Abstract
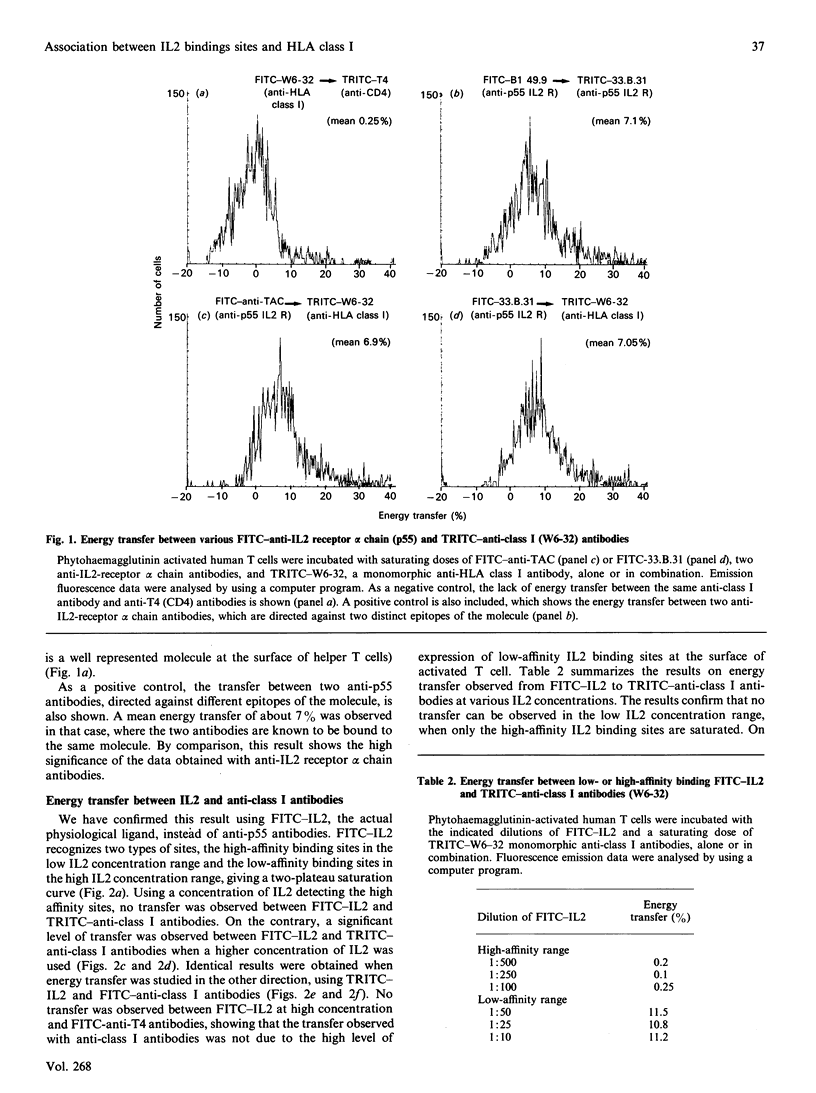
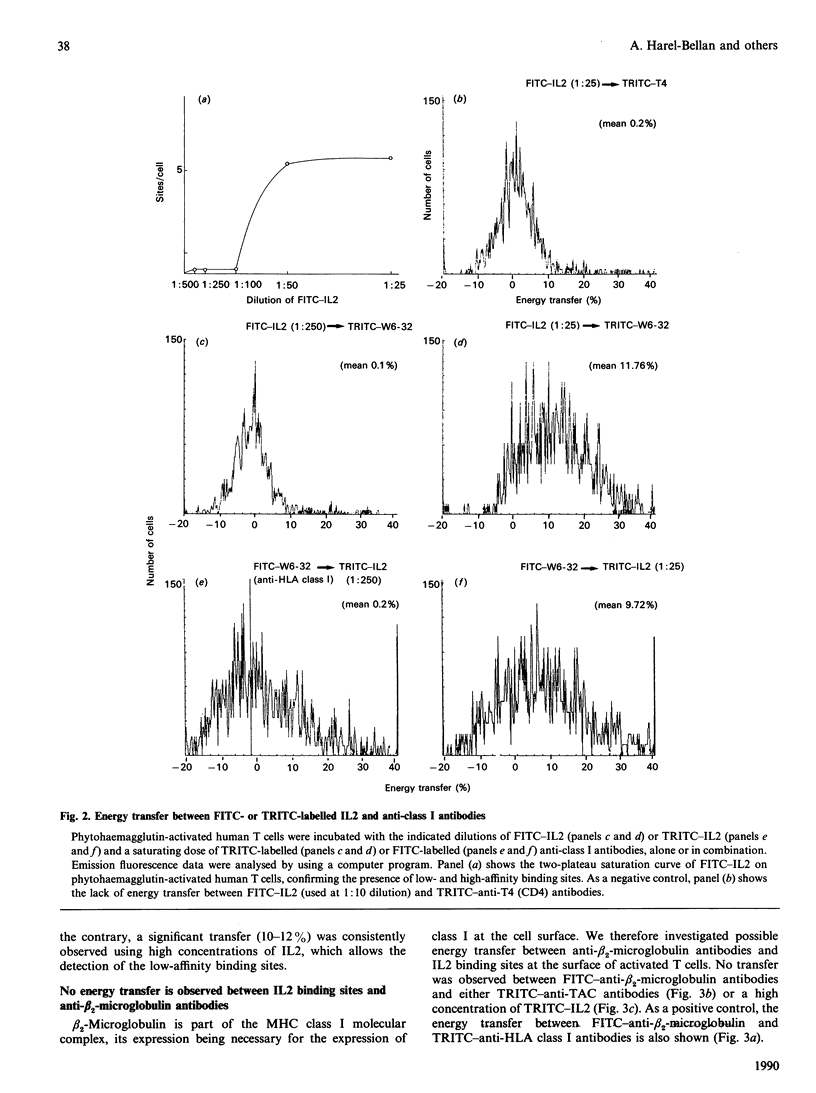
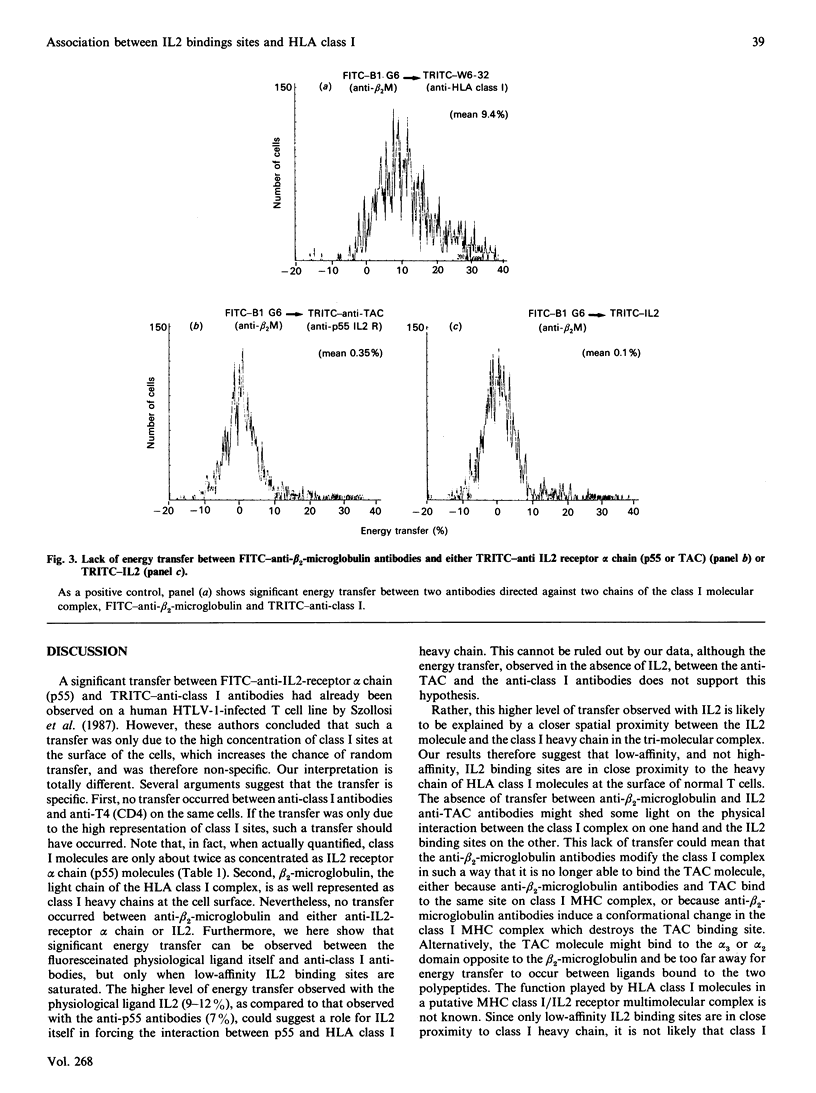
Using flow cytometry energy transfer we have studied the sterical proximity of interleukin 2 receptors and the heavy chain of the major histocompatibility complex at the surface of normal lymphocytes. Our data suggest that class I molecules may be part of a low-affinity interleukin 2 receptor multimolecular complex, where the MHC class I heavy chain is in close proximity to the actual interleukin 2 binding site, in contrast to the light chain (beta 2-microglobulin).
