 65.Sinniah G.D., Adikaram N.K.B., Abayasekara C.L. Differential defense responses expressed in mango (Mangifera indica L.) cultivars resistant and susceptible to Colletotrichum gloeosporioides. Indian Phytopathol. 2012;66:34–40. [Google Scholar]
- 66.Bezirganoglu I., Hwang S.Y., Fang T.J., Shaw J.F. Transgenic lines of melon (Cucumis melo L. var. makuwa cv. ‘Silver Light’) expressing antifungal protein and chitinase genes exhibit enhanced resistance to fungal pathogens. Plant Cell Tissue Organ Cult. 2013;112:227–237. doi: 10.1007/s11240-012-0227-5. [DOI] [Google Scholar]
- 67.Que Y., Su Y., Guo J., Wu Q., Xu L. A global view of transcriptome dynamics during Sporisorium scitamineum challenge in sugarcane by RNA-Seq. PLoS ONE. 2014;9:e106476. doi: 10.1371/journal.pone.0106476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Han Y., Zhang K., Yang J., Zhang N., Fang A., Zhang Y., Liu Y., Chen Z., Hsiang T., Sun W. Differential expression profiling of the early response to Ustilaginoidea virens between false smut resistant and susceptible rice varieties. BMC Genom. 2015;16:1–15. doi: 10.1186/s12864-015-2193-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Edgar R.C. MUSCLE: A multiple sequence alignment method with reduced time and space complexity. BMC Bioinform. 2004;5:113. doi: 10.1186/1471-2105-5-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thompson J.D., Higgins D.G., Gibson T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Saitou N., Nei M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 72.Gearing L.J., Cumming H.E., Chapman R., Finkel A.M., Woodhouse I.B., Luu K., Gould J.A., Forster S.C., Hertzog P.J. CiiiDER: A tool for predicting and analysing transcription factor binding sites. PLoS ONE. 2019;14:e0215495. doi: 10.1371/journal.pone.0215495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Castro-Mondragon J.A., Riudavets-Puig R., Rauluseviciute I., Berhanu Lemma R., Turchi L., Blanc-Mathieu R., Lucas J., Boddie P., Khan A., Pérez N.M., et al. JASPAR 2022: The 9th release of the open-access database of transcription factor binding profiles. Nucleic Acids Res. 2022;50:165–173. doi: 10.1093/nar/gkab1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Álvarez L.V., Hattori Y., Deocaris C.C., Mapanao C.P., Bautista A.B., Cano M.J.B., Nakashima C. Colletotrichum asianum causes anthracnose in philippine mango cv. Carabao. Australas. Plant Dis. Notes. 2020;15:13. doi: 10.1007/s13314-020-00384-x. [DOI] [Google Scholar]
- 75.López-Gómez R., Gomez-Lim M.A. A method for extracting intact RNA from fruits rich in polysaccharides using ripe mango mesocarp. HortScience. 1992;27:440–442. doi: 10.21273/HORTSCI.27.5.440. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are contained within the article.