Abstract
Japanese encephalitis virus (JEV) is a major threat to human health. Bangladesh is considering introducing a JEV vaccine; however, the investment case is hampered by a limited understanding of key aspects of JEV ecology. We conducted a seroprevalence study in a high-incidence region using an assay that limits cross-reactivity with dengue virus. We also trapped mosquitoes and collected information about potential host species. We used mathematical models to recover risk factors for infection and underlying probabilities of severe disease and death. We observed 19.0% [95% confidence interval (CI):17.1 to 21.1] of JEV antibodies. On average, 0.7% (95% CI: 0.2 to 2.0) of the susceptible population gets infected yearly, with pig proximity being the main human infection risk factor. Our traps captured 10 different mosquito species that have been linked with JEV transmission. We estimated that 1 in 1000 infections results in severe disease, 1 in 10,000 results in death, and 76% of severe cases are missed by surveillance.
Optimal deployment of the Japanese encephalitis vaccine may come from focusing on districts with the most pig-raising households.
INTRODUCTION
Japanese encephalitis (JE) is a disease caused by the JE virus (JEV), a flavivirus capable of fatal encephalitis in humans. JEV was first isolated from humans in Japan in 1935 and, 3 years later, from the vector Culex tritaeniorhynchus (1, 2). There are estimated to be 13,600 to 20,400 annual deaths from JE globally across South and Southeast Asia (3). There exist effective human vaccines against JEV, with vaccine efficacies of more than 95% (4). However, not all JEV-affected countries have the vaccine implemented in their routine immunization programs. The decision on whether to use a vaccine will depend on competing health priorities and the availability of resources. Central to decision-making on whether to introduce a vaccine into a national or subnational immunization schedule is a vaccine investment case, which quantifies the potential impact of a vaccine program on human health. Bangladesh does not currently use the JEV vaccine. The first detected case of JE in Bangladesh was in 1977 (5), and there are cases observed each year (6). There is currently renewed interest in vaccinating the population.
A key complication in the development of a vaccine investment case for JEV in Bangladesh is an inadequate understanding of the underlying burden of infection or where risk is concentrated. In general, the disease ecology of JEV is insufficiently understood across the regions where it circulates, to the extent that we are still not sure of the relevance of different vectors and hosts in maintaining transmission. Humans are dead-end hosts and cannot transmit JEV to mosquitoes. Instead, pigs are often cited as the main amplifying hosts; however, it has been suggested that domestic and wading birds may act as reservoirs and also play a key role in transmission (7, 8). Similarly, a number of different vector species, including Culex quinquefasciatus, Aedes albopictus, Culex vishnui, Culex pseudovishnui, and Cx. tritaeniorhynchus, have been shown to be able to transmit JEV; however, their relevance to infection risk in humans remains unclear, which may also differ across transmission settings (9–11).
There are a number of key barriers to studying JEV disease ecology. The vast majority of infections are subclinical and undetected by disease surveillance systems. The World Health Organization (WHO) Global Health Observatory reports (i.e., detected) cases of severe disease, which are strongly dependent on surveillance systems (12). Even when individuals do develop symptoms and seek health care, they can be nonspecific, and confirmatory testing often requires cerebrospinal fluid and convalescent serum samples, which are difficult to obtain. Furthermore, tertiary health care centers that do have appropriate testing facilities are often located far from where infections occur and often not accessed, which means many severe cases remain missed (13). Last, serological studies that look for evidence of historic infection are complicated by the frequent cocirculation of dengue virus (DENV), another flavivirus that cross-reacts with standard commercial JEV serological assays (14). These complications mean that it has been difficult to identify risk factors for JEV infection, as well as quantify the underlying risk of severe disease and death following infection.
To fill the knowledge gap on both infection and disease burden, we focused on the Chapai Nawabganj district in the Rajshahi division, an area of Bangladesh estimated to have the highest incidence of JE, at 2.7 per 100,000 population (6, 15). Bangladesh reports JE cases each year, but the relationship between underlying levels of infection and reported disease remains unclear (12). We used complementary data sources overlapping in this high-incidence region that allowed us to explore different aspects of the disease system. Central to this effort was a seroprevalence study across individuals of all ages, where we used an assay that minimizes cross-reactivity with DENV (16). To capture the potential role of hosts, we used a pig census in the region that identified all pig-owning households and nomadic pig herds (8). Furthermore, we trapped and speciated mosquitoes and used questionnaire data to capture the presence of domestic and wading birds. Last, we use the home location of confirmed JE cases that sought care at the local tertiary care hospital, alongside health care–seeking studies to estimate the number of deaths and severe disease cases missed by surveillance systems (13). This series of studies from a single setting provides a unique opportunity to develop the necessary understanding of infection burden and subsequent severe disease risk to underpin an investment case when applied to the national level.
RESULTS
Human JEV infections and risk factors
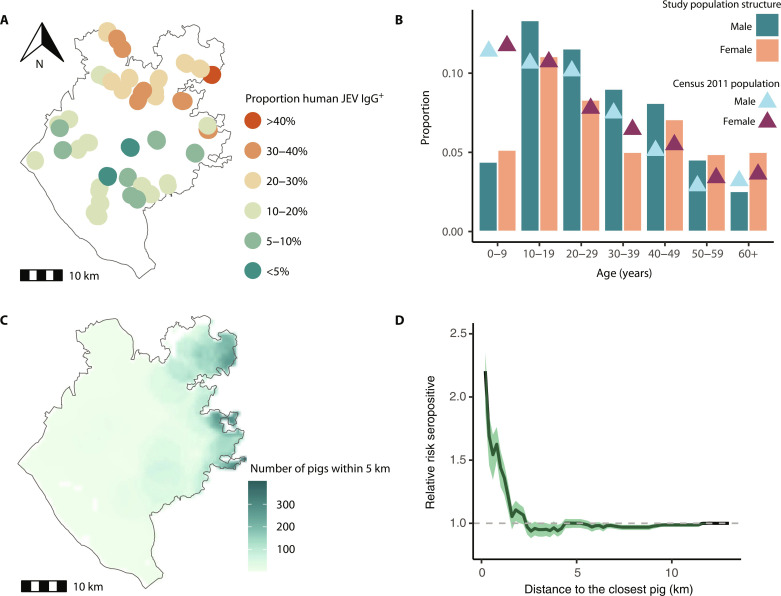
A total of 1455 individuals from 393 households in 39 randomly selected communities in Chapai Nawabganj district in Northwest Bangladesh participated in the cross-sectional seroprevalence study (Table 1). Participants had a median age of 27 years (range: 2 to 90) and 53% were female. The age distribution of sampled individuals was largely representative of the 2011 census, except for those under 5 years old, who were less likely to participate (Fig. 1B). Overall immunoglobulin G seropositivity to JEV was 19.0% [95% confidence interval (CI): 17.1 to 21.1], with no difference in seropositivity by sex (P value: 0.6). Seropositivity increased with age, rising from 2.1% (95% CI: 0.7 to 6.2) in those <10 years to 30% in those >40 years of age (95% CI: 22.2 to 39.1). Mean seropositivity by community was spatially heterogeneous, ranging from 0 to 40%, with communities in the north of Chapai Nawabganj having higher seropositivity than those in the south (Fig. 1A).
Table 1. Individual-, household- and community-level characteristics of participants stratified by serostatus to JEV.
