Introduction
Oral Janus kinase 1 inhibitor upadacitinib at 15-mg and 30-mg daily doses has been approved for moderate-to-severe atopic dermatitis (AD). However, the cost-effectiveness of these 2 regimens is currently unclear. This study aimed to evaluate the impact of tapering upadacitinib from 30 mg daily to 15 mg daily on disease control in AD patients. We retrospectively recruited 7 adult AD patients who received upadacitinib in the Dermatology Department of Kaohsiung Medical University Hospital from 2022 to 2023. This study was approved by the ethics committee of Kaohsiung Medical University Hospital KMUHIRB-E(I)-20230199. The Eczema Area and Severity Index (EASI) and Atopic Dermatitis Control Tool (ADCT) were utilized to assess treatment responses. ADCT is a questionnaire assessing patient-perceived control of their AD [1].
Case Presentation
Seven AD patients had a baseline EASI of 16.6 ± 3.0 and ADCT of 13.9±4.5 before upadacitinib treatment (Table 1). These patients received upadacitinib 30 mg daily treatment for the initial two months (Figure 1). Upadacitinib 30 mg daily showed a rapid effect with a mean EASI being reduced to 2.7 ± 3.4 and ADCT to 3.7 ± 4.2 after 2 months. Notably, 6 patients achieved EASI-90 (≥ 90% improvement from baseline) within 2 months with a mean EASI 1.5±1.3 (Figure 2A). Their dosing was reduced to 15 mg daily in the following 2 months. Step-down treatment maintained an acceptable therapeutic efficacy in these patients (EASI 1.7±1.2 after 4 months), whereas step-down treatment led to slightly increased ADCT scores (3.8±3.1 after 4 months) (Figure 2B). After discontinuation of upadacitinib, 5/7 patients suffered from rapid disease flare within 1 month.
Table 1.
Demographic characteristics of included atopic dermatitis patients and their disease severity at baseline and after upadacitinib treatment
| Patient No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Demographic characteristics | |||||||
| Gender | F | F | F | F | M | M | M |
| Age (y/o) | 20 | 30 | 25 | 22 | 32 | 34 | 30 |
| IgE (IU/mL) | 1508 | 334 | 57.1 | 1526 | 17508 | 1931 | 228 |
| Weight (kg) | 61 | 67 | 49 | 52 | 78 | 77 | 69 |
| Previous treatment | MTX | MTX | UVB | UVB | Systemic CS | UVB | Systemic CS |
| Disease severity | |||||||
| Baseline EASI | 19.8 | 18.2 | 14.1 | 16.1 | 17.4 | 11.3 | 19.4 |
| Baseline ADCT | 14 | 17 | 16 | 8 | 10 | 11 | 21 |
| Response to Upa | |||||||
| EASI Post 2 months 30mg/day (ΔEASI)a | 1.9 (90.4) | 1.8 (90.1) | 0.4 (97.2) | 1.4 (91.3) | 9.9 (43.1) | 0 (100) | 1.9 (90.2) |
| EASI Post 2 months 15mg/day (ΔEASI) | 2.0 (89.9) | 2.5 (86.3) | 0.4 (97.2) | 2.6 (83.9) | 8.9b (48.9) | 0 (100) | 2.8 (85.5) |
| Dosing reduction | Y | Y | Y | Y | N | Y | Y |
| ADCT Post 2 months 30mg/day | 1 | 4 | 0 | 2 | 10 | 0 | 9 |
| ADCT Post 2 months 15mg/day | 3 | 7 | 2 | 3 | 8b | 0 | 8 |
| Discontinuation of UPA | |||||||
| EASI score c | 2.65 | 9.8 | 0.8 | 2.6 | 14.7 | 0 | 12.0 |
| ADCT score c | 13 | 13 | 10 | 1 | 16 | 0 | 22 |
| Treatment required | MTX | MTX+UVB | AZA | AnH | CsA | AnH | Upa + UVB |
ΔEASI: Percentage change in EASI score as compared with baseline EASI.
response to additional 1 month of 30mg/day treatment due to not achieving EASI-90.
within 1 month (before restart of systemic treatment for AD).
ADCT = Atopic Dermatitis Control Tool; AnH = Antihistamine; AZA = azathioprine; CS = corticosteroids; CsA = cyclosporine; EASI = Eczema Area and Severity Index; MTX = methotrexate; Upa = upadacitinib; UVB = narrow-band UVB.
Figure 1.
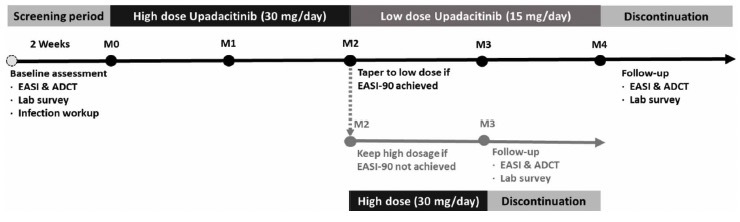
Our department protocol and dosing regimen for patients with moderate-to-severe atopic dermatitis receiving upadacitinib.
