Full text
PDF
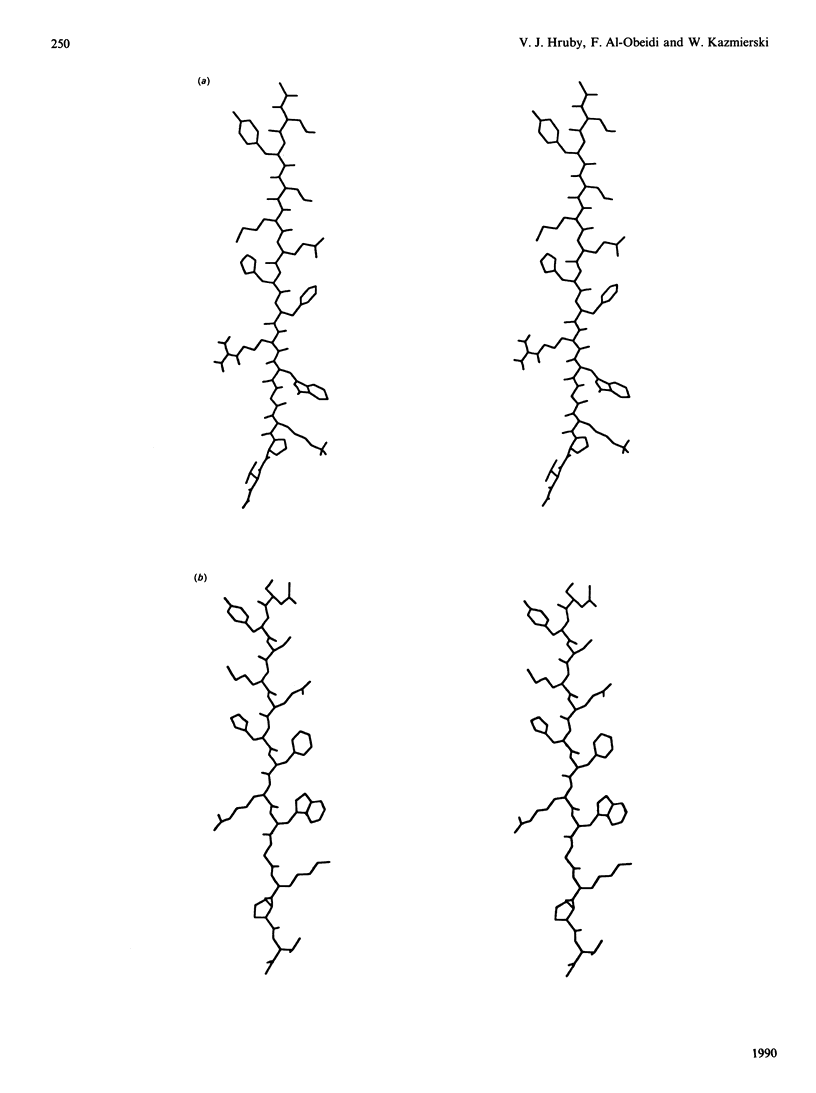
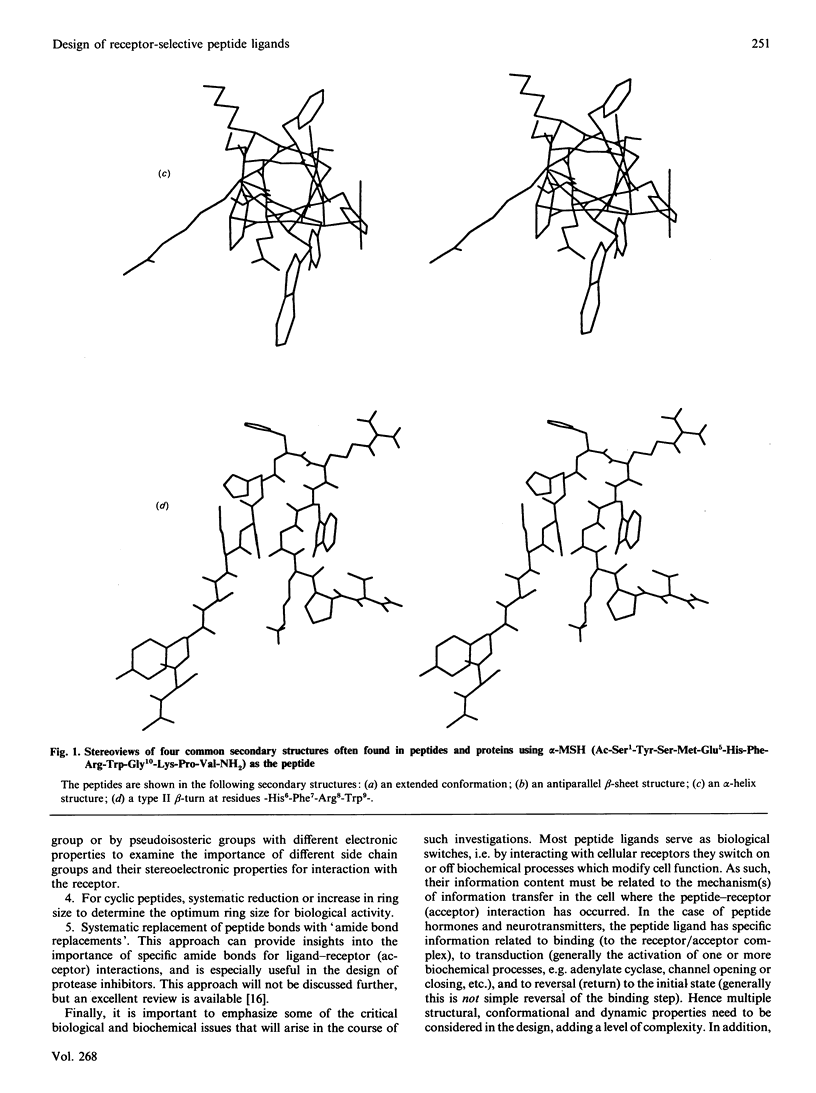





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebi J. D., Guillaume D., Dunlap B. E., Rich D. H. Synthesis, conformation, and immunosuppressive activity of a conformationally restricted cyclosporine lactam analogue. J Med Chem. 1988 Sep;31(9):1805–1815. doi: 10.1021/jm00117a022. [DOI] [PubMed] [Google Scholar]
- Al-Obeidi F., Castrucci A. M., Hadley M. E., Hruby V. J. Potent and prolonged acting cyclic lactam analogues of alpha-melanotropin: design based on molecular dynamics. J Med Chem. 1989 Dec;32(12):2555–2561. doi: 10.1021/jm00132a010. [DOI] [PubMed] [Google Scholar]
- Al-Obeidi F., Hruby V. J., Castrucci A. M., Hadley M. E. Design of potent linear alpha-melanotropin 4-10 analogues modified in positions 5 and 10. J Med Chem. 1989 Jan;32(1):174–179. doi: 10.1021/jm00121a032. [DOI] [PubMed] [Google Scholar]
- Bankowski K., Manning M., Haldar J., Sawyer W. H. Design of potent antagonists of the vasopressor response to arginine-vasopressin. J Med Chem. 1978 Sep;21(9):850–853. doi: 10.1021/jm00207a002. [DOI] [PubMed] [Google Scholar]
- Bryan W. M., Callahan J. F., Codd E. E., Lemieux C., Moore M. L., Schiller P. W., Walker R. F., Huffman W. F. Cyclic enkephalin analogues containing alpha-amino-beta-mercapto-beta,beta-pentamethylenepropionic acid at positions 2 or 5. J Med Chem. 1989 Feb;32(2):302–304. doi: 10.1021/jm00122a005. [DOI] [PubMed] [Google Scholar]
- Burley S. K., Petsko G. A. Aromatic-aromatic interaction: a mechanism of protein structure stabilization. Science. 1985 Jul 5;229(4708):23–28. doi: 10.1126/science.3892686. [DOI] [PubMed] [Google Scholar]
- Charpentier B., Durieux C., Menant I., Roques B. P. Investigation of peripheral cholecystokinin receptor heterogeneity by cyclic and related linear analogues of CCK26-33: synthesis and biological properties. J Med Chem. 1987 Jun;30(6):962–968. doi: 10.1021/jm00389a002. [DOI] [PubMed] [Google Scholar]
- Coy D. H., Horvath A., Nekola M. V., Coy E. J., Erchegyi J., Schally A. V. Peptide antagonists of LH-RH: large increases in antiovulatory activities produced by basic D-amino acids in the six position. Endocrinology. 1982 Apr;110(4):1445–1447. doi: 10.1210/endo-110-4-1445. [DOI] [PubMed] [Google Scholar]
- Darman P. S., Landis G. C., Smits J. R., Hirning L. D., Gulya K., Yamamura H. I., Burks T. F., Hruby V. J. Conformationally restricted cyclic analogues of substance P: insight into the receptor binding process. Biochem Biophys Res Commun. 1985 Mar 15;127(2):656–662. doi: 10.1016/s0006-291x(85)80211-4. [DOI] [PubMed] [Google Scholar]
