Abstract
Introduction
Folliculitis decalvans (FD) is a rare scarring alopecia mainly affecting middle-aged men, characterized by recurring episodes of follicular pustules, crusts, erythema, tufted hairs, and scars.
Objectives
This study investigates the effectiveness of reflectance confocal microscopy (RCM) compared to trichoscopy for diagnosing and monitoring FD.
Methods
The study involved 24 Caucasian patients diagnosed with FD. Patients were examined using trichoscopy and RCM, with a focus on specific features like erythema and inflammatory cell distribution. A subgroup of 16 patients was followed up after 3 months of therapy. The reproducibility of RCM and trichoscopy was assessed using Cohen Kappa Test.
Results
RCM and trichoscopy consistently detected features such as tufted hairs, pustules, and perifollicular fibrosis. However, RCM provided more detailed insights into inflammatory activity and types of fibrosis, often overlooked by trichoscopy. It showed a reduction in vessels and inflammatory cells, which trichoscopy failed to detect. The concordance between RCM evaluations was excellent, indicating high reproducibility.
Conclusions
RCM is effective in diagnosing and monitoring FD, offering detailed insights into inflammation and fibrosis. It complements trichoscopy, especially in aspects where trichoscopy is limited, such as precise measurement of inflammation. The study suggests that combining RCM with trichoscopy could enhance the accuracy of diagnosis and monitoring of FD, leading to tailored therapeutic approaches. Further studies with larger sample sizes and longitudinal designs are recommended to confirm these findings.
Keywords: scarring alopecia, folliculitis decalvans, dermoscopy, trichoscopy, reflectance confocal microscopy
Introduction
Folliculitis decalvans (FD) is a rare scarring alopecia that mainly affects middle-aged men. It is marked by recurring episodes presenting with follicular pustules, crusts, erythema, tufted hairs, and scars, particularly on the vertex and occipital regions [1,2]. These lesions can cause pain or itchiness [3]. The consequences of FD are not just physical but also psychological due to the discomfort and irreversible hair loss [4]. Dermoscopy, named trichoscopy when applied on the scalp, is a validated diagnostic tool for alopecia diagnosis and follow-up evaluation [5]. A scalp biopsy may be needed to establish diagnosis and to characterize the inflammatory infiltrate of the lesion but is not recommended for follow-ups evaluation. FD has both an active phase, marked by erythema and pustules, and a chronic phase characterized by fibrotic areas without follicular openings [3,6]. In vivo reflectance confocal microscopy (RCM), useful in diagnosing and monitoring of skin cancer, has also shown promise for inflammatory skin and scalp diseases [7–11].
Objectives
The present study has two primary endpoints. One is to compare trichoscopy to RCM features of FD to validate the use of RCM in assessing diagnosis of FD. The other is to test the impact of RCM in the disease activity monitoring application on FD, using both trichoscopy and RCM.
Methods
A cohort of 24 Caucasian patients diagnosed with FD was recruited; scalp biopsy was performed when needed to confirm diagnosis [6,12,13]. Clinical characteristics of FD at baseline are summarized in Table 1. No patients were undergoing systemic or topical treatments for FD at baseline (T0). A subgroup of 16 patients was followed up after three months of therapy (T1) with oral antibiotics (eg, tetracyclines, clindamycin) used in monotherapy or in combination with topical corticosteroids. Local Independent Review Boards approval for the study of inflammatory skin diseases using RCM was previously obtained (Prot. CE/237/11). All the patients underwent trichoscopic and RCM examinations after providing signed informed consent. Trichoscopy images have been