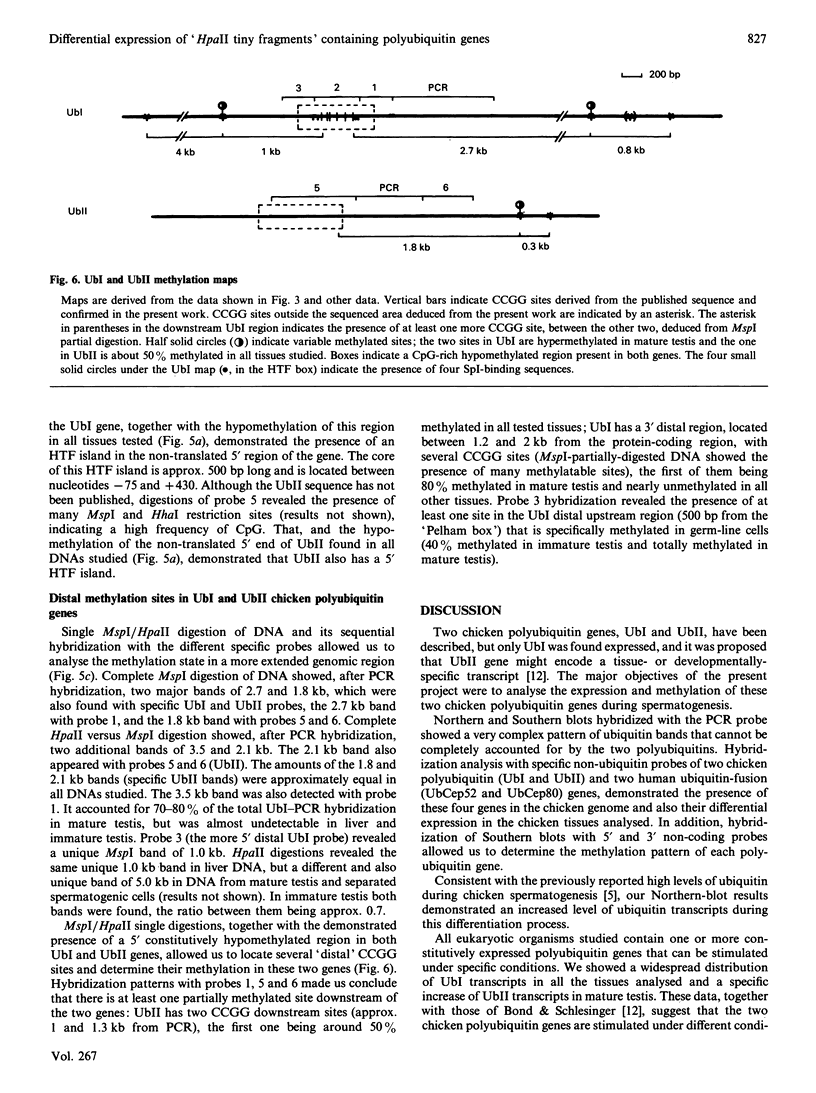
Abstract
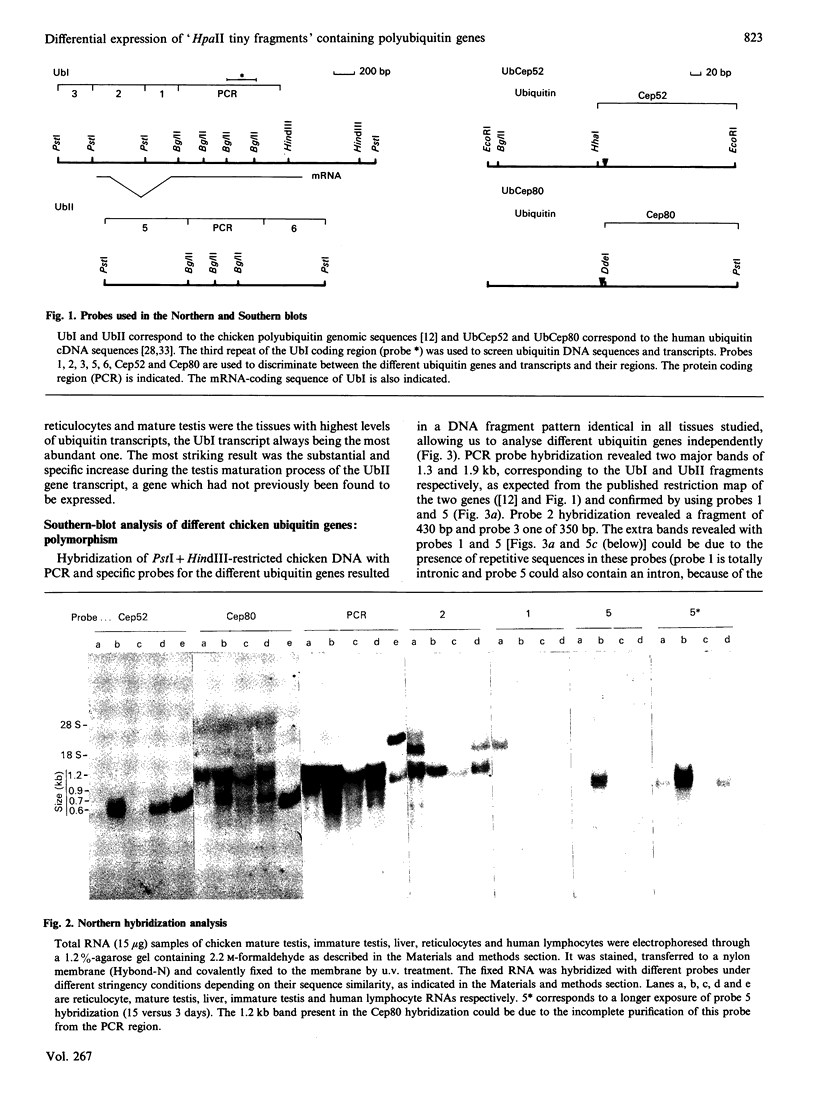
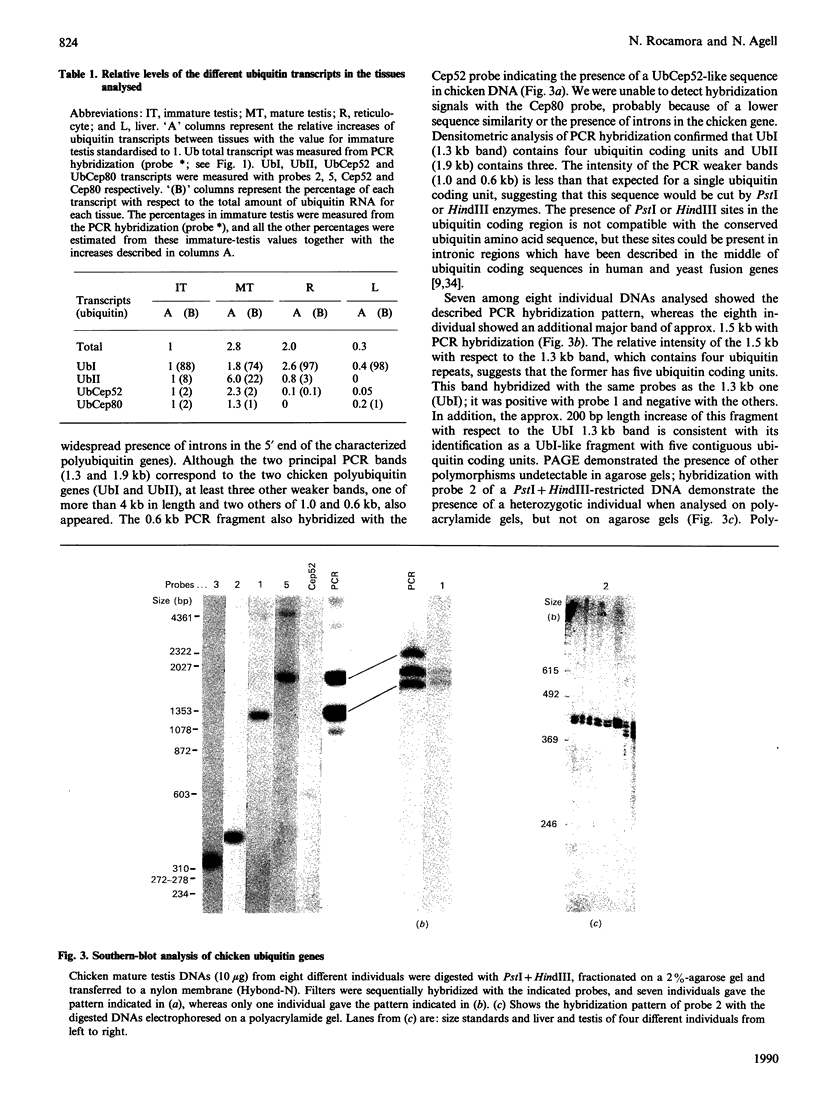
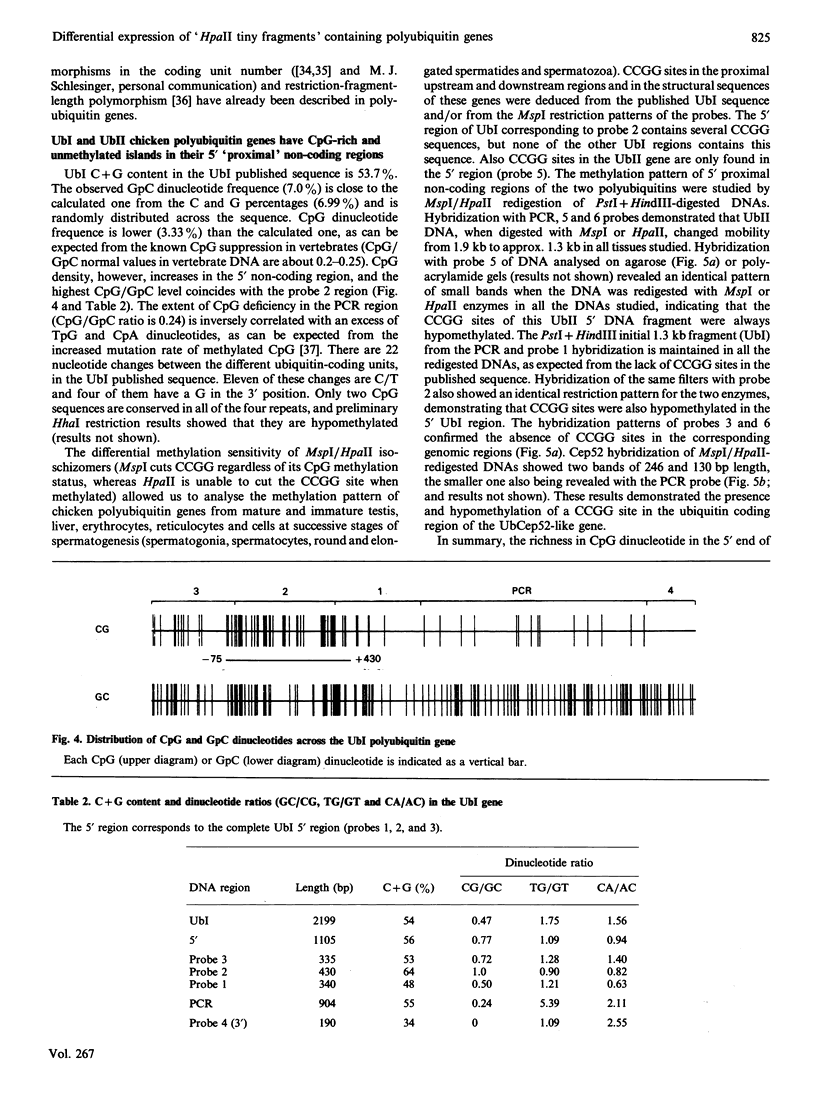
Northern analysis demonstrated that levels of ubiquitin transcript increased during the chicken testis maturation process, in agreement with the previously published increase of ubiquitin during this differentiation process. Specific probes for four different ubiquitin genes (two polyubiquitins, UbI and UbII, and two ubiquitin-fusion genes, UbCep52 and UbCep80) allowed us to analyse the expression of each individual gene. UbI polyubiquitin gene was expressed in all the tissues tested, and its transcript was the most abundant ubiquitin RNA in all of them. Unspliced UbI transcript, already detected in stressed chicken-embryo fibroblast, was also present in immature testis and reticulocytes. UbII, a chicken polyubiquitin gene not previously found expressed and not heat-shock-inducible, was specifically stimulated during the testis maturation process. Two minor ubiquitin fusion transcripts of 0.6 and 0.7 kb, corresponding to UbCep52 and UbCep80 respectively, were also found in chicken testis. Although differentially expressed, it was found that UbI and UbII chicken polyubiquitin genes had an HTF ('HpaII tiny fragments') island (CpG-rich and constitutively unmethylated region) in their 5' proximal non-coding region. In addition, we demonstrated the coexistence of 3' and/or 5' relatively distal methylated sites together with these 5' proximal HTF islands in both chicken polyubiquitin genes. 3' and 5' distal UbI CCGG sites were specifically hypermethylated in mature testis, whereas a 3' distal UbII CCGG site was found to be about 50% methylated in all DNAs tested.