*P < 0.1; **P < 0.05; ***P < 0.001.
| Overall, N = 1455* | Seronegative, N = 1178* | Seropositive, N = 277* | |
|---|---|---|---|
| Age | |||
| <10 years | 139 (9.6%) | 136 (12%) | 3 (1.1%) |
| 10–19 years | 355 (24%) | 304 (26%)*** | 51 (18%)*** |
| 20–29 years | 289 (20%) | 236 (20%)*** | 53 (19%)*** |
| 30–39 years | 204 (14%) | 157 (13%)*** | 47 (17%)*** |
| 40–49 years | 221 (15%) | 163 (14%)*** | 58 (21%)*** |
| 50–59 years | 137 (9.4%) | 105 (8.9%)*** | 32 (12%)*** |
| >60 years | 110 (7.6%) | 77 (6.5%)*** | 33 (12%)*** |
| Female | 778 (53%) | 634 (54%) | 144 (5%) |
| Lived always same village | 1105 (76%) | 904 (77%) | 201 (73%) |
| Travel: | |||
| -Travel in the past 6 months | 827 (57%) | 663 (56%) | 164 (59%) |
| -Travel in the past 30 days | 358 (25%) | 283 (24%) | 75 (27%) |
| -Travel in the past 7 days | 128 (8.8%) | 94 (8.0%)** | 34 (12%)** |
| Electricity (Y/N) | 1090 (76%) | 890 (77%) | 200 (73%) |
| Piped water (Y/N) | 144 (10%) | 107 (9.2%)** | 37 (13%)** |
| Sanitation (Y/N) | 137 (9.5%) | 112 (9.6%) | 25 (9.1%) |
| Household type | |||
| Single house | 210 (15%) | 174 (15%) | 36 (13%) |
| Several separate structures | 884 (62%) | 687 (59%)*** | 197 (72%)*** |
| Flat/apartment | 247 (17%) | 225 (19%)*** | 22 (8.0%)*** |
| Room in a larger dwelling | 95 (6.6%) | 75 (6.5%)*** | 20 (7.3%)*** |
| Rooms in house (≤3) | 1012 (71%) | 877 (70%)*** | 135 (73%)*** |
| Highest household education | |||
| Higher | 1115 (78%) | 893 (77%) | 222 (81%) |
| High school | 129 (9.0%) | 111 (9.6%) | 18 (6.5%) |
| Primary school | 185 (13%) | 150 (13%) | 35 (13%) |
| No education | 7 (0.5%) | 7 (0.6%) | 0 (0%) |
| Animal ownership | |||
| Ducks | 1427 (99%) | 1155 (99%) | 272 (99%) |
| Pigeons | 742 (52%) | 610 (53%) | 132 (48%) |
| Goats | 871 (60%) | 709 (60%) | 162 (58%) |
| Cows | 961 (66%) | 773 (66%) | 188 (68%) |
| Sheep | 22 (1.5%) | 15 (1.3%) | 7 (2.5%) |
| Buffalo | 45 (3.1%) | 34 (2.9%) | 11 (4.0%) |
| Population density (log/km2) | 7.07 (6.74, 7.43) | 7.08 (6.74, 7.48)* | 6.96 (6.71, 7.24)* |
| Migratory birds | 715 (50%) | 583 (50%) | 132 (48%) |
| Number of pigs within 1 km | 0.50 (0, 9) | 0.45 (0, 9)** | 0.74 (0, 6)** |
| Mosquito species | |||
| Cx. quinquefasciatus | 525 (36%) | 413 (35%)* | 112 (40%)* |
| Cx. tritaeniorhynchus | 223 (15%) | 177 (15%) | 46 (17%) |
| Cx. vishnui | 326 (22%) | 266 (23%) | 60 (22%) |
| Cx. pseudovishnui | 285 (20%) | 226 (19%) | 59 (21%) |
| Cx. fuscocephala | 32 (2.2%) | 30 (2.5%)* | 2 (0.7%)* |
| Cx. gelidus | 223 (15%) | 180 (15%) | 43 (16%) |
| Cx. bitaeniorhynchus | 155 (11%) | 131 (11%) | 24 (8.7%) |
| Ar. subalvatus | 121 (8.3%) | 95 (8.1%) | 26 (9.4%) |
| Ae. albopictus | 462 (32%) | 361 (31%)* | 101 (36%)* |
| Ae. vexans | 36 (2.5%) | 22 (1.9%)** | 14 (5.1%)** |
*n (%); median (interquartile range).
Fig. 1. Japanese encephalitis virus seroprevalence, population characteristics, and risk factors.
(A) Map of the 39 sampled communities in Chapai Nawabganj and proportion of JEV immunoglobulin G (IgG) antibodies among the population. (B) Study population structure, represented as a proportion of the total population, by age group and sex (bars), compared with the Census 2011 population structure, depicted as a proportion of the total population, by age group and sex (triangles). (C) Map showing the number of Census pigs (both household and nomadic) within 5 km from each household. (D) Relative risk of having JEV IgG antibodies as a function of Euclidean distance (in kilometers) to the closest pig.
In four communities, households reported that they owned pigs. Virtually, all (98%) communities had domestic duck raisers, 51% had domestic pigeon raisers, and all communities had households raising chickens. As pig ownership is relatively rare in Bangladesh, we used the results of a pig census conducted in Chapai Nawabganj to identify the distance of each individual in our study to the nearest pig-owning household (Fig. 1C). Individuals in our study lived on average 3 km from the closest pig. Among our study participants, individuals had 1.43 times (95% CI: 1.30 to 1.54) the odds of being seropositive if they lived within 1 km of a pig (Fig. 1D). The association was attenuated when the threshold was raised to within 2 km of a pig [odds ratio (OR): 1.10, 95% CI: 1.01 to 1.15].
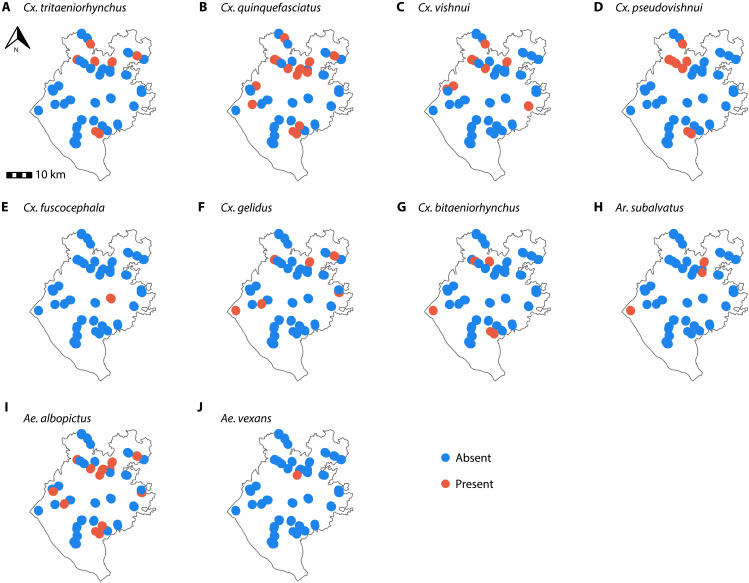
We identified 10 mosquito species that have been identified as competent JEV vectors (Fig. 2) as follows: Cx. quinquefasciatus (identified in 14 of 39 communities), Ae. albopictus (identified in 12 of 39 communities), Cx. vishnui (identified in 9 of 39 communities), Cx. pseudovishnui (identified in 8 of 39 communities), Cx. tritaeniorhynchus (identified in 6 of 39 communities), Culex gelidus (identified in 6 of 39 communities), Culex bitaeniorhynchus (identified in 5 of 39 communities), Armigeres (Ar.) subalvatus (identified in 3 of 39 communities), Culex fuscocephala (identified in 1 of 39 communities), and Aedes vexans (identified in 1 of 39 communities). There was substantial spatial heterogeneity wherein the different mosquito species were found (Fig. 2). The vectors more commonly found in communities with seropositive individuals were Cx. quinquefasciatus (33%), Ae. albopictus (33%), Cx. vishnui (22%), and Cx. pseudovishnui (19%).
Fig. 2. Identification of Japanese encephalitis virus confirmed mosquito vector species using indoor traps.
Panels (A) to (J) show the presence and absence maps of 10 different mosquito species across 39 communities in Chapai Nawabganj district, between August and September 2015.