acquired with the use of Visiomed® integrated Viva CAM from VivaScope, Caliber ID. Confocal images have been acquired with the use of VivaScope 1500 and VivaScope 3000, CaliberID. Description of the technical details of RCM has been reported previously in literature [7–11]. Two different confocal readers (M.A. and C.F.) reviewed all the trichoscopy and RCM images analysis. The distinguishing trichoscopic features of FD were white areas lacking follicular openings (corresponding to dermal fibrosis), perifollicular white ring (white halo surrounding the hair follicle corresponding to perifollicular fibrosis), erythema and honeycomb pattern (hyperchromic grid with irregular lines and holes suggesting sun-damaged skin). The distinguishing RCM features of FD were inflammatory cell infiltrates (corresponding to bright cellular structures in the epidermis, adnexal epithelium, and upper dermis), rimmed dermal papillae (highlighting the integrity and pigmentation of the dermo-epidermal junction), and fibrosis (marked by white papillae and perifollicular and interfollicular thickened collagen bundles). Notably, white papillae, represent a distinct RCM parameter that lacks a trichoscopic equivalent. A semi-quantitative method has been utilized for evaluation of erythema and inflammatory infiltrates. Both trichoscopy (for erythema) and RCM mosaics (for inflammatory cell distribution) were evaluated. Erythema was semi-quantified using trichoscopy, scoring from 0 representing absence of erythema, 1 mild erythema, 2 moderate-to-severe erythema, 3 very severe erythema based on redness intensity (Figure 1). Each 2×2 mm RCM mosaic, comprising 4 individual RCM 1×1 mm frames, was assessed for the inflammatory infiltrate extent, based on number of inflammatory cells per frames scoring from 0 if there is no inflammatory infiltrate in each frame, 1 if inflammatory cells are <5 in one or two frames, 2 if the cells are > 5 in two frames, 3 if the infiltrate is in three or more frames (Figure 1). In both trichoscopy and RCM, tufted hair (corresponding to more than 6 hairs emerging from one ostium), follicular pustules, and dilated blood vessels have been observed. On trichoscopy, these vessels appeared as elongated loops or coiled vessels, while in RCM, they manifested as horizontally oriented canalicular structures in the upper dermis. To assess whether the RCM can detect the same microscopic characteristics of decalvans folliculitis as observed with trichoscopy, we determined the percentage of patients who displayed these features consistently at the beginning of the study (T0) and at follow-up (T1). The agreement between readers was determined using the Cohen Kappa Test.
Table 1.
Clinical Features of Folliculitis Decalvans at Baseline (T0)
| Clinical Features | |
|---|---|
| Percentage of male, N (%) | 16 (67.0%) |
| Age, median [range] | 45 [26–64] |
| Degree of disease extension, N (%) | |
| Degree 1 (<2 cm) | 6 (25%) |
| Degree 2 (2–4.99 cm) | 8 (33.3%) |
| Degree 3 (5 cm or more) | 10 (41.7%) |
| Most frequent localization, N (%) | |
| Vertex | 14 (58.3%) |
| Occipital area | 10 (41.7%) |
| Pruritus, N (%) | 6 (25.0%) |
| Trichodynia, N (%) | 16 (66.7%) |
| Associated androgenetic alopecia, N (%) | 2 (8.0%) |
Figure 1.
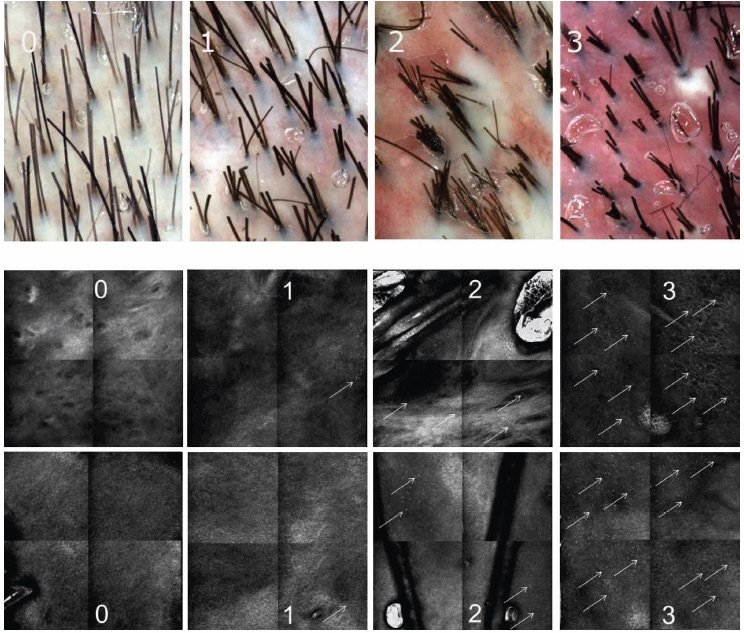
Semi-quantification of erythema evaluated by trichoscopy based on the intensity gradation of red with 0 representing absence of erythema, 1 mild erythema, 2 moderate-to-severe erythema, and 3 very severe erythema (upper section). Semi-quantification of the inflammatory infiltrate evaluated by reflectance confocal microscopy based on number of inflammatory cells (white arrows) per sector with 0 if there is no inflammatory infiltrate in each sector, 1 if inflammatory cells are <5 in 1 or 2 sectors, 2 if the cells are > 5 in 2 sectors, 3 if the infiltrate is in 3 or more sectors (lower section).