- Degrado W. F. Design of peptides and proteins. Adv Protein Chem. 1988;39:51–124. doi: 10.1016/s0065-3233(08)60375-7. [DOI] [PubMed] [Google Scholar]
- Dorow D. S., Shi P. T., Carbone F. R., Minasian E., Todd P. E., Leach S. J. Two large immunogenic and antigenic myoglobin peptides and the effects of cyclisation. Mol Immunol. 1985 Nov;22(11):1255–1264. doi: 10.1016/0161-5890(85)90044-6. [DOI] [PubMed] [Google Scholar]
- Dyckes D. F., Nestor J. J., Jr, Ferger M. F., Du Vigneaud V. (1-Beta-mercapto-beta,beta-diethylpropionic acid)-8-lysine-vasopressin, a potent inhibitor of 8-lysine-vasopressin and of oxytocin. J Med Chem. 1974 Feb;17(2):250–252. doi: 10.1021/jm00248a028. [DOI] [PubMed] [Google Scholar]
- Felix A. M., Wang C. T., Liebman A. A., Delaney C. M., Mowles T., Burghardt B. A., Charnecki A. M., Meienhofer J. Synthesis, biological activity, and tritiation of L-3, 4-dehydroproline-containing peptides. Int J Pept Protein Res. 1977 Oct;10(4):299–310. doi: 10.1111/j.1399-3011.1977.tb02801.x. [DOI] [PubMed] [Google Scholar]
- Filatova M. P., Krit N. A., Komarova O. M., Orekhovich V. N., Reissmann S. Sintez i issledovanie konformatsionno ogranichennykh analogov peptidnykh ingibitorov angiotenzinprevrashchaiushchego fermenta. Bioorg Khim. 1986 Jan;12(1):59–70. [PubMed] [Google Scholar]
- Fisher G. H., Berryer P., Ryan J. W., Chauhan V., Stammer C. H. Dehydrophenylalanyl analogs of bradykinin: synthesis and biological activities. Arch Biochem Biophys. 1981 Oct 1;211(1):269–275. doi: 10.1016/0003-9861(81)90454-9. [DOI] [PubMed] [Google Scholar]
- Hayashi K., Nunami K., Kato J., Yoneda N., Kubo M., Ochiai T., Ishida R. Studies on angiotensin converting enzyme inhibitors. 4. Synthesis and angiotensin converting enzyme inhibitory activities of 3-acyl-1-alkyl-2-oxoimidazolidine-4-carboxylic acid derivatives. J Med Chem. 1989 Feb;32(2):289–297. doi: 10.1021/jm00122a003. [DOI] [PubMed] [Google Scholar]
- Hruby V. J. Conformational restrictions of biologically active peptides via amino acid side chain groups. Life Sci. 1982 Jul 19;31(3):189–199. doi: 10.1016/0024-3205(82)90578-1. [DOI] [PubMed] [Google Scholar]
- Hsieh K. H., LaHann T. R., Speth R. C. Topographic probes of angiotensin and receptor: potent angiotensin II agonist containing diphenylalanine and long-acting antagonists containing biphenylalanine and 2-indan amino acid in position 8. J Med Chem. 1989 Apr;32(4):898–903. doi: 10.1021/jm00124a028. [DOI] [PubMed] [Google Scholar]
