Abstract
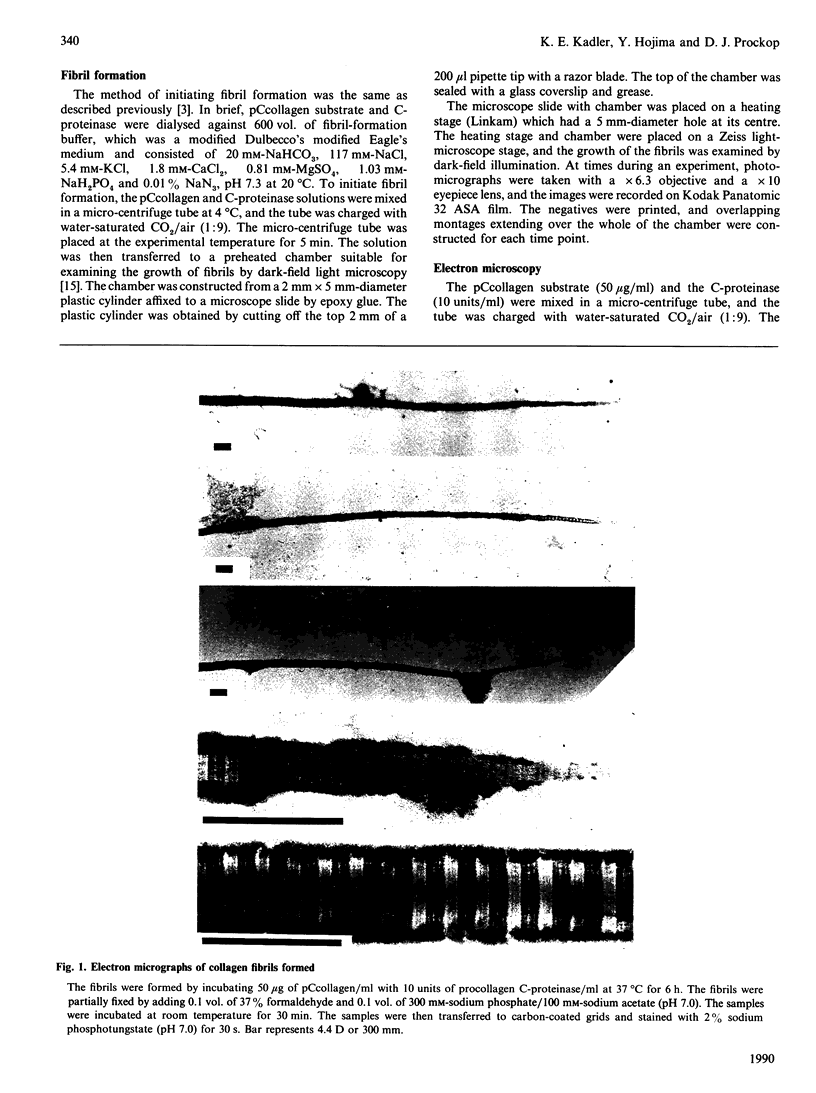
Growth of collagen fibrils was examined in a system in which collagen monomers are generated by specific enzymic cleavage of type IpCcollagen with procollagen C-proteinase. Fibrils formed at 37 degrees C had highly tapered and symmetrical pointed tips. The pattern of cross-striations in the pointed tips indicated that all the molecules were oriented so that the N-termini were directed towards the tip. At 29 degrees C and 32 degrees C, the fibrils formed were thicker. One end of fibrils formed at 29 degrees C was blunt, and the other was pointed. Growth of the fibrils was exclusively from pointed tips. Occasionally a spear-like projection appeared at a blunted end. The spear-like projection then became a new pointed tip for growth in the opposite direction. The results suggested a model for fibril growth with at least three distinct binding sites for monomers. In the model, the pointed tip is the site with the highest affinity for the binding of monomers and most probably defines the critical concentration for fibril assembly. The main shaft of the fibril is a site with very low affinity for binding. The blunted end defines a low-affinity binding site where monomers can bind in opposite orientation to produce growth from a new pointed end.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg R. A. Determination of 3- and 4-hydroxyproline. Methods Enzymol. 1982;82(Pt A):372–398. doi: 10.1016/0076-6879(82)82074-0. [DOI] [PubMed] [Google Scholar]
- Birk D. E., Zycband E. I., Winkelmann D. A., Trelstad R. L. Collagen fibrillogenesis in situ: fibril segments are intermediates in matrix assembly. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4549–4553. doi: 10.1073/pnas.86.12.4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comper W. D., Veis A. The mechanism of nucleation for in vitro collagen fibril formation. Biopolymers. 1977 Oct;16(10):2113–2131. doi: 10.1002/bip.1977.360161004. [DOI] [PubMed] [Google Scholar]
- Cooper A. Thermodynamic studies of the assembly in vitro of native collagen fibrils. Biochem J. 1970 Jul;118(3):355–365. doi: 10.1042/bj1180355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler-Nagy C., Bruckner P., Hayashi T., Prockop D. J. Isolation of unhydroxylated type I procollagen folding of the protein in vitro. Arch Biochem Biophys. 1981 Dec;212(2):668–677. doi: 10.1016/0003-9861(81)90411-2. [DOI] [PubMed] [Google Scholar]
- GROSS J., KIRK D. The heat precipitation of collagen from neutral salt solutions: some rate-regulating factors. J Biol Chem. 1958 Aug;233(2):355–360. [PubMed] [Google Scholar]