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama Y., Zicht R., Ferrone S., Bonnard G. D., Herberman R. B. Effect of monoclonal antibodies (MoAb) to class I and class II HLA antigens on lectin- and MoAb OKT3-induced lymphocyte proliferation. Cell Immunol. 1985 Apr 1;91(2):477–491. doi: 10.1016/0008-8749(85)90245-x. [DOI] [PubMed] [Google Scholar]
- Bonagura V. R., Nordli D. R., Pernis B. Antibodies against monomorphic determinants of the alpha chain of class I human leukocyte antigens inhibit the reaction of human lymphocytes to mitogen and tetanus toxoid. Immunol Invest. 1985 Jun;14(3):233–243. doi: 10.3109/08820138509076147. [DOI] [PubMed] [Google Scholar]
- Chouaib S., Welte K., Dupont B. Differential effect of anti-beta 2-microglobulin on IL 2 production and IL 2 receptor expression in the primary mixed lymphocyte culture reaction. J Immunol. 1985 Feb;134(2):940–948. [PubMed] [Google Scholar]
- Damjanovich S., Trón L., Szöllösi J., Zidovetzki R., Vaz W. L., Regateiro F., Arndt-Jovin D. J., Jovin T. M. Distribution and mobility of murine histocompatibility H-2Kk antigen in the cytoplasmic membrane. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5985–5989. doi: 10.1073/pnas.80.19.5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta J. D., Cemach K., Dubey D. P., Yunis E. J., Amos D. B. The role of class I histocompatibility antigens in the regulation of T-cell activation. Proc Natl Acad Sci U S A. 1987 Feb;84(4):1094–1098. doi: 10.1073/pnas.84.4.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felice M., Turco M. C., Giarrusso P. C., Corbo L., Pizzano R., Martinelli V., Ferrone S., Venuta S. Differential regulatory role of monomorphic and polymorphic determinants of histocompatibility leukocyte antigen class I antigens in monoclonal antibody OKT3-induced T cell proliferation. J Immunol. 1987 Oct 15;139(8):2683–2689. [PubMed] [Google Scholar]
- DeMars R., Chang C. C., Rudersdorf R. A. Dissection of the D-region of the human major histocompatibility complex by means of induced mutations in a lymphoblastoid cell line. Hum Immunol. 1983 Oct;8(2):123–139. doi: 10.1016/0198-8859(83)90008-3. [DOI] [PubMed] [Google Scholar]
- Dukovich M., Wano Y., Le thi Bich Thuy, Katz P., Cullen B. R., Kehrl J. H., Greene W. C. A second human interleukin-2 binding protein that may be a component of high-affinity interleukin-2 receptors. Nature. 1987 Jun 11;327(6122):518–522. doi: 10.1038/327518a0. [DOI] [PubMed] [Google Scholar]
- Gennis R. B., Cantor C. R. Use of nonspecific dye labeling for singlet energy-transfer measurements in complex systems. A simple model. Biochemistry. 1972 Jun 20;11(13):2509–2517. doi: 10.1021/bi00763a020. [DOI] [PubMed] [Google Scholar]
- Greene W. C., Robb R. J., Svetlik P. B., Rusk C. M., Depper J. M., Leonard W. J. Stable expression of cDNA encoding the human interleukin 2 receptor in eukaryotic cells. J Exp Med. 1985 Jul 1;162(1):363–368. doi: 10.1084/jem.162.1.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harel-Bellan A., Bertoglio J., Quillet A., Marchiol C., Wakasugi H., Mishall Z., Fradelizi D. Interleukin 2 (IL 2) up-regulates its own receptor on a subset of human unprimed peripheral blood lymphocytes and triggers their proliferation. J Immunol. 1986 Apr 1;136(7):2463–2469. [PubMed] [Google Scholar]
- Harel-Bellan A., Mishal Z., Willette-Brown J., Farrar W. L. Detection of low and high affinity binding sites with fluoresceinated human recombinant interleukin-2. J Immunol Methods. 1989 Apr 21;119(1):127–133. doi: 10.1016/0022-1759(89)90389-x. [DOI] [PubMed] [Google Scholar]
- Le Bouteiller P. P., Mishal Z., Lemonnier F. A., Kourilsky F. M. Quantification by flow cytofluorimetry of HLA class I molecules at the surface of murine cells transformed by cloned HLA genes. J Immunol Methods. 1983 Jul 29;61(3):301–315. doi: 10.1016/0022-1759(83)90224-7. [DOI] [PubMed] [Google Scholar]