- Kaiser E. T., Kézdy F. J. Amphiphilic secondary structure: design of peptide hormones. Science. 1984 Jan 20;223(4633):249–255. doi: 10.1126/science.6322295. [DOI] [PubMed] [Google Scholar]
- Kazmierski W., Wire W. S., Lui G. K., Knapp R. J., Shook J. E., Burks T. F., Yamamura H. I., Hruby V. J. Design and synthesis of somatostatin analogues with topographical properties that lead to highly potent and specific mu opioid receptor antagonists with greatly reduced binding at somatostatin receptors. J Med Chem. 1988 Nov;31(11):2170–2177. doi: 10.1021/jm00119a019. [DOI] [PubMed] [Google Scholar]
- Krstenansky J. L., Owen T. J., Yates M. T., Mao S. J. Anticoagulant peptides: nature of the interaction of the C-terminal region of hirudin with a noncatalytic binding site on thrombin. J Med Chem. 1987 Sep;30(9):1688–1691. doi: 10.1021/jm00392a030. [DOI] [PubMed] [Google Scholar]
- Krstenansky J. L., Zechel C., Trivedi D., Hruby V. J. Importance of the C-terminal alpha-helical structure for glucagon's biological activity. Int J Pept Protein Res. 1988 Dec;32(6):468–475. doi: 10.1111/j.1399-3011.1988.tb01377.x. [DOI] [PubMed] [Google Scholar]
- Kruszynski M., Lammek B., Manning M., Seto J., Haldar J., Sawyer W. H. [1-beta-Mercapto-beta,beta-cyclopentamethylenepropionic acid),2-(O-methyl)tyrosine ]argine-vasopressin and [1-beta-mercapto-beta,beta-cyclopentamethylenepropionic acid)]argine-vasopressine, two highly potent antagonists of the vasopressor response to arginine-vasopressin. J Med Chem. 1980 Apr;23(4):364–368. doi: 10.1021/jm00178a003. [DOI] [PubMed] [Google Scholar]
- Leszczynski J. F., Rose G. D. Loops in globular proteins: a novel category of secondary structure. Science. 1986 Nov 14;234(4778):849–855. doi: 10.1126/science.3775366. [DOI] [PubMed] [Google Scholar]
- Manning M., Lammek B., Kruszynski M., Seto J., Sawyer W. H. Design of potent and selective antagonists of the vasopressor responses to arginine-vasopressin. J Med Chem. 1982 Apr;25(4):408–414. doi: 10.1021/jm00346a015. [DOI] [PubMed] [Google Scholar]
- Meienhofer J., Trzeciak A., Havran R. T., Walter R. A solid-phase synthesis of (8-arginine)-vasopressin through a crystalline protected nonapeptide intermediate and biological properties of the hormone. J Am Chem Soc. 1970 Dec 2;92(24):7199–7202. doi: 10.1021/ja00727a031. [DOI] [PubMed] [Google Scholar]
