Abstract
Introduction
Infantile hemangioma with minimal or arrested growth (IHMAG) is an unusual subset of infantile hemangioma, difficult to recognize because they are often mistaken for capillary malformation or other entities. Dermoscopic features of IHMAG have been described only in small case series so far.
Objectives
The aim of our study was to evaluate epidemiological, clinical, and dermoscopic features in 79 cases of IHMAG with a specific focus on neonates and toddlers with segmental complicated IHMAG and to provide a remarkable dermoscopic criterion to achieve diagnosis.
Methods
This case series collected all the cases of IHMAG recorded in our Clinical Registry from January 2012 to March 2022.
Results
A total of 79 cases of IHMAG were identified in our study; 53 (67.1%) were localized and 26 (32.9 %) were segmental. Patients showed some complications during follow-up such as ulceration and soft tissue anomalies. One PHACE syndrome and two LUMBAR syndromes were included. Our study highlights the main dermoscopic features differentiating IHMAG from infantile hemangiomas and capillary malformations in neonatal patients, highlighting the presence of enlarged unfocused telangiectatic vessels as remarkable clues.
Conclusions
This is a large case series described in the literature about this rare entity. We emphasize that segmental IHMAG may be associated with structural abnormalities and may pose a diagnostic challenge especially in its rare facial segmental localization. The use of dermoscopy allowed us to find typical signs for IHMAG, thus avoiding the execution of invasive methods and ensuring the prompt suspicion of a syndrome in segmental neonatal cases.
Keywords: IHMAG, infantile hemangioma, dermatoscopy, PHACE syndrome, LUMBAR syndrome, vascular malformations
Introduction
Infantile hemangioma (IH) is the most common tumor of childhood that is usually absent at birth, appears in the first weeks of life, and undergoes a rapid growth phase followed by a period of slow involution with possible residual lesions [1,2]. In addition, IH express an erythrocyte-type glucose transport protein (GLUT-1) in their endothelial cells, which is a highly specific marker of IH [3].
The typical evolution makes them easy to diagnose clinically in most cases.
IH with minimal or arrested growth (S) are an unusual subset of IH that are present at birth and characteristically have little or no proliferation (which has been defined as a proliferative component of <25% of their surface area) [4,5]. This variant of IH has been described with several different names: abortive, precursor, or minimal growth hemangiomas; macular hemangioma with port-wine-stain-like appearance; reticular infantile hemangioma; and plaque-telangiectatic hemangiomas [6,7]. Different from classic IH, the clinical diagnosis of IHMAG can be difficult because they can present as macular infantile hemangiomas with a network-like appearance, and they are often mistaken for vascular malformation [8,9]. The co-occurrence of IHMAG and structural anomalies is uncommon. However, some large segmental IHMAG extending to the face, buttocks, or limbs have been linked with structural anomalies in patients with PHACE, PELVIS, or SACRAL syndromes [5,10]. Other anomalies identified in these tumors are the presence of soft tissue hypotrophy or hypertrophy beneath the surface of the lesion [5,11]. Dermoscopy can be a useful tool for clinicians to better identify this type of hemangioma, allowing for differentiation from other clinically entities such as capillary malformations (CM) or IH. Complications and clinical and dermoscopic features of IHMAG have been previously described only in small case series [6,7,12].
Objective
The aim of our study was to evaluate epidemiological, clinical, and dermoscopic features in 79 cases of IHMAG with a specific focus on neonates and toddlers with segmental complicated IHMAG and to provide a remarkable dermoscopic criterion to achieve diagnosis.
Methods
This single-center retrospective study was conducted on 79 patients with IHMAG at the Pediatric Dermatology Unit of the University XXXXX from January 2012 to March 2022 using medical records and photographs. Clinical and dermoscopic images reported in this paper were all collected by our FotoFinder® video dermatoscopy archive database in our Dermatology Clinic Department. All the patients included were <3 years old. All the parents gave their written consent for scientific use of clinical and dermoscopic images.