To identify risk factors for being seropositive to JEV, we developed a spatially explicit logistic regression model fit to the serostatus of each individual (Table 2). All models that included a spatial field and any combination of random intercepts had essentially the same Watanabe-Akaike information criterion; however, removing the spatial field resulted in a drop in model fit (table S1). We explored the relationship between different individual, household, and community risk factors in both univariate and multivariable analyses. We found the key risk factor linked to infection was the number of pigs within a 1-km radius. For each additional pig within a 1-km radius, we found an increase of 1.14 in the odds of being seropositive (95% CI: 1.05 to 1.24). Furthermore, population density [adjusted OR (aOR): 0.85, 95% credible interval (CrI): 0.75 to 0.95] and the presence of the vector mosquito species Ae. vexans (aOR: 2.03, 95% CrI: 1.41 to 2.92, present in a single community) were linked to seropositivity. By contrast, the location of domestic birds (ducks or pigeons) was not associated with seropositivity.
Table 2. Mixed-effects univariate and multivariate logistic regression model with a complementary log-log link function on the effects of individual, household, and community covariates on the JEV seropositivity.
REF, reference.
| Univariable model, unadjusted odds ratio (95% CrI) | Multivariable model, adjusted odds ratio (95% CrI) | |
|---|---|---|
| Individual-level risk factors | ||
| Female | 0.93 (0.71–1.21) | |
| Lived always same village | 0.79 (0.59–1.07) | |
| Travel: | ||
| -Travel in the past 6 months | 1.14 (0.87–1.49) | |
| -Travel in the past 30 days | 1.18 (0.87–1.6) | |
| -Travel in the past 7 days | 1.64 (1.07–2.52) | |
| Household-level risk factors | ||
| Electricity (Y/N) | 0.8 (0.59–1.09) | |
| Piped water (Y/N) | 1.82 (1.2–2.76) | |
| Sanitation (Y/N) | 0.93 (0.58–1.48) | |
| Household type | ||
| Single house | REF | |
| Several separate structures | 1.09 (0.96–1.57) | |
| Flat/apartment | 0.83 (0.45–1.04) | |
| Room in a larger dwelling | 1.04 (0.82–1.53) | |
| Rooms in house (≤3) | 0.83 (0.62–1.13) | |
| Highest household education | ||
| Higher | REF | |
| High school | 1 (0.98–1.02) | |
| Primary school | 1 (0.98–1.02) | |
| No education | 1 (0.98–1.02) | |
| Animal ownership | ||
| Ducks | 0.93 (0.34–2.54) | |
| Pigeons | 0.82 (0.62–1.07) | |
| Goats | 0.94 (0.71–1.23) | |
| Cows | 1.1 (0.83–1.47) | |
| Sheep | 1.94 (0.75–5.02) | |
| Buffalo | 1.43 (0.71–2.88) | |
| Community-level risk factors | ||
| Population density (log/km2) | 0.77 (0.60–0.98) | 0.85 (0.75–0.95) |
| Migratory birds | 0.94 (0.71–1.23) | |
| Number of pigs within 1 km | 1.14 (1.05–1.24) | 1.08 (1.04–1.13) |
| Mosquito species | ||
| Cx. quinquefasciatus | 1.26 (0.96–1.66) | |
| Cx. tritaeniorhynchus | 1.1 (0.76–1.58) | |
| Cx. vishnui | 0.96 (0.69–1.33) | |
| Cx. pseudovishnui | 1.17 (0.84–1.63) | |
| Cx. fuscocephala | 0.24 (0.06–1.02) | |
| Cx. gelidus | 1.01 (0.69–1.46) | |
| Cx. bitaeniorhynchus | 0.74 (0.46–1.18) | |
| Ar. subalvatus | 1.14 (0.71–1.83) | |
| Ae. albopictus | 1.32 (1.00–1.74) | |
| Ae. vexans | 3.00 (1.51–5.95) | 1.89 (1.33–2.71) |
Underlying JEV infection burden
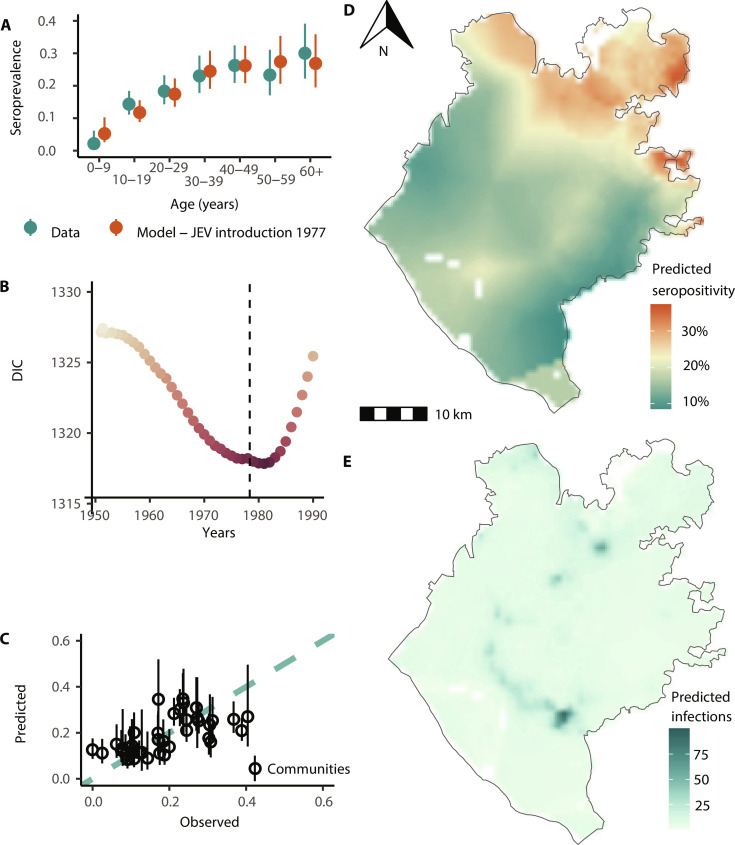
We next used our spatially explicit regression framework to estimate the infection burden from JEV throughout the district of Chapai Nawabganj. The seroprevalence model with a constant force of infection over time aligns with the time of the first introduction of JEV into the region being in the late 1970s (Fig. 3A) and outperforms models where we either assume that JEV has been endemic since the 1950s, or with a more recent introduction (fig. S2). We, therefore, used a model with a date of introduction of 1977 for subsequent analyses, consistent with the observed first introduction in the country. We used a model with distance to the nearest pig (as estimated by the pig census) (8), the spatial distribution of the population in the district as estimated by WorldPop data (17), and the yearly age distribution from the 2015 population estimates (18). We estimated a mean annual force of infection in the district of 0.007 (95% CI: 0.002 to 0.02), leading to an average seropositivity of 16.8% (95% CI: 10.0 to 30.6). The force of infection varied substantially over the region, with infection risk concentrated to the northwest, in the upazilas of Gomastapur and Nachole (Fig. 3B). These estimates suggest that 13,750 individuals (95% CI: 11,990 to 15,615) are infected each year in the district (Fig. 3D). To validate the model, we used leave-one-out cross-validation, where we systematically removed all data from each community from the model fitting process and compared the community-level seropositivity predicted for the held-out community with that actually observed. We found a good correlation (Pearson coefficient r = 0.63) between the predicted and observed values (Fig. 3C).
Fig. 3. Serocatalytic modeling of the Japanese encephalitis virus.
(A) Observed (blue-green points and range) and fitted seroprevalence (orange points and range: spatial model with JEV introduction in 1977) by age group for sampled individuals. (B) Deviance information criterion (DIC) values of each spatial hierarchical model with JEV introduction year from 1950 to 1990. The dashed line represents 1977, the year of the first diagnosed case. (C) Cross-validation using the leave-one-out predictive performance of the modeled seroprevalence by the community. (D) Spatial JEV seroprevalence prediction map using a Matérn covariance structure, Chapai Nawabganj. (E) JEV-predicted annual infections map, 2015, Chapai Nawabganj.