- Meraldi J. P., Hruby V. J., Brewster A. I. Relative conformational rigidity in oxytocin and (1-penicillamine)-oxytocin: a proposal for the relationship of conformational flexibility to peptide hormone agonism and antagonism. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1373–1377. doi: 10.1073/pnas.74.4.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosberg H. I., Hurst R., Hruby V. J., Galligan J. J., Burks T. F., Gee K., Yamamura H. I. [D-Pen2, L-Cys5]enkephalinamide and[D-Pen2, D-Cys5] enkephalinamide, conformationally constrained cyclic enkephalinamide analogs with delta receptor specificity. Biochem Biophys Res Commun. 1982 May 31;106(2):506–512. doi: 10.1016/0006-291x(82)91139-1. [DOI] [PubMed] [Google Scholar]
- Mosberg H. I., Hurst R., Hruby V. J., Gee K., Yamamura H. I., Galligan J. J., Burks T. F. Bis-penicillamine enkephalins possess highly improved specificity toward delta opioid receptors. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5871–5874. doi: 10.1073/pnas.80.19.5871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musso G. F., Assoian R. K., Kaiser E. T., Kézdy F. J., Tager H. S. Heterogeneity of glucagon receptors of rat hepatocytes: a synthetic peptide probe for the high affinity site. Biochem Biophys Res Commun. 1984 Mar 15;119(2):713–719. doi: 10.1016/s0006-291x(84)80309-5. [DOI] [PubMed] [Google Scholar]
- Peggion E., Foffani M. T., Wünsch E., Moroder L., Goodman M., Mammi S. Interaction of calcium ions with gastrin fragments of increasing chain length. Biopolymers. 1986 Jan;25(1):135–152. doi: 10.1002/bip.360250110. [DOI] [PubMed] [Google Scholar]
- Pelton J. T., Gulya K., Hruby V. J., Duckles S. P., Yamamura H. I. Conformationally restricted analogs of somatostatin with high mu-opiate receptor specificity. Proc Natl Acad Sci U S A. 1985 Jan;82(1):236–239. doi: 10.1073/pnas.82.1.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelton J. T., Kazmierski W., Gulya K., Yamamura H. I., Hruby V. J. Design and synthesis of conformationally constrained somatostatin analogues with high potency and specificity for mu opioid receptors. J Med Chem. 1986 Nov;29(11):2370–2375. doi: 10.1021/jm00161a037. [DOI] [PubMed] [Google Scholar]
- Rose G. D., Gierasch L. M., Smith J. A. Turns in peptides and proteins. Adv Protein Chem. 1985;37:1–109. doi: 10.1016/s0065-3233(08)60063-7. [DOI] [PubMed] [Google Scholar]