The term IHMAG is consistently used in this paper to avoid further misunderstandings about this kind of vascular proliferation. An IHMAG is defined as an infantile hemangioma with a proliferative component less than 25% of its total surface area [4,5]. Finally, dermoscopic features of IHMAG were retrospectively assessed by four dermatologists in order to assess remarkable dermoscopic criteria to achieve differential diagnosis from IH and port-wine stain (PWS). A dermoscopic criterion was defined as remarkable in diagnosing IHMAG if at least three out of four dermatologists agreed.
Results
Clinical and Epidemiological Findings of IHMAG
A total of 79 cases of IHMAG in as many subjects were identified in our study. All the clinical characteristics of IHMAG, with their associations and complications, are illustrated in Table 1.
Table 1.
Clinical and Epidemiological Findings of IHMAG Among 79 Patients.
| Characteristics | Value (%) |
|---|---|
|
| |
| Sex | |
| Male | 23 (29.1) |
| Female | 56 (70.9) |
|
| |
| Morphologic subtype | |
| Focal | 53 (67.1) |
| Segmental | 26 (32.9) |
|
| |
| Anatomic distribution | |
| Head and neck | 5 (6.3) |
| Trunk | 21 (26.6) |
| Limb | 37 (46.8) |
| Hand/foot | 16 (20.3) |
|
| |
| Presence of proliferation | |
| Yes | 32 (40.5) |
| No | 47 (59.5) |
|
| |
| Complications | |
| Ulceration | 3 (3.8) |
| Soft tissue hypertrophy | 2 (2.5) |
| Soft tissue hypotrophy | 3 (3.8) |
| Spinal dysraphism | 2 (2.5) |
| PHACE syndrome | 1 (1.3) |
| LUMBAR syndrome | 2 (2.5) |
| No complications | 66 (83.6) |
|
| |
| Associated anomalies | |
| Infantile hemangioma | 7 (8.9) |
| Vascular malformation | 5 (6.3) |
| No other associated anomalies | 67 (84.8) |
|
| |
| Systemic treatment | |
| Yes | 13 (16.5) |
| No | 66 (83.5) |
IHMAG: = infantile hemangiomas with minimal or arrested growth.
All the lesions were noticed at birth as flat, reddish, and asymptomatic macules, with a female-to-male ratio of 2.4:1. The limbs were the most represented anatomical site with 37 (46.8%) IHMAG, 21 (26.6%) were located on the trunk, and 16 (20.3%) on the acral sites (hand or foot). Only five (6.3%) patients presented a lesion on the head and neck region.
IHMAGs were classified as focal or localized if they presented as well-circumscribed small lesions, and segmental if they were larger and affected a specific area of the body such as the head, an acral site, genitalia, or the lumbosacral skin region. Fifty-three (67.1%) localized hemangiomas and 26 (32.9%) segmental lesions were identified, the latter affecting 14 (53.8%) females and 12 (46.2%) males. Limbs represented the most frequently involved site in segmental cases (13/26), while the others segmental lesions are localized on the hands or feet (8/26), the trunk (4/26), and on the head (1/26). Thirty-two cases presented a proliferative component, always < 25% of the total surface area. It clinically appeared as bright red papules, usually located at the periphery of the lesions. In most cases, proliferation started a few days after birth.
In our study, all IHMAGs were characterized by a common clinical presentation, with an erythematous network-like patch and blotchy appearance. These aspects were present either in focal or segmental types (Figure 1A). In seven patients, IHMAGs were associated with the presence of one or more IH, localized in other sites and occurring in the first weeks of life, with a classical growth phase in the first 12 months. Vascular malformations (VM) were observed at birth in five patients with IHMAG, and the differential diagnosis was possible thanks to a different dermoscopic pattern of the two lesions.
Figure 1.