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agell N., Mezquita C. Cellular content of ubiquitin and formation of ubiquitin conjugates during chicken spermatogenesis. Biochem J. 1988 Mar 15;250(3):883–889. doi: 10.1042/bj2500883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen R. L., O'Brien D. A., Jones C. C., Rockett D. L., Eddy E. M. Expression of heat shock proteins by isolated mouse spermatogenic cells. Mol Cell Biol. 1988 Aug;8(8):3260–3266. doi: 10.1128/mcb.8.8.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antequera F., Macleod D., Bird A. P. Specific protection of methylated CpGs in mammalian nuclei. Cell. 1989 Aug 11;58(3):509–517. doi: 10.1016/0092-8674(89)90431-5. [DOI] [PubMed] [Google Scholar]
- Bird A. P. CpG-rich islands and the function of DNA methylation. Nature. 1986 May 15;321(6067):209–213. doi: 10.1038/321209a0. [DOI] [PubMed] [Google Scholar]
- Bond U., Schlesinger M. J. The chicken ubiquitin gene contains a heat shock promoter and expresses an unstable mRNA in heat-shocked cells. Mol Cell Biol. 1986 Dec;6(12):4602–4610. doi: 10.1128/mcb.6.12.4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond U., Schlesinger M. J. Ubiquitin is a heat shock protein in chicken embryo fibroblasts. Mol Cell Biol. 1985 May;5(5):949–956. doi: 10.1128/mcb.5.5.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Coulondre C., Miller J. H., Farabaugh P. J., Gilbert W. Molecular basis of base substitution hotspots in Escherichia coli. Nature. 1978 Aug 24;274(5673):775–780. doi: 10.1038/274775a0. [DOI] [PubMed] [Google Scholar]
- Distel R. J., Kleene K. C., Hecht N. B. Haploid expression of a mouse testis alpha-tubulin gene. Science. 1984 Apr 6;224(4644):68–70. doi: 10.1126/science.6701535. [DOI] [PubMed] [Google Scholar]
- Doerfler W. DNA methylation and gene activity. Annu Rev Biochem. 1983;52:93–124. doi: 10.1146/annurev.bi.52.070183.000521. [DOI] [PubMed] [Google Scholar]
- Dretzen G., Bellard M., Sassone-Corsi P., Chambon P. A reliable method for the recovery of DNA fragments from agarose and acrylamide gels. Anal Biochem. 1981 Apr;112(2):295–298. doi: 10.1016/0003-2697(81)90296-7. [DOI] [PubMed] [Google Scholar]