- Sawyer T. K., Hruby V. J., Darman P. S., Hadley M. E. [half-Cys4,half-Cys10]-alpha-Melanocyte-stimulating hormone: a cyclic alpha-melanotropin exhibiting superagonist biological activity. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1751–1755. doi: 10.1073/pnas.79.6.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer T. K., Sanfilippo P. J., Hruby V. J., Engel M. H., Heward C. B., Burnett J. B., Hadley M. E. 4-Norleucine, 7-D-phenylalanine-alpha-melanocyte-stimulating hormone: a highly potent alpha-melanotropin with ultralong biological activity. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5754–5758. doi: 10.1073/pnas.77.10.5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer W. H., Pang P. K., Seto J., McEnroe M., Lammek B., Manning M. Vasopressin analogs that antagonize antidiuretic responses by rats to the antidiuretic hormone. Science. 1981 Apr 3;212(4490):49–51. doi: 10.1126/science.7209515. [DOI] [PubMed] [Google Scholar]
- Schiller P. W., Nguyen T. M., Maziak L., Lemieux C. A novel cyclic opioid peptide analog showing high preference for mu-receptors. Biochem Biophys Res Commun. 1985 Mar 15;127(2):558–564. doi: 10.1016/s0006-291x(85)80196-0. [DOI] [PubMed] [Google Scholar]
- Schwyzer R. ACTH: a short introductory review. Ann N Y Acad Sci. 1977 Oct 28;297:3–26. doi: 10.1111/j.1749-6632.1977.tb41843.x. [DOI] [PubMed] [Google Scholar]
- Shimohigashi Y., Stammer C. H. Dehydro-enkephalins. VI. Dehydroalanine3-enkephalin: a potent enkephalin analog for the delta opiate receptor. Int J Pept Protein Res. 1982 Sep;20(3):199–206. [PubMed] [Google Scholar]
- Shoemaker K. R., Kim P. S., Brems D. N., Marqusee S., York E. J., Chaiken I. M., Stewart J. M., Baldwin R. L. Nature of the charged-group effect on the stability of the C-peptide helix. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2349–2353. doi: 10.1073/pnas.82.8.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker K. R., Kim P. S., York E. J., Stewart J. M., Baldwin R. L. Tests of the helix dipole model for stabilization of alpha-helices. Nature. 1987 Apr 9;326(6113):563–567. doi: 10.1038/326563a0. [DOI] [PubMed] [Google Scholar]
- Spear K. L., Reinhard E. J., McMahon E. G., Olins G. M., Palomo M. A., Patton D. R. Conformationally restricted analogues of atriopeptin(103-125)amide. J Med Chem. 1989 Jan;32(1):67–72. doi: 10.1021/jm00121a014. [DOI] [PubMed] [Google Scholar]
- Struthers R. S., Rivier J., Hagler A. T. Molecular dynamics and minimum energy conformations of GnRH and analogs. A methodology for computer-aided drug design. Ann N Y Acad Sci. 1985;439:81–96. doi: 10.1111/j.1749-6632.1985.tb25790.x. [DOI] [PubMed] [Google Scholar]
- Taylor J. W., Kaiser E. T. Opioid receptor selectivity of peptide models of beta-endorphin. Int J Pept Protein Res. 1989 Jul;34(1):75–80. [PubMed] [Google Scholar]