Results
Results from evaluations of RCM and trichoscopic data were considered to verify the reproducibility of newly detected confocal criteria. These criteria were then compared to trichoscopy features to define a consistent confocal pattern of FD. The fraction of patients presenting with the trichoscopic and RCM features during the baseline (T0) and follow up visit (T1) are depicted in Figures 2 and 3. Two different patients belonging to the therapeutic subgroup are shown in Figures 4 and 5 where the variation of inflammatory cells infiltrate is not associated with the same variation of erythema. A similar behavior has been observed for all patients (not shown). Note that the patient in Figure 4 shows a slight decrease in the erythema score on trichoscopy whereas on RCM it shows a pronounced decrease of inflammatory cells. Contrary, the patient in Figure 5 presents on trichoscopy a decrease of erythema score to T0 and T1, while on RCM inflammatory cells are still present as sign of disease activity. After three months of treatment (T1), both trichoscopy and RCM agreed on findings like tuft hairs, pustules, and perifollicular fibrosis. However, at T1, RCM showed a reduction at 88% vessels, while trichoscopy observed no significant changes (100%). Discrepancies between RCM and trichoscopy were also noted in features like interfollicular fibrosis/ white ring and rimmed dermal papillae/honeycomb. The reproducibility of confocal features was tested by comparing the concordance between the evaluations performed by the 2 readers (M.A. and C.F.). The concordance was excellent overall with κ values ranging from 0.79 (white papillae) to 0.94 (tufted hair) with a P value statistically significant for all the confocal features (Table 2).
Figure 2.
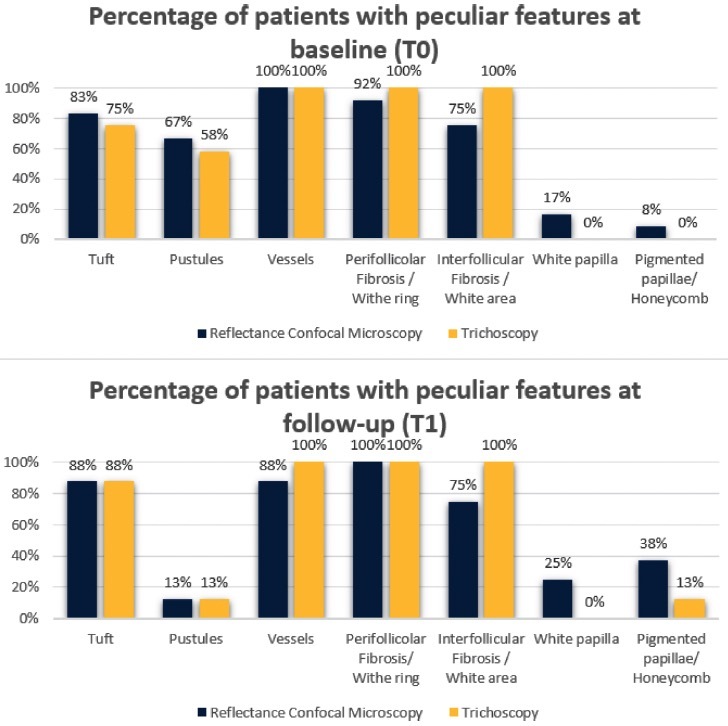
Results of reflectance confocal microscopy (RCM) features of folliculitis decalvans and the corresponding features visualized by trichoscopy at baseline (T0) and at follow-up after three months of therapy (T1). The perifollicular fibrosis seen with RCM matched the white rings visualized by trichoscopy. Similarly, the interfollicular fibrosis detected by RCM was aligned with the white areas found by trichoscopy. The pigmented papillae evident in RCM analysis corresponded to the honeycomb patterns identified by trichoscopy. However, the white papillae identified by RCM did not have a trichoscopic counterpart.