- Gelman R. A., Williams B. R., Piez K. A. Collagen fibril formation. Evidence for a multistep process. J Biol Chem. 1979 Jan 10;254(1):180–186. [PubMed] [Google Scholar]
- Hojima Y., McKenzie J. A., van der Rest M., Prockop D. J. Type I procollagen N-proteinase from chick embryo tendons. Purification of a new 500-kDa form of the enzyme and identification of the catalytically active polypeptides. J Biol Chem. 1989 Jul 5;264(19):11336–11345. [PubMed] [Google Scholar]
- Hojima Y., van der Rest M., Prockop D. J. Type I procollagen carboxyl-terminal proteinase from chick embryo tendons. Purification and characterization. J Biol Chem. 1985 Dec 15;260(29):15996–16003. [PubMed] [Google Scholar]
- Holmes D. F., Chapman J. A. Axial mass distributions of collagen fibrils grown in vitro: results for the end regions of early fibrils. Biochem Biophys Res Commun. 1979 Apr 27;87(4):993–999. doi: 10.1016/s0006-291x(79)80005-4. [DOI] [PubMed] [Google Scholar]
- Hulmes D. J., Holmes D. F., Cummings C. Crystalline regions in collagen fibrils. J Mol Biol. 1985 Aug 5;184(3):473–477. doi: 10.1016/0022-2836(85)90295-5. [DOI] [PubMed] [Google Scholar]
- Hulmes D. J., Miller A. Quasi-hexagonal molecular packing in collagen fibrils. Nature. 1979 Dec 20;282(5741):878–880. doi: 10.1038/282878a0. [DOI] [PubMed] [Google Scholar]
- Kadler K. E., Hojima Y., Prockop D. J. Assembly of collagen fibrils de novo by cleavage of the type I pC-collagen with procollagen C-proteinase. Assay of critical concentration demonstrates that collagen self-assembly is a classical example of an entropy-driven process. J Biol Chem. 1987 Nov 15;262(32):15696–15701. [PubMed] [Google Scholar]
- Kadler K. E., Hojima Y., Prockop D. J. Assembly of type I collagen fibrils de novo. Between 37 and 41 degrees C the process is limited by micro-unfolding of monomers. J Biol Chem. 1988 Jul 25;263(21):10517–10523. [PubMed] [Google Scholar]
- Kivirikko K. I., Laitinen O., Prockop D. J. Modifications of a specific assay for hydroxyproline in urine. Anal Biochem. 1967 May;19(2):249–255. doi: 10.1016/0003-2697(67)90160-1. [DOI] [PubMed] [Google Scholar]
- Peltonen L., Palotie A., Prockop D. J. A defect in the structure of type I procollagen in a patient who had osteogenesis imperfecta: excess mannose in the COOH-terminal propeptide. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6179–6183. doi: 10.1073/pnas.77.10.6179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver F. H., Langley K. H., Trelstad R. L. Type I collagen fibrillogenesis: initiation via reversible linear and lateral growth steps. Biopolymers. 1979 Oct;18(10):2523–2535. doi: 10.1002/bip.1979.360181011. [DOI] [PubMed] [Google Scholar]