Abstract
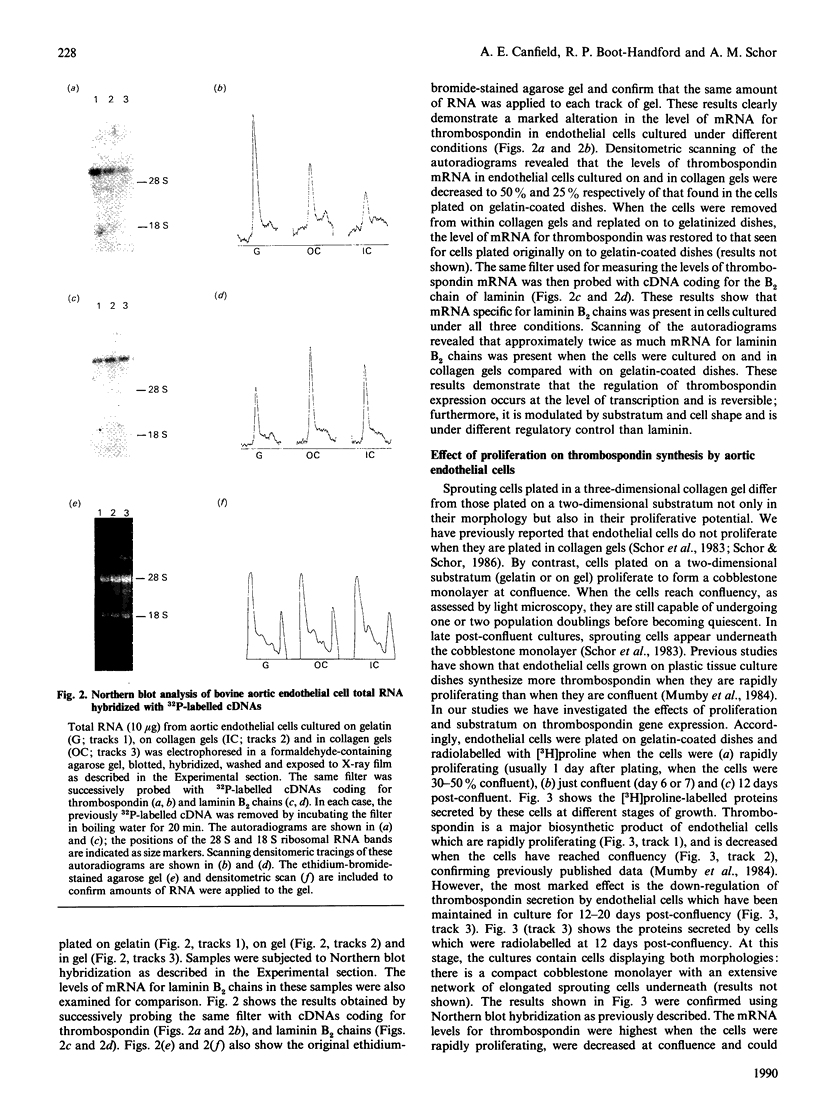
Endothelial cells plated on the surface of a two-dimensional substratum (gelatin-coated dishes, dishes coated with native type I collagen or collagen gels) form a cobblestone monolayer at confluence, whereas cells plated within a three-dimensional gel matrix elongate into a sprouting morphology and self-associate into tube-like structures. In this study, we have compared the synthesis of thrombospondin by quiescent endothelial cells displaying (a) the same morphological phenotype (cobblestone) on different substrata (gelatin and collagen) and (b) different morphological phenotypes (cobblestone and sprouting) on the same substratum (collagen). We demonstrate that thrombospondin is a major biosynthetic product of confluent, quiescent cells cultured on dishes coated with either gelatin or collagen, and that the synthesis of this protein is markedly decreased when cells are plated on or in three-dimensional collagen gels. Moreover, we demonstrate that cells plated in gel (sprouting) secrete less thrombospondin than do cells plated on the gel surface (cobblestone). The regulation of thrombospondin synthesis is reversible and occurs at the level of transcription, as steady-state mRNA levels for thrombospondin decrease in a manner comparable with the levels of protein secreted by these cells. We also show that mRNA levels for laminin B2 chains are increased when cells are cultured on and in collagen gels compared with on gelatin-coated dishes, suggesting that the syntheses of thrombospondin and laminin are regulated by different mechanisms. When cells are cultured on gelatin- or collagen-coated dishes, thrombospondin gene expression is directly proportional to the proliferative state of the cultures. By contrast, the synthesis of thrombospondin by cells cultured on collagen gels remains at equally low levels whether they are labelled when they are sparse and rapidly proliferating or when they are confluent and quiescent. Fibronectin synthesis was found to increase with increasing confluency of the cells plated on all three substrata. These results demonstrate that thrombospondin gene expression is modulated by cell shape, cell proliferation and the nature of the substratum used for cell culture.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baenziger N. L., Brodie G. N., Majerus P. W. A thrombin-sensitive protein of human platelet membranes. Proc Natl Acad Sci U S A. 1971 Jan;68(1):240–243. doi: 10.1073/pnas.68.1.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baenziger N. L., Brodie G. N., Majerus P. W. Isolation and properties of a thrombin-sensitive protein of human platelets. J Biol Chem. 1972 May 10;247(9):2723–2731. [PubMed] [Google Scholar]
- Barlow D. P., Green N. M., Kurkinen M., Hogan B. L. Sequencing of laminin B chain cDNAs reveals C-terminal regions of coiled-coil alpha-helix. EMBO J. 1984 Oct;3(10):2355–2362. doi: 10.1002/j.1460-2075.1984.tb02140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Canfield A. E., Schor A. M., Loskutoff D. J., Schor S. L., Grant M. E. Plasminogen activator inhibitor-type I is a major biosynthetic product of retinal microvascular endothelial cells and pericytes in culture. Biochem J. 1989 Apr 15;259(2):529–535. doi: 10.1042/bj2590529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canfield A. E., Schor A. M., Schor S. L., Grant M. E. The biosynthesis of extracellular-matrix components by bovine retinal endothelial cells displaying distinctive morphological phenotypes. Biochem J. 1986 Apr 15;235(2):375–383. doi: 10.1042/bj2350375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canfield A. E., Schor A. M., West D. C., Schor S. L., Grant M. E. Identification and partial characterization of two major proteins of Mr 47,000 synthesized by bovine retinal endothelial cells in culture. Biochem J. 1987 Aug 15;246(1):121–129. doi: 10.1042/bj2460121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Clezardin P., Malaval L., Ehrensperger A. S., Delmas P. D., Dechavanne M., McGregor J. L. Complex formation of human thrombospondin with osteonectin. Eur J Biochem. 