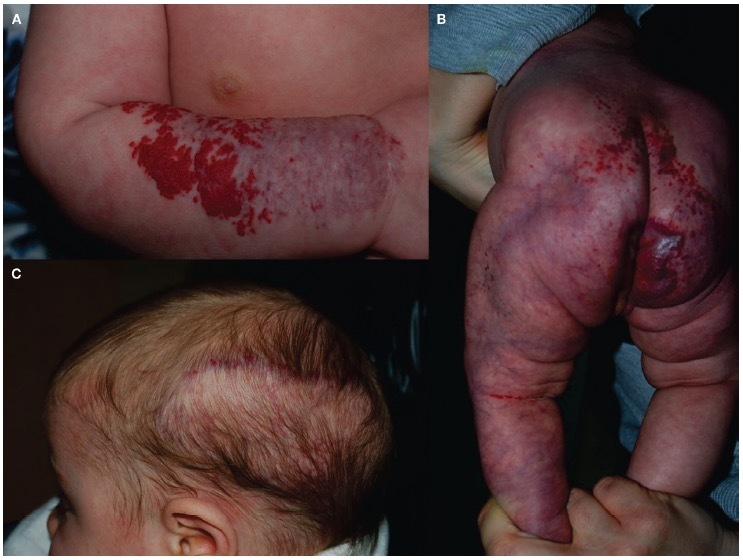
Clinical characteristics of uncomplicated and complicated infantile hemangiomas with minimal or arrested growth (IHMAG). (A) Segmental uncomplicated IHMAG of the arm, with a minimal proliferative component. (B) Segmental patch of the left limb, perianal, and sacral region with a minimal proliferative component in this boy affected by LUMBAR syndrome. A subcutaneous swelling on the sacral area, consistent with spinal dysraphism, can be observed. (C) Large plaque of the left temporal side of the scalp, present at birth. Minimal growth can be seen at the peripheral region in this girl affected by PHACE syndrome.
Dermoscopic Features of IHMAG and Differential Diagnosis
On dermoscopic evaluation, red, dotted, or globular vessels, alone (51/79, 64.6%) or combined with dilated linear and tortuous vessels (28/79, 35.4%), were associated with enlarged unfocused telangiectatic vessels, present in all IHMAGs as a remarkable sign of the neoplasia. An erythematous background and structureless whitish areas were also observed, enlarging over time during the follow-up period, until it represented almost the entire lesion (Figure 2, A-D). Indeed, CMs were also characterized by red, dotted, or globular vessels, alone or combined with dilated linear and tortuous vessels but without the presence of unfocused large telangiectatic vessels (Figure 2, E and F).
Figure 2.
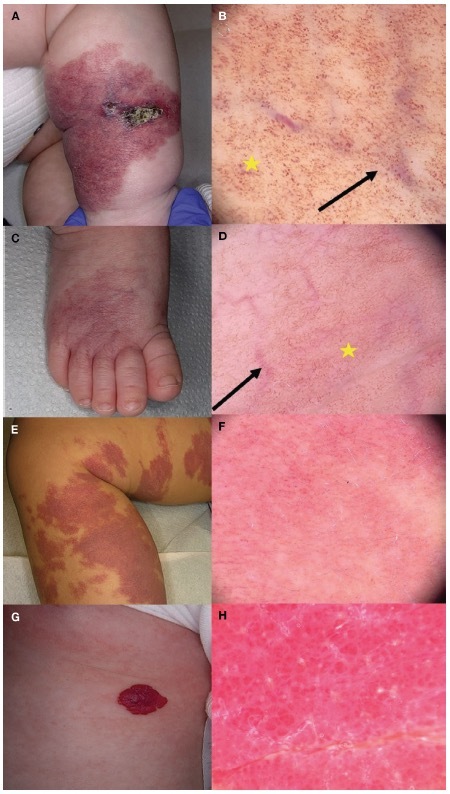
Clinical and dermoscopic characteristics of infantile hemangiomas with minimal or arrested growth (IHMAG), capillary malformation, and infantile hemangioma. (A) Ulcerated segmental IHMAG of the right leg in a toddler, which showed (B) enlarged unfocused telangiectasia (black arrow) on an erythematous background composed of dotted globular vessels (yellow star) on dermoscopy. (C) Uncomplicated segmental IHMAG of the right foot in a toddler with (D) multiple unfocused large telangiectasias (black arrow) on an erythematous background combined with dilated linear and tortuous vessels (yellow star) on dermoscopy. (E) Large segmental capillary malformation of the right leg in a toddler with (F) multiple red, dotted, or globular vessels on an erythematous background on dermoscopy. (G) Single infantile hemangiomas with a proliferative dermal component, with the (H) peculiar lacunae on dermoscopy.
