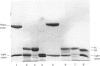
Abstract
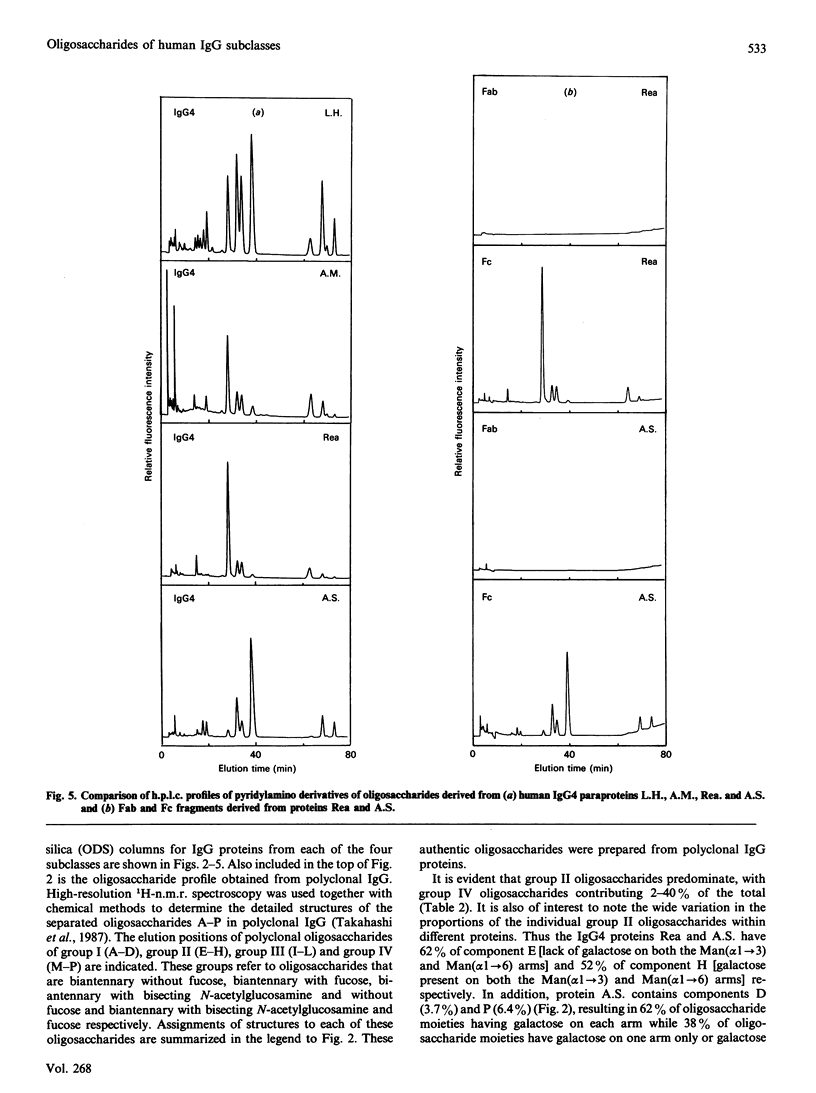