Severe case burden in humans
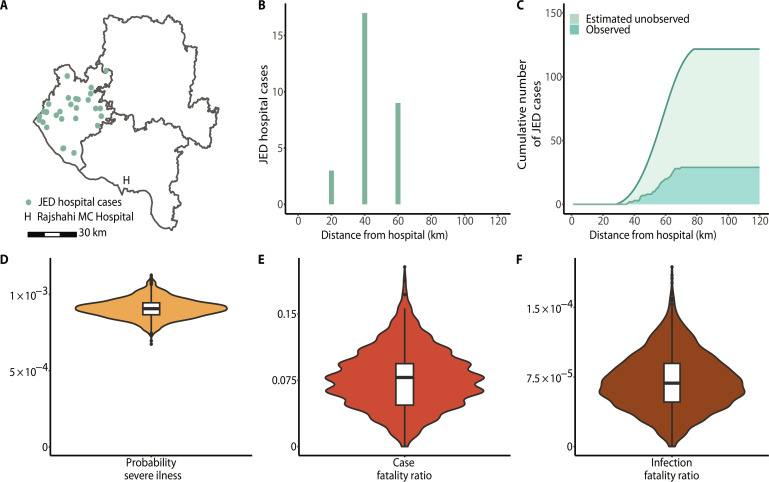
The government of Bangladesh maintains a hospital-based acute meningitis-encephalitis syndrome surveillance for JE, which means that it depends on hospital attendance to detect JE severe cases. From 2007 to 2016, there were 29 confirmed JE cases from Chapai Nawabganj attending the closest tertiary care surveillance hospital, located in neighboring Rajshahi (6). The mean age of JE cases was 35 years (range: 0 to 70 years). Patients were followed up between 2007 and 2011 and five deaths were reported among 64 severe JE cases that presented at the surveillance hospital. There were differences in the number of JE severe cases by distance from the hospital, with most cases (66%) located within 60 km of the Rajshahi surveillance hospital (Fig. 4, A and B).
Fig. 4. Japanese encephalitis burden.
(A) Map of Chapai Nawabganj (left district) and the two neighboring districts, Naogoan (northeast) and Rajshahi (southeast), where each green point corresponds to a hospital-attended JE severe case (n = 29) resident in Chapai Nawabganj district, along with its location relative to the Rajshahi Medical College Hospital (surveillance hospital). (B) Hospital severe cases as a function of distance (in kilometers) from the surveillance hospital. (C) Cumulative numbers of observed and estimates of unobserved JE severe cases using a health care–seeking behavior regression model. (D) Probability of JE severe illness in Chapai Nawabganj using hospital-attended (observed) severe cases and estimated JEV infections. (E) JE case fatality ratio in Chapai Nawabganj using deaths observed in hospital and hospital-attended (observed) JE severe cases. (F) JE infection fatality ratio in Chapai Nawabganj using the probability of JE severe illness, obtained from the total estimated JE severe cases over the estimated number of infections in the district, and previously estimated case fatality ratio.
To estimate the number of JE cases that did not attend this surveillance hospital, we made use of a health care–seeking study conducted in the region (15). We estimated that between 2007 and 2016, there were an additional 92 severe JE cases (95% CI: 87 to 95) from Chapai Nawabganj that did not seek care at the surveillance hospital, resulting in 121 total severe cases (95% CI: 117 to 124) over this period. We estimated that there were overall nine deaths from JEV over this period (95% CI: 2 to 19), five of which were detected. This results in an average of 12 severe cases and 1 death (95% CI: 0 to 2) in the district per year. The resulting estimated JE incidence of severe cases in Chapai is 0.7 cases per 100,000 inhabitants. It also suggests that 24% (95% CI: 23 to 25) of severe JE cases are detected by the surveillance system. Comparing the number of severe cases with the estimated number of annual infections suggested that 0.09% (95% CI: 0.08 to 0.10) of infections result in severe disease (Fig. 4C). Furthermore, we estimated the case fatality ratio to be 7.8% (95% CI: 1.6 to 14.1) and the infection fatality ratio (IFR) to be 0.007% (95% CI: 0.001 to 0.013) (Fig. 4, D and E).
Comparison of assay response to DENV
Our seropositivity results are reliant on an assay that uses just the E protein III domains of the flaviviruses, which are more specific than the whole E protein (16). To assess whether the assay can specifically identify JEV infections and is not affected by DENV infections, we compared the relative fluorescence intensity (RFI) to the JEV antigen with the mean RFI to the four DENV1–4 antigens (fig. S3). We find a mean correlation of 0.17 across the four serotypes (DENV1 0.07, DENV2 0.25, DENV3 0.07, and DENV4 0.28). Overall seropositivity to any DENV as measured by the Luminex was 19.5% (95% CI: 17.6 to 21.6) and higher in the more urban south of the district, as compared to the more rural north, consistent with JEV and DENV occupying different ecological niches in this area (fig. S4).
DISCUSSION
In this study, we have combined a population-based serology study, an assay with limited cross-reactivity, and detailed descriptions of candidate host species as well as human case data and health care–seeking data to obtain a detailed characterization of the drivers of infection risk of a major, but poorly understood, burden on public health. We have also generated estimates of the underlying level of infection and the probability of severe disease and death following infection. In our seroprevalence data, we observe a very steady increase in seropositivity from age up to the age of 40 years, followed by a leveling off. This indicates that individuals who are 40 years or older have had a similar cumulative lifetime risk of infection. We use serocatalytic models to directly understand which patterns of infection are consistent with this observed seropositivity by age. Our findings show that simple models, which assume an introduction of the virus in the late 1970s followed by a steady force of infection, can accurately replicate the observed patterns of seropositivity by age. These confirm the endemic nature of JEV in this district of Bangladesh and suggest JEV emergence in a previously naive population around 1977, consistent with the first detected case in the country (5).
The estimated incidence of severe JE disease in the district (approximately 1 case per 100,000) is similar to other low-endemic countries before the introduction of the vaccine and lower than many highly endemic regions (3, 19, 20). On average, under 1% of the population gets infected annually, around 1 in 1000 infections results in severe disease, and 1 in 10,000 results in death. Our estimates of the case fatality ratio (CFR) and the asymptomatic/symptomatic ratio are largely consistent with existing estimates (21, 22). The most comprehensive prior estimates come from Cheng et al. (22), who estimated a CFR in Bangladesh of 18% (95% CI: 8 to 35) between 2000 and 2018, slightly higher than our estimate, however, with overlapping uncertainty estimates. We note that the estimate from Bangladesh in the prior study is largely based on only 20 cases from a single year (2004) in a newly implemented hospital-based surveillance, and therefore, differences from our estimates may come from stochastic variability in small sample sizes. However, previous estimates of the asymptomatic proportion have relied on enzyme-linked immunosorbent assay (ELISA) conducted between 1950 and 1990, during which cross-reactivity issues with other flaviviruses were potentially encountered (21, 23, 24). While low on average, infection risk was spatially heterogeneous and was higher in the vicinity of pig-raising communities. The population in Bangladesh is predominantly Muslim, with pig-raising households usually in small, ethnic minority rural communities (8). Optimal deployment of a vaccine and impact on health per dose may come from focusing on districts where risk is greatest, likely in regions with the greatest number of pig-raising households. However, subnational vaccination efforts may be difficult for national vaccine programs to implement. There also may be a risk of further marginalizing the ethnic minorities engaged in pig raising (25). A vaccine program that focuses on the pigs themselves may also be considered, although the rapid turnover in pigs due to the short life span and the presence of maternal antibodies, which last 2 to 3 months, may make this unfeasible (8, 26). While infection risk appears to have been relatively stable since JEV’s introduction in the region, the large number of competent vectors coupled with a warming climate may lead to increases in future transmission risk (27, 28).