Figure 3.
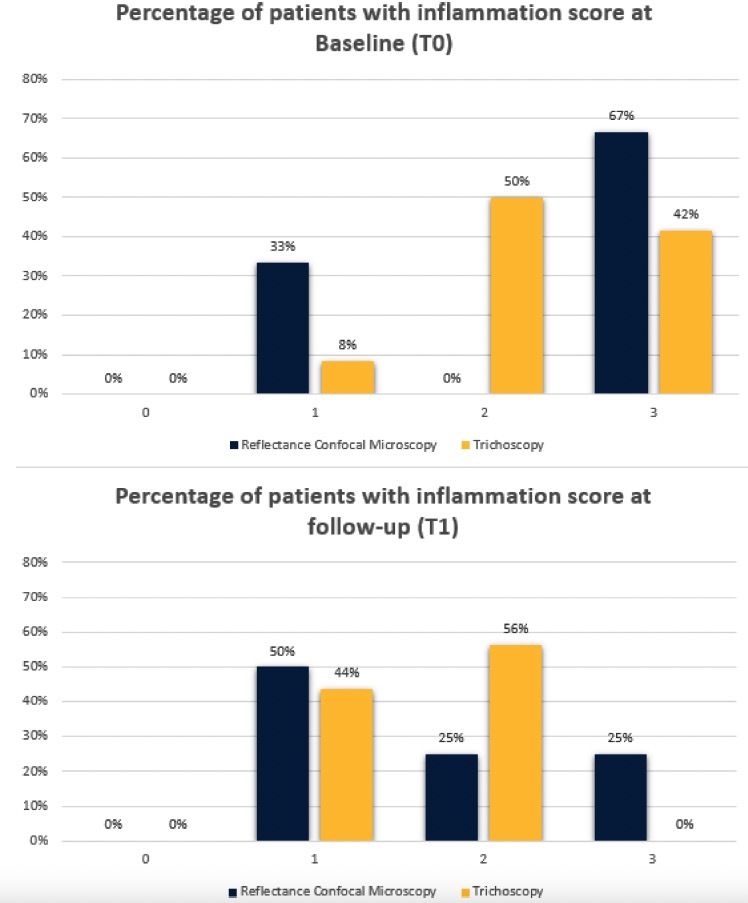
Results of erythema semi-quantification based on trichoscopy and inflammatory infiltrate semi-quantification based on reflectance confocal microscopy at baseline (T0) and at follow-up after 3 months of therapy (T1).
Figure 4.
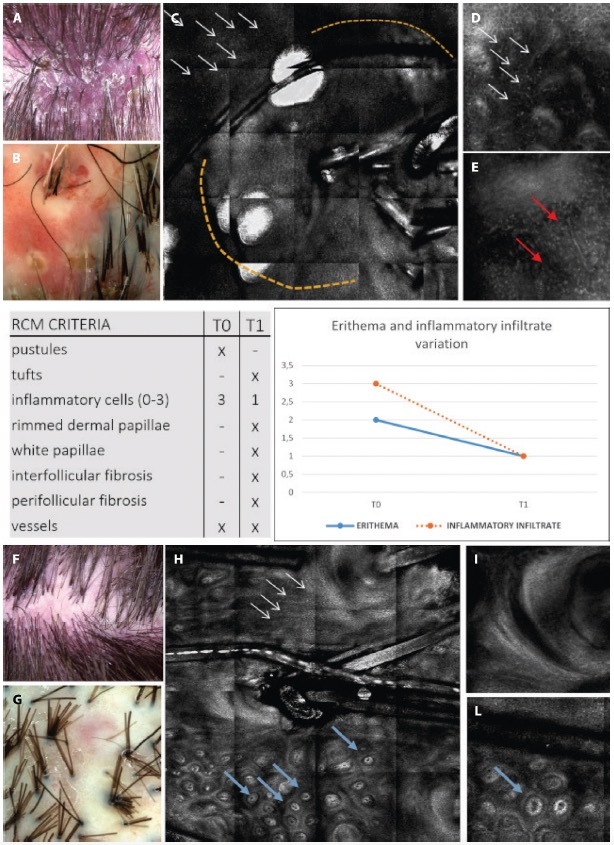
Clinical, trichoscopic and confocal aspects of patient 1 at (A–E) T0 and (F–I) after treatment at T1. (A,B) Clinical and trichoscopic appearance at baseline. (C) Reflectance confocal microscopy (RCM) shows follicular pustule (yellow dashes) and inflammatory cells infiltrate (white arrows). (D,E) Confocal magnification demonstrates the presence of numerous inflammatory cells (white arrows) and dilated superficial vessels (red arrows). (F,G) Clinical and trichoscopic aspects after treatment. (H,I) RCM shows the presence of white papillae (blue arrows) and perifollicular fibrosis (white arrows). It should be noted that the patient shows (B,G) a slight decrease in the erythema score on trichoscopy, whereas on RCM it shows (D,I) a marked decrease of inflammatory cells.