- Liabeuf A., le Borgne de Kaouel C., Kourilsky F. M., Malissen B., Manuel Y., Sanderson A. R. An antigenic determinant of human beta 2-microglobulin masked by the association with HLA heavy chains at the cell surface: analysis using monoclonal antibodies. J Immunol. 1981 Oct;127(4):1542–1548. [PubMed] [Google Scholar]
- Reinherz E. L., Meuer S., Fitzgerald K. A., Hussey R. E., Levine H., Schlossman S. F. Antigen recognition by human T lymphocytes is linked to surface expression of the T3 molecular complex. Cell. 1982 Oct;30(3):735–743. doi: 10.1016/0092-8674(82)90278-1. [DOI] [PubMed] [Google Scholar]
- Robb R. J., Greene W. C., Rusk C. M. Low and high affinity cellular receptors for interleukin 2. Implications for the level of Tac antigen. J Exp Med. 1984 Oct 1;160(4):1126–1146. doi: 10.1084/jem.160.4.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb R. J., Rusk C. M., Yodoi J., Greene W. C. Interleukin 2 binding molecule distinct from the Tac protein: analysis of its role in formation of high-affinity receptors. Proc Natl Acad Sci U S A. 1987 Apr;84(7):2002–2006. doi: 10.1073/pnas.84.7.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon M., Klausner R. D., Cullen B. R., Chizzonite R., Leonard W. J. Novel interleukin-2 receptor subunit detected by cross-linking under high-affinity conditions. Science. 1986 Nov 14;234(4778):859–863. doi: 10.1126/science.3095922. [DOI] [PubMed] [Google Scholar]
- Siegel J. P., Sharon M., Smith P. L., Leonard W. J. The IL-2 receptor beta chain (p70): role in mediating signals for LAK, NK, and proliferative activities. Science. 1987 Oct 2;238(4823):75–78. doi: 10.1126/science.3116668. [DOI] [PubMed] [Google Scholar]
- Szöllösi J., Damjanovich S., Goldman C. K., Fulwyler M. J., Aszalos A. A., Goldstein G., Rao P., Talle M. A., Waldmann T. A. Flow cytometric resonance energy transfer measurements support the association of a 95-kDa peptide termed T27 with the 55-kDa Tac peptide. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7246–7250. doi: 10.1073/pnas.84.20.7246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szöllösi J., Mátyus L., Trón L., Balázs M., Ember I., Fulwyler M. J., Damjanovich S. Flow cytometric measurements of fluorescence energy transfer using single laser excitation. Cytometry. 1987 Mar;8(2):120–128. doi: 10.1002/cyto.990080204. [DOI] [PubMed] [Google Scholar]
- Szöllösi J., Trón L., Damjanovich S., Helliwell S. H., Arndt-Jovin D., Jovin T. M. Fluorescence energy transfer measurements on cell surfaces: a critical comparison of steady-state fluorimetric and flow cytometric methods. Cytometry. 1984 Mar;5(2):210–216. doi: 10.1002/cyto.990050216. [DOI] [PubMed] [Google Scholar]
- Taylor D. S., Nowell P. C., Kornbluth J. Functional role of HLA class I cell-surface molecules in human T-lymphocyte activation and proliferation. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4446–4450. doi: 10.1073/pnas.83.12.4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teshigawara K., Wang H. M., Kato K., Smith K. A. Interleukin 2 high-affinity receptor expression requires two distinct binding proteins. J Exp Med. 1987 Jan 1;165(1):223–238. doi: 10.1084/jem.165.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trón L., Szöllósi J., Damjanovich S., Helliwell S. H., Arndt-Jovin D. J., Jovin T. M. Flow cytometric measurement of fluorescence resonance energy transfer on cell surfaces. Quantitative evaluation of the transfer efficiency on a cell-by-cell basis. Biophys J. 1984 May;45(5):939–946. doi: 10.1016/S0006-3495(84)84240-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turco M. C., De Felice M., Corbo L., Morrone G., Mertelsmann R., Ferrone S., Venuta S. Regulatory role of a monomorphic determinant of HLA Class I antigens in T cell proliferation. J Immunol. 1985 Oct;135(4):2268–2273. [PubMed] [Google Scholar]