- Thaisrivongs S., Pals D. T., Turner S. R., Kroll L. T. Conformationally constrained renin inhibitory peptides: gamma-lactam-bridged dipeptide isostere as conformational restriction. J Med Chem. 1988 Jul;31(7):1369–1376. doi: 10.1021/jm00402a021. [DOI] [PubMed] [Google Scholar]
- Theodoropoulos D., Poulos C., Gatos D., Cordopatis P., Escher E., Mizrahi J., Regoli D., Dalietos D., Furst A., Lee T. D. Conformationally restricted C-terminal peptides of substance P. Synthesis, mass spectral analysis and pharmacological properties. J Med Chem. 1985 Oct;28(10):1536–1539. doi: 10.1021/jm00148a029. [DOI] [PubMed] [Google Scholar]
- Thornber C. W., Shaw J. S., Miller L., Hayward C. F., Morley J. S., Timms D., Wilkinson A. New delta-receptor antagonists. NIDA Res Monogr. 1986;75:177–180. [PubMed] [Google Scholar]
- Toniolo C. Conformationally restricted peptides through short-range cyclizations. Int J Pept Protein Res. 1990 Apr;35(4):287–300. doi: 10.1111/j.1399-3011.1990.tb00052.x. [DOI] [PubMed] [Google Scholar]
- Torchiana M. L., Cook P. G., Wiese S. R., Saperstein R., Veber D. F. Subcutaneous administration of somatostatin analogs as a major factor in the enhancement of the duration of action. Arch Int Pharmacodyn Ther. 1978 Sep;235(1):170–176. [PubMed] [Google Scholar]
- Vander Elst P., van den Berg E., Pepermans H., vander Auwera L., Zeeuws R., Tourwe D., van Binst G. Synthesis and conformational study of a cyclic hexapeptide analogue of somatostatin: cyclo(Phe-D-Trp-Lys-Thr-o-AMPA). Int J Pept Protein Res. 1987 Mar;29(3):318–330. doi: 10.1111/j.1399-3011.1987.tb02259.x. [DOI] [PubMed] [Google Scholar]
- Varughese K. I., Srinivasan A. R., Stammer C. H. Conformational preferences of cyclopropyl peptides. Crystal structure of (E)-DL-1-benzamido-1-methoxycarbonyl-2-chlorcyclopropane (BCP). Int J Pept Protein Res. 1985 Sep;26(3):242–251. [PubMed] [Google Scholar]
- Veber D. F., Freidlinger R. M., Perlow D. S., Paleveda W. J., Jr, Holly F. W., Strachan R. G., Nutt R. F., Arison B. H., Homnick C., Randall W. C. A potent cyclic hexapeptide analogue of somatostatin. Nature. 1981 Jul 2;292(5818):55–58. doi: 10.1038/292055a0. [DOI] [PubMed] [Google Scholar]
- Zajac J. M., Gacel G., Petit F., Dodey P., Rossignol P., Roques B. P. Deltakephalin, Tyr-D-Thr-Gly-Phe-Leu-Thr: a new highly potent and fully specific agonist for opiate delta-receptors. Biochem Biophys Res Commun. 1983 Mar 16;111(2):390–397. doi: 10.1016/0006-291x(83)90318-2. [DOI] [PubMed] [Google Scholar]