We identified multiple mosquito species that are known competent vectors for JEV. Some vector species, such as Cx. pseudovishnui and Ar. subalvatus, were previously collected in Bangladesh mosquito surveys with high frequency, specifically in Chapai Nawabganj (29). In addition, both Aedes species highlighted in our analysis have been profiled in Chapai Nawabganj, nevertheless in low frequency (<5% of the collection efforts), using light traps near pig hosts. Although only detected in one community, we found Ae. vexans to be positively associated with JEV seropositivity, and this species has shown a host preference for pigs, horses, and deer in field studies (30–32). Although different species were associated with JEV seropositivity, Cx. quinquefasciatus was the most common species in communities where JEV seropositivity was observed. Previous field studies around cattle in the same region identified Cx. tritaeniorhynchus as the most frequently collected known JEV vector (29). We did not find an important role of these two vectors in our analysis, highlighting that sampling around nonhuman dead-end hosts, such as cattle, might not identify the vector’s role in human JEV risk. The presence of cattle in a community did not appear to be protective, as has previously been suggested, although, given the overall low level of JEV transmission, we may not have had the power to detect such a protective effect (33). Our findings suggest that multiple mosquito species may contribute to driving infection risk in this region. We note that our traps were left in indoor spaces only, and therefore, we did not capture the potential other vectors that only are found outside the household.
We identify relative proximity to pigs as the most important risk factor for human infection. While domestic and peri-domestic birds can become infected by JEV (34–36), they do not appear to be important in maintaining transmission in this region. Essentially, all the sampled communities had a large number of domestic birds (ducks or chickens). If they were driving JEV transmission, then we would expect to observe a much more even pattern of seropositivity across the region. There were nevertheless human cases and seropositive individuals far from pig-raising households. It remains unclear whether travel to endemic regions, the presence of nomadic pig herds that travel across the wider region, the presence of other host species (e.g., wading birds), or long travel of infected mosquitoes from elsewhere could explain these infections. We also identified population density as negatively correlated with JEV seroprevalence, aligning with our understanding of JEV disease ecology. JEV is more prevalent in rural areas with lower population densities, where pig-raising communities, paddy fields, and other flooded environments suitable for mosquito vectors are commonly found (3, 37).
Our findings confirm that the WHO Global Health Observatory data do not provide an accurate picture of the JEV disease burden since the observed number of confirmed JE cases by the surveillance system only represents a minority of the true number of severe cases, with around three-quarters of severe cases being missed. In particular, we note that the tertiary care surveillance hospital in the region, which is relied on for confirmatory JEV testing (near the southeast of the district), is farthest from the part of the region with the greatest infection risk (the north). Health care seeking in Bangladesh is dominated by the informal sector, especially at the early stages of disease (13). Limited availability of public transport severely hampers access to tertiary care governmental health care facilities. An important strength of our approach to estimating the true underlying level of severe JE cases is that it is not biased by any underlying heterogeneity in infection risk over space. This is because the location of the observed JE cases will also be determined by the underlying infection risk so locations that have a higher infection risk will result in more observed cases and will contribute to more estimated unobserved cases than another location equally far away from the surveillance hospital with a lower infection risk. This robustness to spatial heterogeneity in infection risk has previously been demonstrated through simulations (38). Our findings support the targeted expansion of JEV surveillance systems to the north of the district. They also demonstrate the utility of combining community-based health care–seeking surveys with surveillance data using existing platforms to obtain estimates of underlying disease burden (39). Similar approaches have been conducted to estimate the number of missed Nipah virus cases, which also circulate in the same region (38).
Our study highlights the strength of spatial community-based seroprevalence studies to quantify the burden of infection and identify at-risk populations, especially for pathogens where asymptomatic infection is common. Similar studies from the country have explored the spatial distribution of risk for multiple pathogens using the same blood samples (40–42). The recent growth in the use of multiplex technology, especially via the Luminex platform, alongside advances in the development of antigens that limit cross-reactivity is central to these efforts (43). Our samples were tested against the four DENVs at the same time, and the resultant titers had a correlation of around 0.2. We also found low overall seropositivity to DENV as compared to other parts of Bangladesh (e.g., Dhaka and Chittagong) and other countries in the region where DENV circulates, and DENV seropositivity appeared concentrated in different locations than JEV seropositivity, consistent with the two pathogens occupying different ecological niches in the district. Furthermore, by triangulating disease data from surveillance, we can obtain a better understanding of the probability of severe outcomes following infection. Our study also highlights the role that mathematical modeling can play in supplementing the information provided by serological studies. In particular, we note that the crude seroprevalence estimates (19%) were slightly different from the modeled estimates (17%), which incorporated information on the population distribution across the district and the underlying demography in the population as compared to the individuals we sampled.
Our study results should be interpreted considering some limitations. Our datasets do not overlap in time. The pig census data are from 2009, the health care–seeking study from 2011, and the seroprevalence study from 2015 and, therefore, do not capture the most recent epidemiological situation in the district. They also only represent single snapshots of the situation at the time of data collection. However, we note that the location of pig-raising communities is unlikely to have changed over these time frames, we also note that these past studies provide critical insights into the disease ecology of a major pathogen, including risk factors and the underlying probability of severe disease and death, which remain relevant today. We assumed a constant force of infection following the introduction of the virus. There will certainly be year-to-year variability in the force of infection; however, we did not have the power to detect any annual changes. Nevertheless, understanding the average annual infection risk represents a key metric to understanding the infection burden in the region. We also predicted the burden in unsampled communities using covariates of interest. While our sampling strategy to select communities for inclusion was random, we may have missed pockets of transmission. Cross-validation of our model showed only a moderate correlation between observed and predicted seroprevalence suggesting that some drivers of infection were not captured by our model. Other potential drivers of infection may include human mobility differences, the movement of nomadic pig herds, preventative action against mosquitoes, or factors relevant to mosquito habitat. There may be residual cross-reactivity with flavivirus circulating in the region. In particular, we estimated a substantial number of infections in the more populated southern part of the district. Even a lower level of cross-reactivity with DENV could explain these infections, which would mean the true JEV burden may be slightly lower than our estimates. Improved assays or analytical approaches may improve the resolution of the test results.
Despite these limitations, this study presents a comprehensive assessment of an important and understudied burden on health, identifies risk factors for infection, and quantifies the probability of severe disease and death. Our methodology framework applied to national-level data on JE severe disease cases, national representative seroprevalence studies, and risk factors exposure could have direct implications for the development of investment cases for a vaccine introduction at a country level by identifying core areas for vaccine introduction.
MATERIALS AND METHODS
Data
This study brings together the results of a cross-sectional seroprevalence study, mosquito trapping study, hospitalization data, health care–seeking study, and a pig census.
Cross-sectional seroprevalence study
We conducted a cross-sectional seroprevalence study in Chapai Nawabganj district between March and May 2013. We randomly sampled 40 communities from the list of all communities in the district from the 2011 national census. We defined a community as a village in rural areas, whereas in urban areas, as a city ward. Here, data from 39 of these communities were used (samples from one community were not tested for JEV antibodies). Study teams visited each community and randomly selected households for inclusion in the study. The randomization process used the following approach: The study team first identified the house where the most recent wedding took place and then selected the closest neighbor. To select the first household of the community, they counted six houses in a random direction. The last step was repeated to find the next study household. All individuals over 6 months of age were invited to participate. Following an informed consent process, individuals who agreed to participate provided a blood sample and answered a series of questions about demographic information and behavior (e.g., travel). The household head also answered a separate questionnaire that covered characteristics of the household (e.g., household structure and animal ownership). Parents/guardians responded on behalf of individuals who were too young to answer for themselves. We organized times at later dates to return to households to invite individuals from the household who were absent during the initial visit. Trained phlebotomists collected blood, which was spun down in the field. We sent the sera to icddr,b laboratories in Dhaka in nitrogen dry shippers for serological testing. Additional households were recruited until 40 blood samples and at least 10 households had been recruited from each community. We determined the sample size based on sufficient precision to estimate the overall seroprevalence with a margin of error of under 0.05 where the true seropositivity is 0.5 (i.e., the 95% CI of seropositivity would be narrower than 0.45 to 0.55). We provided blood group typing as a benefit for all participants.