- Dworkin-Rastl E., Shrutkowski A., Dworkin M. B. Multiple ubiquitin mRNAs during Xenopus laevis development contain tandem repeats of the 76 amino acid coding sequence. Cell. 1984 Dec;39(2 Pt 1):321–325. doi: 10.1016/0092-8674(84)90010-2. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Finley D., Bartel B., Varshavsky A. The tails of ubiquitin precursors are ribosomal proteins whose fusion to ubiquitin facilitates ribosome biogenesis. Nature. 1989 Mar 30;338(6214):394–401. doi: 10.1038/338394a0. [DOI] [PubMed] [Google Scholar]
- Finley D., Ozkaynak E., Varshavsky A. The yeast polyubiquitin gene is essential for resistance to high temperatures, starvation, and other stresses. Cell. 1987 Mar 27;48(6):1035–1046. doi: 10.1016/0092-8674(87)90711-2. [DOI] [PubMed] [Google Scholar]
- Giorda R., Ennis H. L. Structure of two developmentally regulated Dictyostelium discoideum ubiquitin genes. Mol Cell Biol. 1987 Jun;7(6):2097–2103. doi: 10.1128/mcb.7.6.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groudine M., Conkin K. F. Chromatin structure and de novo methylation of sperm DNA: implications for activation of the paternal genome. Science. 1985 May 31;228(4703):1061–1068. doi: 10.1126/science.2986289. [DOI] [PubMed] [Google Scholar]
- Hershko A., Ciechanover A. The ubiquitin pathway for the degradation of intracellular proteins. Prog Nucleic Acid Res Mol Biol. 1986;33:19-56, 301. doi: 10.1016/s0079-6603(08)60019-7. [DOI] [PubMed] [Google Scholar]
- Jones P. A. Altering gene expression with 5-azacytidine. Cell. 1985 Mar;40(3):485–486. doi: 10.1016/0092-8674(85)90192-8. [DOI] [PubMed] [Google Scholar]