IH were characterized by well-demarcated round or oval areas in which the color ranged from red to reddish-brown or reddish-blue, and the size varied within the lesion, called lacunae. Their presence is quite a constant dermoscopic finding in IH (Figure 2, G and H). Clinical and dermoscopic features and outcome of the described vascular lesions are reported in Table 2.
Table 2.
Clinical and Dermoscopic Features and Outcomes of IHMAG, IH, and Capillary Malformation.
| Onset | IHMAG | IH | CM |
|---|---|---|---|
| At Birth | First Weeks of Life | At Birth | |
| Clinical | Focal or segmental flat reddish asymptomatic macules; a proliferative component, regarding <25% of the total surface area can be present | Variable (red, finely lobulated plaque or bluish subcutaneous mass) | Reddish and asymptomatic macules |
| Dermoscopy | Red, dotted, or globular vessels, alone or combined with dilated linear and tortuous vessels, were associated with enlarged unfocused telangiectatic vessels | Well-demarcated round or oval areas called lacunae, in which the color can range from red to reddish-brown or reddish blue | Multiple red, dotted, or globular vessels, reticular and sausage-like vessels as well as white circles and whitish veil |
| Outcome | Spontaneous regression | Rapid growth phase, followed by a period of slow involution with possible residual lesions | It grows proportionally to the child’s development, without showing signs of regression over time |
CM = capillary malformation; IH = infantile hemangioma; IHMA: = infantile hemangiomas with minimal or arrested growth.
Complications of IHMAG
In our study, 13/79 patients showed some complications during follow-up in the first year of life. IHMAG with skin ulceration was detected in three patients of our analysis (Figure 2A); in two of these cases, the ulceration was associated with the presence of other complications, such as spinal dysraphism and hypotrophy of the subcutaneous tissues. The peculiar aspect of ulcerated IHMAG lies in the difficulty of management: local dressings and systemic treatment with propranolol showed poor response in our cases as well as residual scarring.
Two patients presented with segmental IHMAG associated with underlying soft tissue hypertrophy, localized on the right limb and left hand, respectively. Soft tissue hypertrophy was clinically characterized by a soft doughy palpable mass underlying the skin lesion. The absence of underlying vascular and muscle anomalies on magnetic resonance imaging (MRI) supported the conclusion that this hypertrophy was primarily due to soft tissue overgrowth. Large telangiectasias and prominent veins were also observed, but the clinical appearance did not change over time.
Three children presented localized soft tissue hypotrophy of the unilateral limb, where imaging investigations found only a reduction in the subcutaneous fatty tissue without other local abnormalities.
One case reported a segmental vascular lesion in the left temporal region of the scalp, present at birth with minimal peripheral proliferation (Figure 2C). Specific dermoscopic criteria raised the suspicion of IHMAG, and an MRI of the head and neck was performed, which confirmed anomalies of the intracranial structures of the posterior fossa as well as the presence of ipsilateral neck carotid artery malformations. This confirmed the clinical suspicion of PHACE (posterior fossa brain malformations, large facial hemangiomas, anatomical anomalies of the cerebral arteries, aortic coarctation, and other cardiac anomalies, and eye abnormalities) syndrome, for which ophthalmological, neurological, cardiological, and endocrinological evaluations were also performed.
In addition, four patients in our series developed segmental IHMAG in the lumbosacral skin at birth (Figure 2B). After performing an imaging assessment of the pelvis and lumbosacral spinal cord, two patients presented with isolated spinal dysraphism, without any other anatomical malformations, while the others were diagnosed with LUMBAR (lower body congenital infantile hemangiomas and other skin defects; urogenital anomalies and ulceration; myelopathy; bony deformities; anorectal malformations and arterial anomalies; and rectal anomalies) syndrome. All the patients underwent surgical correction of spinal dysraphism and treatment of anatomical malformations.