1988 Aug 1;175(2):275–284. doi: 10.1111/j.1432-1033.1988.tb14194.x. [DOI] [PubMed] [Google Scholar]
- Dawes J., Pratt D. A., Dewar M. S., Preston F. E. Do extra-platelet sources contribute to the plasma level of thrombospondin? Thromb Haemost. 1988 Apr 8;59(2):273–276. [PubMed] [Google Scholar]
- Dixit V. M., Hennessy S. W., Grant G. A., Rotwein P., Frazier W. A. Characterization of a cDNA encoding the heparin and collagen binding domains of human thrombospondin. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5449–5453. doi: 10.1073/pnas.83.15.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyfus M., Lahav J. The build-up of the thrombospondin extracellular matrix. An apparent dependence on synthesis and on preformed fibrillar matrix. Eur J Cell Biol. 1988 Dec;47(2):275–282. [PubMed] [Google Scholar]
- Frazier W. A. Thrombospondin: a modular adhesive glycoprotein of platelets and nucleated cells. J Cell Biol. 1987 Aug;105(2):625–632. doi: 10.1083/jcb.105.2.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter N. R., Dawes J., MacGregor I. R., Pepper D. S. Quantitation by radioimmunoassay of thrombospondin synthesised and secreted by human endothelial cells. Thromb Haemost. 1984 Dec 29;52(3):288–291. [PubMed] [Google Scholar]
- Ketis N. V., Lawler J., Hoover R. L., Karnovsky M. J. Effects of heat shock on the expression of thrombospondin by endothelial cells in culture. J Cell Biol. 1988 Mar;106(3):893–904. doi: 10.1083/jcb.106.3.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lawler J., Hynes R. O. The structure of human thrombospondin, an adhesive glycoprotein with multiple calcium-binding sites and homologies with several different proteins. J Cell Biol. 1986 Nov;103(5):1635–1648. doi: 10.1083/jcb.103.5.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler J. The structural and functional properties of thrombospondin. Blood. 1986 May;67(5):1197–1209. [PubMed] [Google Scholar]
- Lawler J., Weinstein R., Hynes R. O. Cell attachment to thrombospondin: the role of ARG-GLY-ASP, calcium, and integrin receptors. J Cell Biol. 1988 Dec;107(6 Pt 1):2351–2361. doi: 10.1083/jcb.107.6.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung L. L. Role of thrombospondin in platelet aggregation. J Clin Invest. 1984 Nov;74(5):1764–1772. doi: 10.1172/JCI111595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majack R. A., Cook S. C., Bornstein P. Control of smooth muscle cell growth by components of the extracellular matrix: autocrine role for thrombospondin. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9050–9054. doi: 10.1073/pnas.83.23.9050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majack R. A., Cook S. C., Bornstein P. Platelet-derived growth factor and heparin-like glycosaminoglycans regulate thrombospondin synthesis and deposition in the matrix by smooth muscle cells. J Cell Biol. 1985 Sep;101(3):1059–1070. doi: 10.1083/jcb.101.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majack R. A., Goodman L. V., Dixit V. M. Cell surface thrombospondin is functionally essential for vascular smooth muscle cell proliferation. J Cell Biol. 1988 Feb;106(2):415–422. doi: 10.1083/jcb.106.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson J., Sage H., Bornstein P. Isolation and characterization of a glycoprotein secreted by aortic endothelial cells in culture. Apparent identity with platelet thrombospondin. J Biol Chem. 1981 Nov 10;256(21):11330–11336. [PubMed] [Google Scholar]