We tested all serum samples using a multiplex assay developed by the Institut Pasteur on a Luminex platform using a Bio-Plex 200 (16). We used a panel of beads that covered antigens from different flaviviruses (dengue serotypes 1 to 4, tick-borne encephalitis, yellow fever, JE, and West Nile virus). We constructed each antigen using the domain III of the E protein. This domain has been shown to have limited cross-reactivity across flaviviruses, especially as compared to commercial ELISA assays that typically use the whole E protein (16). The output is a measure of RFI that is derived from the individual level control (mean FI/control). We considered individuals to be seropositive (i.e., had been infected with JEV) if they had an RFI of 3 or greater. Luminex thresholds for JEV infection have not been defined yet, so we based this threshold on our experience with other flaviviruses (optimal cut point range of 1.5 to 2.1 for DENV1–4) using the same platform (44). We conducted a sensitivity analysis with higher/lower cut points to define seropositivity (fig. S1).
Mosquito trapping study
Between August and September 2015, study teams revisited the same communities as the seroprevalence study. In each community, eight randomly selected households were selected for inclusion, whether they had been previously included in the seroprevalence study. In households that agreed, we left a BG-Sentinel trap running for 24 hours in an indoor space. The mosquitoes were then collected and sent to icddr,b laboratories to train entomologists for morphological identification using standard keys (45). We recorded the number of mosquitoes by species (across males and females) trapped in each community.
Pig census data
We used data from a large pig census conducted between May and September 2009 through a snowball sampling technique. The results from this study have been previously published (8). This study identified 11,364 pigs (Sus scrofa) in the districts of Chapai Nawabganj, alongside neighboring Rajshahi and Naogaon districts, and their coordinates were registered. We include pigs in neighboring districts due to the potential of vector movement between districts.
Hospitalization data
To help quantify the underlying level of severe disease in the region, we used data from the regional tertiary care surveillance hospital, located in Rajshahi city. This is a public hospital that receives all severe cases of referred encephalitis from the wider Rajshahi division, within which Chapai Nawabganj district is located. We conducted confirmatory testing, consisting of both polymerase chain reaction and ELISA testing at both a local laboratory and at the United States of America Centers for Disease Control and Prevention in Atlanta (6). In this study, we use the year and subdistrict for all confirmed JE cases that were presented at Rajshahi Medical College Hospital (RMCH) between 2007 and 2016. Five out of 64 of these individuals died during the initial follow-up period (2007–2011)(15).
Health care utilization study
To help estimate the number of severe JE cases from Chapai Nawabganj that did not attend the hospital (RMCH) and were therefore missed by the surveillance system, we used the results of a health care utilization study from the region, where study teams visited communities in the catchment area of three surveillance hospitals around the country (including RMCH) between June 2008 and March 2009. The details of this study have been published (13). Briefly, study teams visited public meeting spaces (markets and mosques) in communities around surveillance hospitals and asked who in the community had symptoms of severe neurological disease in the past 12 months compatible with JE infection. We identified severe neurological disease using syndromic criteria, including fever with altered mental status for more than 6 hours or with unconsciousness for one or more hours, or fever with altered mental status, unconsciousness, or a new onset seizure that resulted in death. They then identified the individual and interviewed them (or their family members if they had deceased) to identify the individual care-seeking pathway. From that information, we have previously quantified the probability of a severe encephalitis case being detected by the surveillance hospital as a function of the distance from the hospital (15, 38).
Analysis
Factors associated with seropositivity
We use logistic regression to identify individual-, household- and community-level factors associated with seropositivity. To account for residual spatial correlation in seroprevalence, we used a spatial random effect as implemented in integrated nested Laplace approximation (INLA) (46). We modeled it as a zero-mean Gaussian process with a Matérn covariance function representing the residual spatial variation not explained by the covariates included. Because of the likely collinearity between covariates and spatial effects and to address spatial confounding in the effect estimates, we accounted for spatial dependence on covariates of interest, constraining the effect of the spatial random intercept on the outcome (47). For that, we built a design matrix with the covariates of interest and applied a QR decomposition. We used the resulting orthogonal matrix as a constraint in the spatial random effects model. We considered models that had household and community random effects, alongside the spatial field, and decided on the best model using the deviance information criterion (DIC). Models with DIC differences of ≤5 were considered equivalent and so we selected the more parsimonious model. We initially took all covariates, in turn, in univariate analysis. We then identified all covariates that were significant at the 0.05 P value level for inclusion in a multivariable model.
Serocatalytic modeling
To quantify the JEV force of infection in the district, we used a serocatalytic model fit to the age-dependent variation in seropositivity (48). The catalytic approach assumes that the risk of infection of an individual is proportional to the time of exposure (determined by their age), and therefore, the probability Pp(a) that an individual is seropositive under endemic transmission is given by the following expression (49)
| (1) |
where a is the age of the individual. To estimate the force of infection in each sampled community, we fit the serostatus of each individual in a binomial model with a complementary log-log link function using the logarithm of age as an offset (48). We used INLA to approximate posterior estimates, implemented in the R package R-INLA (46). To account for spatial correlation, we used a stochastic partial differential equation approach and fitted the model in a Bayesian framework using INLA. We included the number of household pigs found in a 5-km radius as a covariate.
The first report of JE in Bangladesh dates from 1977 (5); however, as JEV may have been introduced earlier, we considered separate models where we assumed that JEV had been first introduced at different times ranging between 1950 and 1990. Under this assumption, all individuals who were alive at the assumed time of JEV introduction will have experienced the same lifetime risk of infection. We determined the best-fitting model using DIC.
From the force of infection model, we extracted the estimated force of infection in each 1 km by 1 km grid cell in the district. We next estimated the number of infections in each cell using the number of people living in the cell, the age distribution, and the estimated level of immunity
| (2) |
where Na, i is the number of individuals of age a living in cell i, as estimated by WorldPop data (17), and [1 − exp ( − λia)] represents the proportion of individuals of age a living in cell i that are immune due to historic infection. This approach assumes that individuals have lifelong immunity and consequently can only get infected once.
Estimate disease burden using hospital-cases data
We used the results of a large health care utilization study in Bangladesh to estimate the probability that an individual with severe encephalitis would present at the Rajshahi Medical College Hospital and, therefore, be detected in our human JE dataset (13, 15). The methods for estimating the probability of detecting a case from the surveillance system using this data are published in detail elsewhere (13). Briefly, there is a strong distance effect with individuals living farther from the surveillance hospital less likely to seek treatment there. By using the changing probability of seeking care at the surveillance hospital as a function of distance, we can estimate the number of missed cases by the surveillance system (13). This approach is robust to underlying heterogeneity in infection risk.
Estimate the infection fatality ratio
The IFR quantifies the average number of deaths of individuals infected by a pathogen. We calculated the IFR of JEV in Chapai Nawabganj as the average number of JE deaths among all infected individuals with JEV. We estimated the IFR using the estimated average annual number of infections and the average annual number of JE deaths reported in the Chapai Nawabganj population
Ethical approval
This study was approved by the ethical review boards at CDC, icddr,b (PR-13001), Johns Hopkins Bloomberg School of Public Health, and Institut Pasteur. We obtained informed consent, and assent when appropriate, from all individuals.
Acknowledgments
We thank all the participants who took part in these studies across Bangladesh for willingness to contribute to this research. In addition, we also thank the field and laboratory staff for committed efforts.