All 13 cases associated with complications underwent systemic treatment with propranolol hydrochloride in oral solution, as recommended by the current guidelines on IH [13]. It is important to underline that ten complicated cases were associated with segmental IHMAG (38.5% of all segmental cases) but only three with focal ones.
The other 66 patients underwent wait-and-see management through clinical follow-up, or therapy with topical beta blockers was prescribed to reduce the risk of proliferation and associated complications.
Discussion
During the drafting of this paper, the authors experienced difficulty in carrying out a complete literature search about IHMAG as already described. This may be explained by the small number of lesions described over the years, especially since IHMAG has only recently been classified as a specific subtype of classical IH. In addition, this subtype of IH has historically been defined using heterogeneous and poorly coded nomenclature, as clarified in the text above, leading to further difficulties in collecting cases from the literature.
In this series, 70.9% of the patients were female, another known epidemiological characteristic of these lesions, which is in common with IH [13]. The anatomical distribution of these lesions is consistent with other reports in the literature [6]; in particular, IHMAG was mostly represented on the limbs (46.8% of the total) as focal or segmental lesions than on the face or trunk, in contrast with IH [14,15]. The large number of segmental IHMAGs (32.9 % of the total) makes the differential diagnosis with other entities, such as CM and segmental IH, more difficult. Clinically, they both present as well-demarcated red macular stains, sometimes covered by fine telangiectasias [2]. At dermoscopy, IHMAG presents with enlarged unfocused telangiectasias on an erythematous background composed of capillary dilations, not observed in CM [2]. In CMs, on the other hand, multiple red, dotted, or globular vessels, alone or combined with dilated linear and tortuous vessels, are the predominant signs on an erythematous whitish background, usually associated with a gray whitish veil (Figure 2, E and F) [16]. These aspects can easily distinguish between these two vascular lesions [17]. In doubtful cases, skin biopsy may be useful, as GLUT-1 results negative in vascular malformation, but in IHMAG, it is positive for this marker at immunochemistry [1,18].
In addition, over time, IHMAG and CM have different clinical courses: IHMAG has a slight amount of proliferation, which is characterized by peripheral small vascular papules in the context of the lesion. The proliferative component tends to stabilize in the first year of life, then it slowly regresses spontaneously [19]. CM, on the other hand, maintains the clinical aspect of a flat lesion which grows proportionally to the child’s development, without showing signs of regression over time. Finally, CM and IHMAG can coexist in the same patient, as described in five cases of our retrospective analysis. The early distinction between IHMAG and CM through dermoscopy allows for the initiation of a clinical follow-up during the first year of life. This is crucial to identifying potential complications associated with IHMAG, which do not arise in the case of CM.
In our retrospective analysis, 11 patients presented IHMAG associated with several complications. However, as shown in the literature, segmental IH could also be associated with ulceration, spinal dysraphism, LUMBAR syndrome, and PHACE syndrome. Ulcerated IHMAG, usually localized in the anogenital area, has been reported in larger series [6]. However, there are no data regarding the prognosis and complications of these lesions. In the literature, there are some cases consisting of IHMAG associated with and soft tissue hypertrophy and hypotrophy [1,6]. Planas-Ciudad et al. [5] described ten patients with similar characteristics, localized on the arms or legs. Prognosis is similar to the other IHMAG without subcutaneous involvement, but these rare cases have to be differentiated from other conditions, such as venous, arteriovenous, or CM, which can show different complications over time. Bessis et al. [11] reported seven IHMAG cases with hypotrophy, where the clinical characteristics are superimposable. According to the literature, MRI imaging should be performed on all the patients with IHMAG-associated abnormality of soft tissue [20].
PHACE and LUMBAR syndromes are usually reported with the presence of a segmental IH, while cases of association with segmental IHMAG are rare or misdiagnosed [25,28]. This emphasizes the importance of early recognition of this association in order to carry out a complete diagnostic investigation and start systemic treatment, avoiding the onset of complications [21].