- Kaput J., Sneider T. W. Methylation of somatic vs germ cell DNAs analyzed by restriction endonuclease digestions. Nucleic Acids Res. 1979 Dec 20;7(8):2303–2322. doi: 10.1093/nar/7.8.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolsto A. B., Kollias G., Giguere V., Isobe K. I., Prydz H., Grosveld F. The maintenance of methylation-free islands in transgenic mice. Nucleic Acids Res. 1986 Dec 22;14(24):9667–9678. [PMC free article] [PubMed] [Google Scholar]
- Kreitman M., Aguadé M. Genetic uniformity in two populations of Drosophila melanogaster as revealed by filter hybridization of four-nucleotide-recognizing restriction enzyme digests. Proc Natl Acad Sci U S A. 1986 May;83(10):3562–3566. doi: 10.1073/pnas.83.10.3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund P. K., Moats-Staats B. M., Simmons J. G., Hoyt E., D'Ercole A. J., Martin F., Van Wyk J. J. Nucleotide sequence analysis of a cDNA encoding human ubiquitin reveals that ubiquitin is synthesized as a precursor. J Biol Chem. 1985 Jun 25;260(12):7609–7613. [PubMed] [Google Scholar]
- Meehan R. R., Lewis J. D., McKay S., Kleiner E. L., Bird A. P. Identification of a mammalian protein that binds specifically to DNA containing methylated CpGs. Cell. 1989 Aug 11;58(3):499–507. doi: 10.1016/0092-8674(89)90430-3. [DOI] [PubMed] [Google Scholar]
- Mezquita C., Teng C. S. Studies on sex-organ development. Changes in nuclear and chromatin composition and genomic activity during spermatogenesis in the maturing rooster testis. Biochem J. 1977 Apr 15;164(1):99–111. doi: 10.1042/bj1640099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozkaynak E., Finley D., Solomon M. J., Varshavsky A. The yeast ubiquitin genes: a family of natural gene fusions. EMBO J. 1987 May;6(5):1429–1439. doi: 10.1002/j.1460-2075.1987.tb02384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponzetto-Zimmerman C., Wolgemuth D. J. Methylation of satellite sequences in mouse spermatogenic and somatic DNAs. Nucleic Acids Res. 1984 Mar 26;12(6):2807–2822. doi: 10.1093/nar/12.6.