Figure 5.
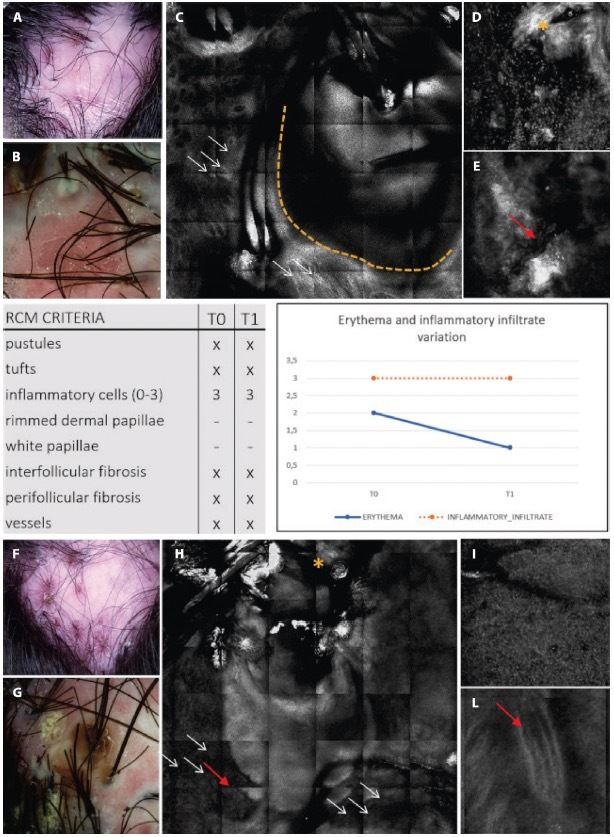
Clinical, trichoscopic and confocal aspects of patient 2 at (A–E) T0 and (F–I) after treatment at T1. (A,B) This patient presents on trichoscopy a decrease of erythema score to T0 and T1, while on reflectance confocal microscopy (RCM) inflammatory cells are still present as sign of disease activity. Clinical and trichoscopic appearance at T0. (C) Follicular pustule (yellow dashes) and perifollicular fibrosis (white arrows) seen with RCM. (D,E) RCM shows inter follicular fibrosis (yellow asterisk) and superficial dilated vessels (red arrow). (F,G) Clinical and trichoscopic features of active FD at T1. (H,I) Confocal magnification demonstrates the presence of numerous inflammatory cells (white arrows), interfollicular fibrosis (yellow asterisk), and dilated superficial vessels (red arrows).
Table 2.