Funding: This work was supported by the Centers for Disease Prevention and Control (U01 CI0000628), Gates Cambridge Trust (OPP1144, to M.P.D.), European Union’s Horizon 2020 Research and Innovation Programme (804744, to H.S.), Labex IBEID (ANR-10-LABX-62-IBEID, to S.C.), and European Union’s Horizon 2020 Research and Innovation Programme (874735, to S.C.).
Author contributions: Conceptualization: H.S., E.S.G., and M.P.D. Methodology: M.P.D., G.R.d.S., M.O., J.V., S.P.L., E.S.G., and H.S. Investigation: A.M.N., K.K.P., M.R., M.S.A., H.M.A.-A., M.Z.R., M.E.H., R.C.P., S.P.L., E.S.G., and H.S. Visualization: M.P.D. Supervision: H.S. and E.S.G. Writing—original draft: M.P.D. and H.S. Writing—review and editing: All authors.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions of the paper are present in the paper and/or the supplementary materials. The data and code for this study are available at 10.5281/zenodo.11242717. To protect privacy, we grouped ages into age categories and grouped communities into the above administrative level.
Supplementary Materials
This PDF file includes:
Table S1
Figs. S1 to S4
REFERENCES AND NOTES
- 1.Mitamura T., Kitaoka M., Mori K., Okubo K., Isolation of the virus of Japanese encephalitis from mosquitoes caught in nature. Reports to the Ninth Meeting of the Committee on encephalitis. Tokyo Iji Shinshi 3076, 820–824 (1938). [Google Scholar]
- 2.Mitamura T., Kitaoka M., Watanabe M., Okuba K., Tenjin S., Yamada S., Mori K., Asada J., Study on Japanese encephalitis virus. Animal experiments and mosquito transmission experiments. Kansai Iji 1, 260–261 (1936). [Google Scholar]
- 3.Campbell G. L., Hills S. L., Fischer M., Jacobson J. A., Hoke C. H., Hombach J. M., Marfin A. A., Solomon T., Tsai T. F., Tsu V. D., Ginsburg A. S., Estimated global incidence of Japanese encephalitis: A systematic review. Bull. World Health Organ. 89, 774A–774E (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vannice K. S., Hills S. L., Schwartz L. M., Barrett A. D., Heffelfinger J., Hombach J., Letson G. W., Solomon T., Marfin A. A., Japanese Encephalitis Vaccination Experts Panel , The future of Japanese encephalitis vaccination: Expert recommendations for achieving and maintaining optimal JE control. NPJ Vaccines 6, 82 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khan A. M., Khan A. Q., Dobrzynski L., Joshi G. P., Myat A., A Japanese encephalitis focus in Bangladesh. J. Trop. Med. Hyg. 84, 41–44 (1981). [PubMed] [Google Scholar]
- 6.Paul K. K., Sazzad H. M. S., Rahman M., Sultana S., Hossain M. J., Ledermann J. P., Burns P., Friedman M. S., Flora M. S., Fischer M., Hills S., Luby S. P., Gurley E. S., Hospital-based surveillance for Japanese encephalitis in Bangladesh, 2007-2016: Implications for introduction of immunization. Int. J. Infect. Dis. 99, 69–74 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lord J. S., Gurley E. S., Pulliam J. R. C., Rethinking Japanese encephalitis virus transmission: A framework for implicating host and vector species. PLoS Negl. Trop. Dis. 9, e0004074 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khan S. U., Salje H., Hannan A., Islam M. A., Bhuyan A. A. M., Islam M. A., Rahman M. Z., Nahar N., Hossain M. J., Luby S. P., Gurley E. S., Dynamics of Japanese encephalitis virus transmission among pigs in Northwest Bangladesh and the potential impact of pig vaccination. PLoS Negl. Trop. Dis. 8, e3166 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Auerswald H., Maquart P.-O., Chevalier V., Boyer S., Mosquito vector competence for Japanese encephalitis virus. Viruses 13, 1154 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hernández-Triana L. M., Folly A. J., Sewgobind S., Lean F. Z. X., Ackroyd S., Nuñez A., Delacour S., Drago A., Visentin P., Mansfield K. L., Johnson N., Susceptibility of Aedes albopictus and Culex quinquefasciatus to Japanese encephalitis virus. Parasit. Vectors 15, 210 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu W.-T., Chen Y.-J., Chen C.-C., Liao K.-M., Tzeng H.-Y., Tu W.-C., Impact of temperature on infection with Japanese encephalitis virus of three potential urban vectors in Taiwan; Aedes albopictus, Armigeres subalbatus, and Culex quinquefasciatus. Acta Trop. 237, 106726 (2023). [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization, Japanese encephalitis–Reported Cases by Country. The Global Health Observatory (World Health Organization, 2015); www.who.int/data/gho/data/indicators/indicator-details/GHO/japanese-encephalitis---number-of-reported-cases.
- 13.Nikolay B., Salje H., Sturm-Ramirez K., Azziz-Baumgartner E., Homaira N., Ahmed M., Iuliano A. D., Paul R. C., Rahman M., Hossain M. J., Luby S. P., Cauchemez S., Gurley E. S., Evaluating hospital-based surveillance for outbreak detection in Bangladesh: Analysis of healthcare utilization data. PLoS Med. 14, e1002218 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.A-Nuegoonpipat A., Panthuyosri N., Anantapreecha S., Chanama S., Sa-Ngasang A., Sawanpanyalert P., Kurane I., Cross-reactive IgM responses in patients with dengue or Japanese encephalitis. J. Clin. Virol. 42, 75–77 (2008). [DOI] [PubMed] [Google Scholar]
- 15.Luby S. P., Rahman M., Gurley E. S., Paul R. C., Fischer M., Diorditsa S., Alamgir A. S. M., Hossain M. J., Rahman M. A., Hasan A. S. M. M., Banu S. S., Sandhu H., A novel low-cost approach to estimate the incidence of Japanese encephalitis in the catchment area of three hospitals in Bangladesh. Am. J. Trop. Med. Hyg. 85, 379–385 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beck C., Desprès P., Paulous S., Vanhomwegen J., Lowenski S., Nowotny N., Durand B., Garnier A., Blaise-Boisseau S., Guitton E., Yamanaka T., Zientara S., Lecollinet S., A high-performance multiplex immunoassay for serodiagnosis of flavivirus-associated neurological diseases in horses. Biomed. Res. Int. 2015, 678084 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.WorldPop, Bangladesh 100m population, version 2, University of Southampton, (2017); 10.5258/SOTON/WP00533. [DOI]
- 18.International database, United States Census Bureau; www.census.gov/data-tools/demo/idb/#/pop?COUNTRY_YEAR=2023&COUNTRY_YR_ANIM=2023&CCODE=BD&popPages=BYAGE&menu=popViz&POP_YEARS=2015&CCODE_SINGLE=BD.