- Miller R. R., McDevitt C. A. Thrombospondin is present in articular cartilage and is synthesized by articular chondrocytes. Biochem Biophys Res Commun. 1988 Jun 16;153(2):708–714. doi: 10.1016/s0006-291x(88)81152-5. [DOI] [PubMed] [Google Scholar]
- Mosher D. F., Doyle M. J., Jaffe E. A. Synthesis and secretion of thrombospondin by cultured human endothelial cells. J Cell Biol. 1982 May;93(2):343–348. doi: 10.1083/jcb.93.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumby S. M., Abbott-Brown D., Raugi G. J., Bornstein P. Regulation of thrombospondin secretion by cells in culture. J Cell Physiol. 1984 Sep;120(3):280–288. doi: 10.1002/jcp.1041200304. [DOI] [PubMed] [Google Scholar]
- Roberts D. D., Sherwood J. A., Ginsburg V. Platelet thrombospondin mediates attachment and spreading of human melanoma cells. J Cell Biol. 1987 Jan;104(1):131–139. doi: 10.1083/jcb.104.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robey P. G., Young M. F., Fisher L. W., McClain T. D. Thrombospondin is an osteoblast-derived component of mineralized extracellular matrix. J Cell Biol. 1989 Feb;108(2):719–727. doi: 10.1083/jcb.108.2.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schor A. M., Schor S. L., Allen T. D. Effects of culture conditions on the proliferation, morphology and migration of bovine aortic endothelial cells. J Cell Sci. 1983 Jul;62:267–285. doi: 10.1242/jcs.62.1.267. [DOI] [PubMed] [Google Scholar]
- Schor A. M., Schor S. L., Allen T. D. The synthesis of subendothelial matrix by bovine aortic endothelial cells in culture. Tissue Cell. 1984;16(5):677–691. doi: 10.1016/0040-8166(84)90002-8. [DOI] [PubMed] [Google Scholar]
- Schor A. M., Schor S. L. Inhibition of endothelial cell morphogenetic interactions in vitro by alpha- and beta-xylosides. In Vitro Cell Dev Biol. 1988 Jul;24(7):659–668. doi: 10.1007/BF02623603. [DOI] [PubMed] [Google Scholar]
- Schor A. M., Schor S. L. The isolation and culture of endothelial cells and pericytes from the bovine retinal microvasculature: a comparative study with large vessel vascular cells. Microvasc Res. 1986 Jul;32(1):21–38. doi: 10.1016/0026-2862(86)90041-5. [DOI] [PubMed] [Google Scholar]
- Schor S. L. Cell proliferation and migration on collagen substrata in vitro. J Cell Sci. 1980 Feb;41:159–175. doi: 10.1242/jcs.41.1.159. [DOI] [PubMed] [Google Scholar]
- Tuszynski G. P., Gasic T. B., Rothman V. L., Knudsen K. A., Gasic G. J. Thrombospondin, a potentiator of tumor cell metastasis. Cancer Res. 1987 Aug 1;47(15):4130–4133. [PubMed] [Google Scholar]
- Tuszynski G. P., Rothman V. L., Murphy A., Siegler K., Knudsen K. A. Thrombospondin promotes platelet aggregation. Blood. 1988 Jul;72(1):109–115. [PubMed] [Google Scholar]
- Tuszynski G. P., Rothman V., Murphy A., Siegler K., Smith L., Smith S., Karczewski J., Knudsen K. A. Thrombospondin promotes cell-substratum adhesion. Science. 1987 Jun 19;236(4808):1570–1573. doi: 10.1126/science.2438772. [DOI] [PubMed] [Google Scholar]
- Wight T. N., Raugi G. J., Mumby S. M., Bornstein P. Light microscopic immunolocation of thrombospondin in human tissues. J Histochem Cytochem. 1985 Apr;33(4):295–302. doi: 10.1177/33.4.3884704. [DOI] [PubMed] [Google Scholar]