Results of Cohen Kappa Test Between Readers
| Reflectance Confocal Microscopy Features | Concordance Between Readers (k) | P Value |
|---|---|---|
| Inflammatory cell infiltrate | 0.88 | <0.0001 |
| Rimmed dermal papillae | 0.90 | <0.0001 |
| Interfollicular fibrosis | 0.86 | <0.0001 |
| Perifollicular fibrosis | 0.85 | <0.0001 |
| White papillae | 0.79 | <0.0001 |
| Tufted hair | 0.94 | <0.0001 |
| Yellow follicular pustules | 0.90 | <0.0001 |
| Dilated blood vessels | 0.80 | <0.0001 |
Conclusions
Trichoscopy is considered the gold standard for non-invasive diagnosis and management of hair and scalp diseases and is widely used to support the clinical diagnosis of FD [14,15]. Trichoscopy, while simple and noninvasive, lacks the detailed microscopic insights required for monitoring FD disease activity. Previous studies have highlighted correlations between dermoscopy and RCM in skin conditions, but trichoscopy often shows inconsistency results with histology [11,16,17]. To confirm RCM effectiveness in FD management, certain trichoscopic features must be consistently observable with RCM. We found that both trichoscopy and RCM can detect features like vessels, pustules, and tufted hair equally well. Notably, erythema identified by trichoscopy corresponds to RCM detection of dilated vessels, with or without inflammatory cells [4]. Our study also identified three patterns of fibrosis: perifollicular, interfollicular, and white papillae. The latter appears as dermal papillae with an inner bright white ring which may indicate an abnormal deposition of dermal collagen bundles representing an early stage of fibrosis [18,19]. While trichoscopy struggles to provide a precise measure of inflammation, RCM allows real-time observation of inflammatory cells. Trichoscopy may overestimate disease activity, with erythema influenced by factors like topical treatments, temperature changes, injuries, and the use of items like prostheses and hats. The disparity in detecting inflammation underlines the complementary nature of trichoscopy and RCM. Specifically, RCM can reveal microscopic details, such as the precise type of fibrosis (perifollicular, interfollicular and white papillae) and can visualize each individual cell composing the inflammatory infiltrate, undetectable by trichoscopy. Our primary endpoint was successfully achieved, as the FD features identified by trichoscopy correspond to, or are associated with, those detected by RCM (Figures 2 and 3). Furthermore, a co-primary endpoint was also achieved as RCM surpasses trichoscopy in monitoring inflammatory disease activity of FD (Figures 4 and 5). Incorporating RCM in the evaluation of scalp conditions aids in both diagnosis and therapeutic monitoring, offering the possibility to adjust treatment accordingly by assessing the inflammatory infiltrate. Given FD complex management, combining trichoscopy and RCM could lead to more reliable evaluations and enable tailored therapeutic approaches. More precise information can be added with the use of other non-invasive diagnostic techniques such as line-field confocal optical coherence tomography (LC-OCT), that can visualize deeper levels of the dermis. Our experience supports the use of RCM in diagnosis and monitoring FD disease activity. However, additional studies, possibly with longitudinal designs and larger sample size, are needed to further assess the complementary role of RCM in scalp disease monitoring.
Footnotes
Funding: None.
Competing Interests: None.
Authorship: All authors have contributed significantly to this publication.
References
- 1.Brooke RC, Griffiths CE. Folliculitis decalvans. Clin Exp Dermatol. 2001;26(1):120–122. doi: 10.1046/j.1365-2230.2001.00746.x. [DOI] [PubMed] [Google Scholar]