- 19.Lopez A. L., Raguindin P. F., Aldaba J. G., Avelino F., Sy A. K., Heffelfinger J. D., Silva M. W. T., Epidemiology of Japanese encephalitis in the Philippines prior to routine immunization. Int. J. Infect. Dis. 102, 344–351 (2021). [DOI] [PubMed] [Google Scholar]
- 20.Tandale B. V., Deshmukh P. S., Tomar S. J., Narang R., Qazi M. S., Venkata P. G., Jain M., Jain D., Guduru V. K., Jain J., Gosavi R. V., Valupadas C. S., Deshmukh P. R., Raut A. V., Narlawar U. W., Jha P. K., Bondre V. P., Sapkal G. N., Damle R. G., Khude P. M., Niswade A. K., Talapalliwar M., Rathod P., Balla P. S., Muttineni P. K., Janakiram K. K. K., Rajderkar S. S., Incidence of Japanese encephalitis and acute encephalitis syndrome hospitalizations in the medium-endemic region in central India. J. Epidemiol. Glob. Health. 13, 173–179 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vaughn D. W., Hoke C. H. Jr., The epidemiology of Japanese encephalitis: Prospects for prevention. Epidemiol. Rev. 14, 197–221 (1992). [DOI] [PubMed] [Google Scholar]
- 22.Cheng Y., Tran Minh N., Tran Minh Q., Khandelwal S., Clapham H. E., Estimates of Japanese encephalitis mortality and morbidity: A systematic review and modeling analysis. PLoS Negl. Trop. Dis. 16, e0010361 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Halstead S., Grosz C., Japanese S., encephalitis. I., Subclinical Japanese encephalitis. I. Infection of Americans with limited residence in Korea. Am. J. Hyg. 75, 190–201 (1962). [PubMed] [Google Scholar]
- 24.van den Hurk A. F., Ritchie S. A., Mackenzie J. S., Ecology and geographical expansion of Japanese encephalitis virus. Annu. Rev. Entomol. 54, 17–35 (2009). [DOI] [PubMed] [Google Scholar]
- 25.Nahar N., Uddin M., Gurley E. S., Jahangir Hossain M., Sultana R., Luby S. P., Cultural and economic motivation of pig raising practices in Bangladesh. Ecohealth 12, 611–620 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cappelle J., Duong V., Pring L., Kong L., Yakovleff M., Prasetyo D. B., Peng B., Choeung R., Duboz R., Ong S., Sorn S., Dussart P., Tarantola A., Buchy P., Chevalier V., Intensive circulation of Japanese encephalitis virus in peri-urban sentinel pigs near Phnom Penh, Cambodia. PLoS Negl. Trop. Dis. 10, e0005149 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bai Y., Xu Z., Zhang J., Mao D., Luo C., He Y., Liang G., Lu B., Bisesi M. S., Sun Q., Xu X., Yang W., Liu Q., Regional impact of climate on japanese encephalitis in areas located near the three gorges dam. PLOS ONE 9, e84326 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tu T., Xu K., Xu L., Gao Y., Zhou Y., He Y., Liu Y., Liu Q., Ji H., Tang W., Association between meteorological factors and the prevalence dynamics of Japanese encephalitis. PLOS ONE 16, e0247980 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lord J. S., Al-Amin H. M., Chakma S., Alam M. S., Gurley E. S., Pulliam J. R. C., Sampling design influences the observed dominance of Culex tritaeniorhynchus: Considerations for future studies of Japanese encephalitis virus transmission. PLoS Negl. Trop. Dis. 10, e0004249 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stelder J. J., Mihalca A. D., Olesen A. S., Kjær L. J., Boklund A. E., Rasmussen T. B., Marinov M., Alexe V., Balmoş O. M., Bødker R., Potential mosquito vector attraction to-and feeding preferences for pigs in Romanian backyard farms. Front. Vet. Sci. 9, 1046263 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Biteye B., Fall A. G., Seck M. T., Ciss M., Diop M., Gimonneau G., Host-feeding patterns of Aedes (Aedimorphus) vexans arabiensis, a Rift Valley Fever virus vector in the Ferlo pastoral ecosystem of Senegal. PLOS ONE 14, e0215194 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schönenberger A. C., Wagner S., Tuten H. C., Schaffner F., Torgerson P., Furrer S., Mathis A., Silaghi C., Host preferences in host-seeking and blood-fed mosquitoes in Switzerland. Med. Vet. Entomol. 30, 39–52 (2016). [DOI] [PubMed] [Google Scholar]
- 33.G. W. Beran, Handbook of Zoonoses, Section B: Viral Zoonoses (CRC Press, 2017). [Google Scholar]
- 34.Auerswald H., Ruget A.-S., Ladreyt H., In S., Mao S., Sorn S., Tum S., Duong V., Dussart P., Cappelle J., Chevalier V., Serological evidence for Japanese encephalitis and West Nile virus infections in domestic birds in Cambodia. Front. Vet. Sci. 7, 15 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Connor B., Bunn W. B., The changing epidemiology of Japanese encephalitis and new data: The implications for new recommendations for Japanese encephalitis vaccine. Trop. Dis. Travel. Med. Vaccines 3, 14 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Page M. J., Cleton N. B., Bowen R. A., Bosco-Lauth A., Age-related susceptibility to Japanese encephalitis virus in domestic ducklings and chicks. Am. J. Trop. Med. Hyg. 90, 242–246 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Erlanger T. E., Weiss S., Keiser J., Utzinger J., Wiedenmayer K., Past, present, and future of Japanese encephalitis. Emerg. Infect. Dis. 15, 1–7 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hegde S. T., Salje H., Sazzad H. M. S., Hossain M. J., Rahman M., Daszak P., Klena J. D., Nichol S. T., Luby S. P., Gurley E. S., Using healthcare-seeking behaviour to estimate the number of Nipah outbreaks missed by hospital-based surveillance in Bangladesh. Int. J. Epidemiol. 48, 1219–1227 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anwar H. B., Mazumder Y., Nujhat S., Islam B. Z., Kalbarczyk A., Alonge O. O., Sarker M., Acute flaccid paralysis surveillance in polio eradication and beyond: Learning from Bangladesh. Res. Square 10.21203/rs.3.rs-680893/v1, (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salje H., Paul K. K., Paul R., Rodriguez-Barraquer I., Rahman Z., Alam M. S., Rahman M., Al-Amin H. M., Heffelfinger J., Gurley E., Nationally-representative serostudy of dengue in Bangladesh allows generalizable disease burden estimates. eLife 8, e42869 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Azman A. S., Lauer S. A., Bhuiyan T. R., Luquero F. J., Leung D. T., Hegde S. T., Harris J. B., Paul K. K., Khaton F., Ferdous J., Lessler J., Salje H., Qadri F., Gurley E. S., Vibrio cholerae O1 transmission in Bangladesh: Insights from a nationally representative serosurvey. Lancet Microbe. 1, e336–e343 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Azman A., Paul K. K., Bhuiyan T. R., Koyuncu A., Salje H., Qadri F., Gurley E. S., Hepatitis E in Bangladesh: Insights from a national serosurvey. J. Infect. Dis. 224, S805–S812 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wiens K. E., Jauregui B., Arnold B. F., Banke K., Wade D., Hayford K., Costero-Saint Denis A., Hall R. H., Salje H., Rodriguez-Barraquer I., Azman A. S., Vernet G., Leung D. T., on behalf of the Collaboration on Integrated Biomarkers Surveillance , Building an integrated serosurveillance platform to inform public health interventions: Insights from an experts’ meeting on serum biomarkers. PLoS Negl. Trop. Dis. 16, e0010657 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bailly S., Rousset D., Fritzell C., Hozé N., Ben Achour S., Berthelot L., Enfissi A., Vanhomwegen J., Salje H., Fernandes-Pellerin S., Saout M., Lavergne A., Manuguerra J.-C., Carod J.-F., Djossou F., Cauchemez S., Flamand C., Spatial distribution and burden of emerging arboviruses in French Guiana. Viruses. 13, 1299, ( 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reuben R., Tewari S. C., Hiriyan J., Akiyama J., Illustrated keys to species of Culex (Culex) associated with Japanese encephalitis in Southeast Asia (Diptera: Culicidae). Mosq. Syst. 26, 75–96 (1994). [Google Scholar]
- 46.Lindgren F., Rue H., Bayesian spatial modelling with R-INLA. JSS J. Stat. Softw. 63, 1–25 (2015). [Google Scholar]
- 47.Hanks E. M., Schliep E. M., Hooten M. B., Hoeting J. A., Restricted spatial regression in practice: Geostatistical models, confounding, and robustness under model misspecification. Environ. 26, 243–254 (2015). [Google Scholar]
- 48.O. N. Bjørnstad, Epidemics: Models and Data Using R (Springer Nature, 2023). [Google Scholar]
- 49.H. Muench, Catalytic Models in Epidemiology (Harvard Univ. Press, 1959). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Figs. S1 to S4