Figure 2.
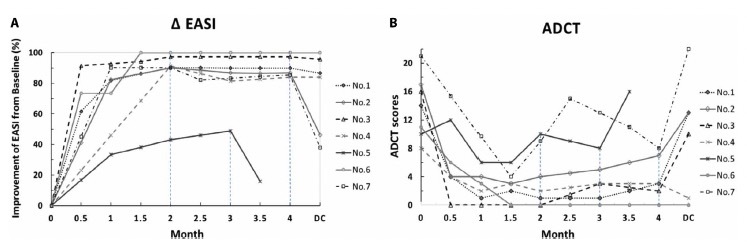
Improvement of disease severity assessed through percentage change in Eczema Area and Severity Index (ΔEASI) (A) and Atopic Dermatitis Control Tool (ADCT) scores (B) after treatment with upadacitinib. Disease severity was followed up within one month after discontinuation of upadacitinib. The improvement in EASI scores generally correlates with improvement in ADCT scores. Notably, patients #5 and #7 had relatively fluctuated disease severity (ADCT score) during upadacitinib treatment. Patients #4 and #6 had stable EASI and ADCT scores after discontinuation of upadacitinib. ADCT score above 7 points is thought to indicate inadequate control over atopic dermatitis, whereas an increase of 5 points indicates a worsening of disease control.
Discussion
Previous large-scale clinical trials showed EASI-90 response rate of upadacitinib 30 mg daily dose was about 60–61% in patients with moderate-to-severe AD at week 8 [2–4]. Meanwhile, EASI-90 response rates in patients receiving 15 mg was 36% at week 8 and 43%–48% at week 16 [2,3]. Therefore, upadacitinib 30-mg daily dose was used initially to achieve rapid disease control. Then, this study demonstrated the impacts of dose reduction on skin lesional severity and subjective symptoms in patients receiving upadacitinib. Tapering upadacitinib dosage in patients achieving EASI-90 led to persistent EASI-90 effect in 2/6 patients after 4 months (patients #3 and #6). These 2 patients were found to have lower baseline EASI scores under previous treatment with phototherapy. This observation suggests that in AD patients with less severe skin lesions, EASI-90 response may be maintained after upadacitinib dosage is tapered. Of note, patient 5 failed to achieve EASI-90 after high-dose upadacitinib treatment for 3 months. Serum total Immunoglobulin E (IgE) has been indicated as a potential biomarker for treatment response of AD 5,6. Patient 5 was found to show elevation of serum IgE from 17508 to 24860 IU/mL during upadacitinib treatment. This observation suggests that extremely high IgE level and failure to show reducing IgE level during treatment may indicate a poor response.
Conclusions
Upadacitinib 15 mg daily can be used as a maintenance therapy in patients with moderate AD after achieving EASI-90 to balance the efficacy and cost in real-world practice.
Footnotes
Funding: None.
Competing Interests: None.
Authorship: All authors have contributed significantly to this publication.
References
- 1.Pariser DM, Simpson EL, Gadkari A, et al. Evaluating patient-perceived control of atopic dermatitis: design, validation, and scoring of the Atopic Dermatitis Control Tool (ADCT) Curr Med Res Opin. 2020;36(3):367–376. doi: 10.1080/03007995.2019.1699516. [DOI] [PubMed] [Google Scholar]
- 2.Reich K, Teixeira HD, de Bruin-Weller M, et al. Safety and efficacy of upadacitinib in combination with topical corticosteroids in adolescents and adults with moderate-to-severe atopic dermatitis (AD Up): results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2021 Jun 5;397(10290):2169–2181. doi: 10.1016/s0140-6736(21)00589-4. [DOI] [PubMed] [Google Scholar]
- 3.Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021 Jun 5;397(10290):2151–2168. doi: 10.1016/s0140-6736(21)00588-2. [DOI] [PubMed] [Google Scholar]
- 4.Blauvelt A, Teixeira HD, Simpson EL, et al. Efficacy and Safety of Upadacitinib vs Dupilumab in Adults With Moderate-to-Severe Atopic Dermatitis: A Randomized Clinical Trial. JAMA Dermatol. 2021 Sep 1;157(9):1047–1055. doi: 10.1001/jamadermatol.2021.3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kiiski V, Karlsson O, Remitz A, Reitamo S. High serum total IgE predicts poor long-term outcome in atopic dermatitis. Acta Derm Venereol. 2015 Nov;95(8):943–7. doi: 10.2340/00015555-2126. [DOI] [PubMed] [Google Scholar]
- 6.Huang TH, Chen YC, Lin SY, et al. Treatment of atopic dermatitis with dupilumab in Taiwan: dynamic changes of IgE levels as a potential response biomarker. Eur J Dermatol. 2019 Dec 1;29(6):658–659. doi: 10.1684/ejd.2019.3661. [DOI] [PubMed] [Google Scholar]