Until about ten years ago, it was a common opinion to discriminate between three different forms of syndromes associated with segmental IH of the pelvic and lumbosacral area: PELVIS, LUMBAR, and SACRAL. Currently, to avoid confusion, the denomination LUMBAR is preferred to indicate forms of segmental IH of the lower body, associated with regional congenital anomalies [22,23]. Cases of LUMBAR associated with IHMAG are very rare in the literature [24,25]. Suh and Frieden [6] reported a single case of lumbar IHMAG associated with regional anomalies in their study, emphasizing the role of this apparently benign lesion as a marker of serious regional abnormalities.
Seven patients in our study presented with the coexistence of IHMAG and IH, occurring mostly in the weeks after birth as fast-growing erythematous plaques often associated with a proportion of subcutaneous proliferation [26,27]. In sporadic cases, IH may present a precursor lesion similar to IHMAG, as it is characterized by an erythematous and telangiectatic patch without a proliferating component. However, these lesions differ in the presence of an anemic halo in the peripheral area, which is not seen in IHMAG [28]. The development of these two lesions in the subsequent weeks will lead to a definitive diagnosis.
IH at dermoscopy are characterized by well-demarcated round or oval areas in which the color can range from red to reddish brown or reddish blue, and the size can vary within the lesion, called lacunae (Figure 2, G and H). The deep, mixed IH, unlike the exclusively superficial ones, present a more bluish background on dermatoscopy [17]. The natural history of these lesions presents some differences: IHMAG, having a low or absent proliferating amount, tend to persist longer as telangiectatic macules, unlike superficial IH, which regress completely, leaving only few dilated vessels [4]. Clinical and dermoscopic features and outcome of the described vascular lesions are reported in Table 2.
The dermoscopic findings in our study population are comparable to the observations reported in the literature [29]. Fernández-Domper et al. [12] found the presence of vascular lacunae in 100% of lesions diagnosed as IHMAG, while not observing the presence of telangiectatic vessels on an erythematous background. These differences are likely due to the fact that dermoscopic examination in their patients was performed at least one year after the onset of lesions, with changes in the pattern attributed to the physiological remodeling and spontaneous absorption of vascular proliferative components, while our data are referred to new onset IHMAG, in the first weeks after birth. The presence of lacunae may also be attributed to the minority proliferative component usually present in the peripheral areas of IHMAG but absent in the remaining flat part of the skin lesion.
The main limitations are that this was a single-center study and the retrospective nature like other studies. Finally, dermoscopic differential diagnosis of IHMAG with capillary malformation and infantile hemangioma is only a descriptive analysis and not a case-control comparative study.
Conclusions
This paper presents a large retrospective study of IHMAG reported in a single-center experience with epidemiological, clinical, and dermoscopic features focused on neonates and toddlers. This large case series lets us better describe IHMAG, highlighting its clinical appearance and associated structural anomalies, above all with segmental lesions and dermoscopic features. Further investigations are needed to better understand the etiology of this condition as well as any negative prognostic factors. Dermoscopy allowed us to find the signs for IHMAG in order to achieve a prompt diagnosis and to avoid a diagnostic pitfall with CM and local and segmental IH, thus avoiding the execution of invasive methods or promptly suspecting a syndrome such as the PHACE syndrome on neonatal cases.
Footnotes
Funding: None.
Competing Interests: None.
Authorship: All authors have contributed significantly to this publication.