- 2.Tan E, Martinka M, Ball N, Shapiro J. Primary cicatricial alopecias: clinicopathology of 112 cases. J Am Acad Dermatol. 2004;50(1):25–32. doi: 10.1016/j.jaad.2003.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Otberg N, Kang H, Alzolibani AA, Shapiro J. Folliculitis decalvans. Dermatol Ther. 2008;21(4):238–244. doi: 10.1111/j.1529-8019.2008.00204.x. [DOI] [PubMed] [Google Scholar]
- 4.Pindado-Ortega C, Saceda-Corralo D, Miguel-Gómez L, et al. Impact of Folliculitis Decalvans on Quality of Life and Subjective Perception of Disease. Skin Appendage Disord. 2018;4(1):34–36. doi: 10.1159/000478053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rudnicka L, Olszewska M, Rakowska A, Slowinska M. Trichoscopy update 2011. J Dermatol Case Rep. 2011;5(4):82–88. doi: 10.3315/jdcr.2011.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu WY, Otberg N, McElwee KJ, Shapiro J. Diagnosis and management of primary cicatricial alopecia: part II. Skinmed. 2008;7(2):78–83. doi: 10.1111/j.1751-7125.2008.07586.x. [DOI] [PubMed] [Google Scholar]
- 7.Ulrich M, Lange-Asschenfeldt S, Gonzalez S. Clinical applicability of in vivo reflectance confocal microscopy in dermatology. G Ital Dermatol Venereol. 2012;147(2):171–178. [PubMed] [Google Scholar]
- 8.Agozzino M, Donadio C, Franceschini C, Ardigò M. Therapeutic follow-up of Lichen Planopilaris using in vivo reflectance confocal microscopy: a case report. Skin Res Technol. 2015;21(3):380–383. doi: 10.1111/srt.12203. [DOI] [PubMed] [Google Scholar]
- 9.Agozzino M, Tosti A, Barbieri L, et al. Confocal microscopic features of scarring alopecia: preliminary report. Br J Dermatol. 2011;165(3):534–540. doi: 10.1111/j.1365-2133.2011.10424.x. [DOI] [PubMed] [Google Scholar]
- 10.Ardigò M, El Shabrawi-Caelen L, Tosti A. In vivo reflectance confocal microscopy assessment of the therapeutic follow-up of cutaneous T-cell lymphomas causing scalp alopecia. Dermatol Ther. 2014;27(4):248–251. doi: 10.1111/dth.12129. [DOI] [PubMed] [Google Scholar]
- 11.Ardigò M, Tosti A, Cameli N, Vincenzi C, Misciali C, Berardesca E. Reflectance confocal microscopy of the yellow dot pattern in alopecia areata. Arch Dermatol. 2011;147(1):61–64. doi: 10.1001/archdermatol.2010.288. [DOI] [PubMed] [Google Scholar]
- 12.Vañó-Galván S, Molina-Ruiz AM, Fernández-Crehuet P, et al. Folliculitis decalvans: a multicentre review of 82 patients. J Eur Acad Dermatol Venereol. 2015;29(9):1750–1757. doi: 10.1111/jdv.12993. [DOI] [PubMed] [Google Scholar]
- 13.Fernández-Crehuet P, Vañó-Galván S, Molina-Ruiz AM, et al. Trichoscopic Features of Folliculitis Decalvans: Results in 58 Patients. Int J Trichology. 2017;9(3):140–141. doi: 10.4103/ijt.ijt_85_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kowalska-Oledzka E, Slowinska M, Rakowska A. Sensitivity and specificity of the trichoscopy. Indian J Dermatol Venereol Leprol. 2012;78(5):636–637. doi: 10.4103/0378-6323.100591. [DOI] [PubMed] [Google Scholar]
- 15.Ardigò M, Torres F, Abraham LS, et al. Reflectance confocal microscopy can differentiate dermoscopic white dots of the scalp between sweat gland ducts or follicular infundibulum. Br J Dermatol. 2011;164(5):1122–1124. doi: 10.1111/j.1365-2133.2011.10242.x. [DOI] [PubMed] [Google Scholar]
- 16.Ardigò M, Torres F, Abraham LS, et al. Reflectance confocal microscopy can differentiate dermoscopic white dots of the scalp between sweat gland ducts or follicular infundibulum. Br J Dermatol. 2011;164(5):1122–1124. doi: 10.1111/j.1365-2133.2011.10242.x. [DOI] [PubMed] [Google Scholar]
- 17.Peccerillo F, Mandel VD, Greco M, Ciardo S, Pellacani G. A headstrong case of folliculitis decalvans: Treatment options and evaluation with dermoscopy, reflectance confocal microscopy and optical coherence tomography. Dermatol Ther. 2020;33(6):e14049. doi: 10.1111/dth.14049. [DOI] [PubMed] [Google Scholar]
- 18.Al-Chaer RN, Bouazzi D, Jemec G, Mogensen M. Confocal microscopy and optical coherence tomography of inflammatory skin diseases in hairs and pilosebaceous units: A systematic review. Exp Dermatol. 2023;32(7):945–954. doi: 10.1111/exd.14830. [DOI] [PubMed] [Google Scholar]