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappold G. A., Stubbs L., Labeit S., Crkvenjakov R. B., Lehrach H. Identification of a testis-specific gene from the mouse t-complex next to a CpG-rich island. EMBO J. 1987 Jul;6(7):1975–1980. doi: 10.1002/j.1460-2075.1987.tb02460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechsteiner M. Ubiquitin-mediated pathways for intracellular proteolysis. Annu Rev Cell Biol. 1987;3:1–30. doi: 10.1146/annurev.cb.03.110187.000245. [DOI] [PubMed] [Google Scholar]
- Rocamora N., Mezquita C. Chicken spermatogenesis is accompanied by a genomic-wide loss of DNA methylation. FEBS Lett. 1989 Apr 24;247(2):415–418. doi: 10.1016/0014-5793(89)81382-1. [DOI] [PubMed] [Google Scholar]
- Salvesen G., Lloyd C., Farley D. cDNA encoding a human homolog of yeast ubiquitin 1. Nucleic Acids Res. 1987 Jul 10;15(13):5485–5485. doi: 10.1093/nar/15.13.5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapienza C., Peterson A. C., Rossant J., Balling R. Degree of methylation of transgenes is dependent on gamete of origin. Nature. 1987 Jul 16;328(6127):251–254. doi: 10.1038/328251a0. [DOI] [PubMed] [Google Scholar]
- Schlesinger M. J., Bond U. Ubiquitin genes. Oxf Surv Eukaryot Genes. 1987;4:77–91. [PubMed] [Google Scholar]
- Toniolo D., Persico M., Alcalay M. A "housekeeping" gene on the X chromosome encodes a protein similar to ubiquitin. Proc Natl Acad Sci U S A. 1988 Feb;85(3):851–855. doi: 10.1073/pnas.85.3.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yost H. J., Lindquist S. RNA splicing is interrupted by heat shock and is rescued by heat shock protein synthesis. Cell. 1986 Apr 25;45(2):185–193. doi: 10.1016/0092-8674(86)90382-x. [DOI] [PubMed] [Google Scholar]
- Zhang X. Y., Wang R. Y., Ehrlich M. Human DNA sequences exhibiting gamete-specific hypomethylation. Nucleic Acids Res. 1985 Jul 11;13(13):4837–4851. doi: 10.1093/nar/13.13.4837. [DOI] [PMC free article] [PubMed] [Google Scholar]