References
- 1.Corella F, Garcia-Navarro X, Ribe A, Alomar A, Baselga E. Abortive or Minimal-Growth Hemangiomas: Immunohistochemical Evidence That They Represent True Infantile Hemangiomas. JAAD. 2008;58(4):685–690. doi: 10.1016/j.jaad.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 2.Toledo-Alberola F, Betlloch-Mas I, Cuesta-Montero L, et al. Abortive Hemangiomas. Description of Clinical and Pathological Findings with Special Emphasis on Dermoscopy. Eur J Dermatol. 2010;20(4):497–500. doi: 10.1684/ejd.2010.0959. [DOI] [PubMed] [Google Scholar]
- 3.North PE, Waner M, Mizeracki A, Mihm MC. GLUT1: A Newly Discovered Immunohistochemical Marker for Juvenile Hemangiomas. Human Pathology. 2000;31(1):11–22. doi: 10.1016/S0046-8177(00)80192-6. [DOI] [PubMed] [Google Scholar]
- 4.Ma EH, Robertson SJ, Chow CW, Bekhor PS. Infantile Hemangioma with Minimal or Arrested Growth: Further Observations on Clinical and Histopathologic Findings of This Unique but Under-recognized Entity. Pediatr Dermatol. 2017;34(1):64–71. doi: 10.1111/pde.13022. [DOI] [PubMed] [Google Scholar]
- 5.Planas-Ciudad S, Roé Crespo E, Sánchez-Carpintero I, et al. Infantile Hemangiomas with Minimal or Arrested Growth Associated with Soft Tissue Hypertrophy: A Case Series of 10 Patients. J Eur Acad Dermatol Venereol. 2017;31(11):1924–1929. doi: 10.1111/jdv.14457. [DOI] [PubMed] [Google Scholar]
- 6.Suh K-Y, Frieden IJ. Infantile Hemangiomas With Minimal or Arrested Growth: A Retrospective Case Series. Arch Dermatol. 2010;146(9) doi: 10.1001/archdermatol.2010.197. [DOI] [PubMed] [Google Scholar]
- 7.Martin JM, Sanchez S, González V, Cordero P, Ramon D. Infantile Hemangiomas with Minimal or Arrested Growth: A Retrospective Case Series. Pediatr Dermatol. 2019;36(1):125–131. doi: 10.1111/pde.13695. [DOI] [PubMed] [Google Scholar]
- 8.Vega Mata N, López Gutiérrez JC, Vivanco Allende B, Fernández García MS. Different Clinical Features of Acral Abortive Hemangiomas. Case Rep Dermatolog Med. 2017;2017:1–5. doi: 10.1155/2017/2897617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martín-Santiago A, Bauzá A, del Pozo LJ, Carrillo P. Hemangiomas abortivos o mínimamente proliferativos. Revisión de 14 casos. Actas Dermo-Sifiliográficas. 2012;103(3):246–250. doi: 10.1016/j.ad.2011.03.030. [DOI] [PubMed] [Google Scholar]
- 10.Metry DW, Haggstrom AN, Drolet BA, et al. A Prospective Study of PHACE Syndrome in Infantile Hemangiomas: Demographic Features, Clinical Findings, and Complications. Am J Med Genet. 2006;140A(9):975–986. doi: 10.1002/ajmg.a.31189. [DOI] [PubMed] [Google Scholar]
- 11.Bessis D, Bigorre M, Labrèze C. Reticular Infantile Hemangiomas with Minimal or Arrested Growth Associated with Lipoatrophy. JAAD. 2015;72(5):828–833. doi: 10.1016/j.jaad.2015.01.028. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez-Domper L, Silva Diaz E, Ballesteros Redondo M, Martín Hernández JM, Revert Fernandez A. Dermoscopy of Abortive Hemangioma: Morphological Study of 11 Cases. Dermatol Pract Concept. 2023:e2023098. doi: 10.5826/dpc.1302a98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodríguez Bandera AI, Sebaratnam DF, Wargon O, Wong L-CF. Infantile Hemangioma. Part 1: Epidemiology, Pathogenesis, Clinical Presentation and Assessment. JAAD. 2021;85(6):1379–1392. doi: 10.1016/j.jaad.2021.08.019. [DOI] [PubMed] [Google Scholar]
- 14.Haggstrom AN, Drolet BA, Baselga E, et al. Prospective Study of Infantile Hemangiomas: Clinical Characteristics Predicting Complications and Treatment. Pediatrics. 2006;118(3):882–887. doi: 10.1542/peds.2006-0413. [DOI] [PubMed] [Google Scholar]
- 15.Chiller KG, Passaro D, Frieden IJ. Hemangiomas of Infancy: Clinical Characteristics, Morphologic Subtypes, and Their Relationship to Race, Ethnicity, and Sex. Arch Dermatol. 2002;138:12. doi: 10.1001/archderm.138.12.1567. [DOI] [PubMed] [Google Scholar]
- 16.Lanigan SW. Videomicroscopy Predicts Outcome in Treatment of Port-Wine Stains. Arch Dermatol. 1997;133(7):921. doi: 10.1001/archderm.1997.03890430143029. [DOI] [PubMed] [Google Scholar]
- 17.Piccolo V, Russo T, Moscarella, Brancaccio G, Alfano R, Argenziano G. Dermatoscopy of Vascular Lesions. Dermatolog Clin. 2018;36(4):389–395. doi: 10.1016/j.det.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 18.Harik, Kalaria R, Andersson L, Lundahl P, Perry G. Immunocytochemical Localization of the Erythroid Glucose Transporter: Abundance in Tissues with Barrier Functions. J Neurosci. 1990;10(12):3862–3872. doi: 10.1523/JNEUROSCI.10-12-03862.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bae K-N, Shin K, Kim H-S, Kim B-S, Kim M-B, Ko H-C. Infantile Hemangiomas with Minimal and Arrested Growth: Clinical Features and Treatment Outcomes with 0.5% Topical Timolol Maleate. Ann Dermatol. 2021;33(5):448. doi: 10.5021/ad.2021.33.5.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stillo F, Mattassi R, Diociaiuti, et al. Guidelines for Vascular Anomalies by the Italian Society for the Study of Vascular Anomalies (SISAV) Int Angiol. 2022;41(2 Suppl 1) doi: 10.23736/S0392-9590.22.04902-1. [DOI] [PubMed] [Google Scholar]
- 21.Leuzzi M, Sechi A, Filippi F, Di Altobrando A, Gurioli C, Neri I. Infantile Hemangioma with Minimal or Arrested Growth and Isolated Spinal Dysraphism: A New or Underrecognized Entity? Indian J Dermatol. 2021;66(5):559. doi: 10.4103/ijd.IJD_267_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iacobas I, Burrows PE, Frieden IJ, et al. LUMBAR: Association between Cutaneous Infantile Hemangiomas of the Lower Body and Regional Congenital Anomalies. J Pediatr. 2010;157(5):795–801.e7. doi: 10.1016/j.jpeds.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 23.Frade F, Kadlub N, Soupre V, Cassier S, Vazquez M-P, Picard A. Du PELVIS au LUMBAR syndrome : à propos de 2 cas. Archives de Pédiatrie. 2012;19(1):55–58. doi: 10.1016/j.arcped.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 24.Martínez-Criado Y, Fernández-Pineda I, Merchante E, Bernabeu-Wittel J. Lumbosacral Abortive Hemangioma with Intradural Extension. Pediatr Dermatol. 2014;31(3):e80–e81. doi: 10.1111/pde.12234. [DOI] [PubMed] [Google Scholar]
- 25.Calderón-Castrat X, Peceros-Escalante J, Velásquez F, Lipa-Chancolla RM, Ballona R. Hemangioma infantil de crecimiento mínimo o detenido segmentario en un síndrome LUMBAR. Actas Dermo-Sifiliográficas. 2017;108(5):475–477. doi: 10.1016/j.ad.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 26.Chang LC, Haggstrom AN, Drolet BA, et al. for the Hemangioma Investigator Group. Growth Characteristics of Infantile Hemangiomas: Implications for Management. Pediatrics. 2008;122(2):360–367. doi: 10.1542/peds.2007-2767. [DOI] [PubMed] [Google Scholar]
- 27.Bruckner AL, Frieden IJ. Hemangiomas of Infancy. JAAD. 2003;48(4):477–496. doi: 10.1067/mjd.2003.200. [DOI] [PubMed] [Google Scholar]
- 28.Payne MM, Moyer F, Marcks KM, Trevaskis AE. The Precursor to the Hemangioma. PlastReconstr Surg. 1966;38(1):64–67. doi: 10.1097/00006534-196607000-00013. [DOI] [PubMed] [Google Scholar]
- 29.Oiso N, Kimura M, Kawara S, Kawada A. Clinical, Dermoscopic, and Histopathologic Features in a Case of Infantile Hemangioma without Proliferation. Pediatr Dermatol. 2011;28(1):66–68. doi: 10.1111/j.1525-1470.2010.01363.x. [DOI] [PubMed] [Google Scholar]