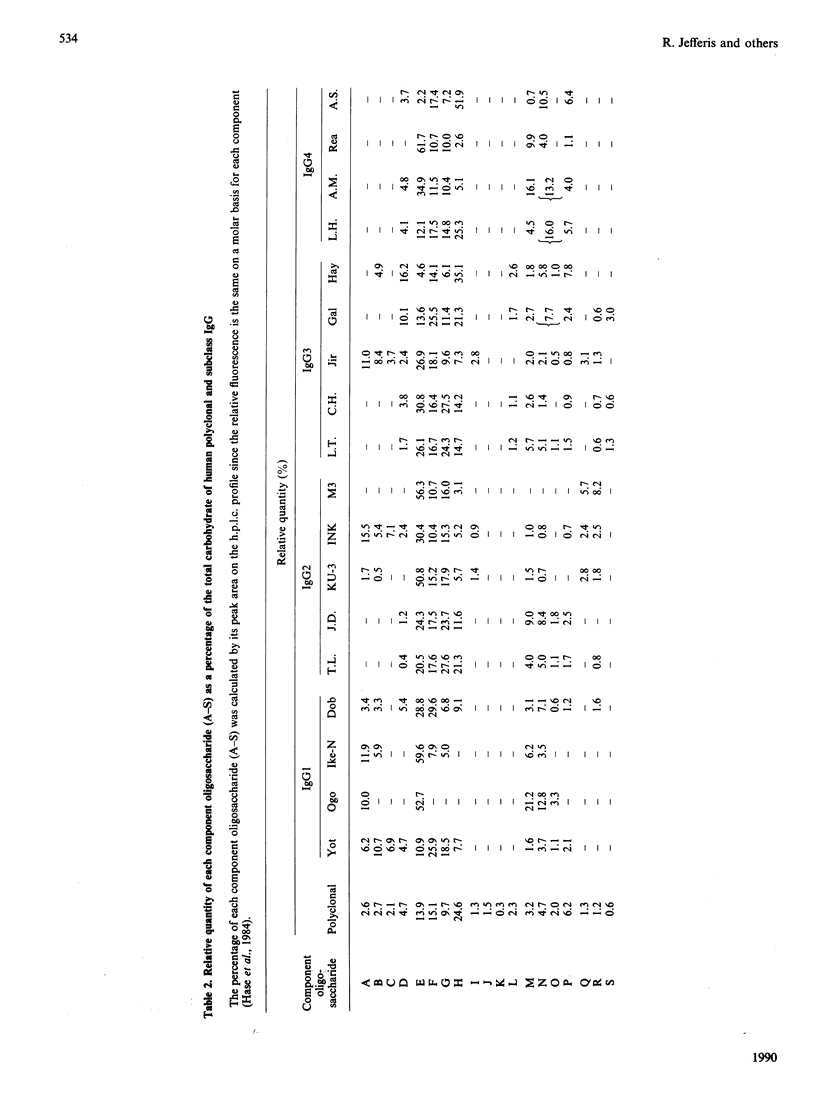
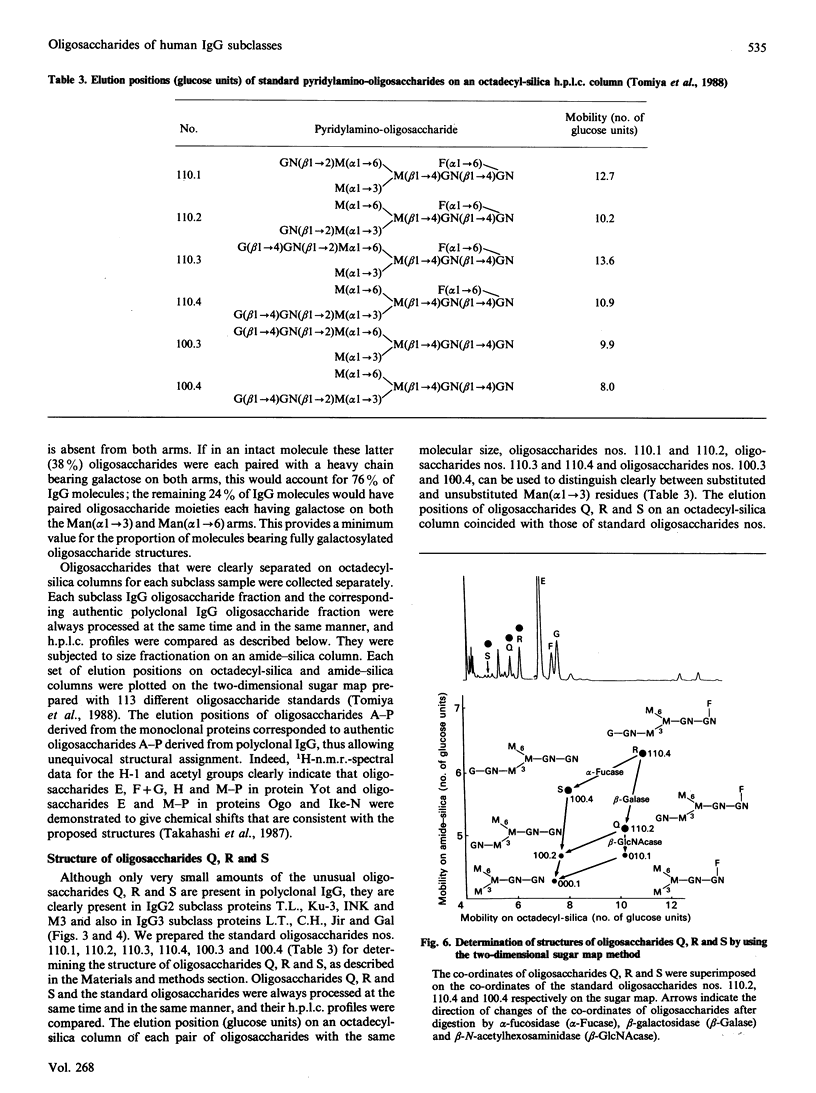
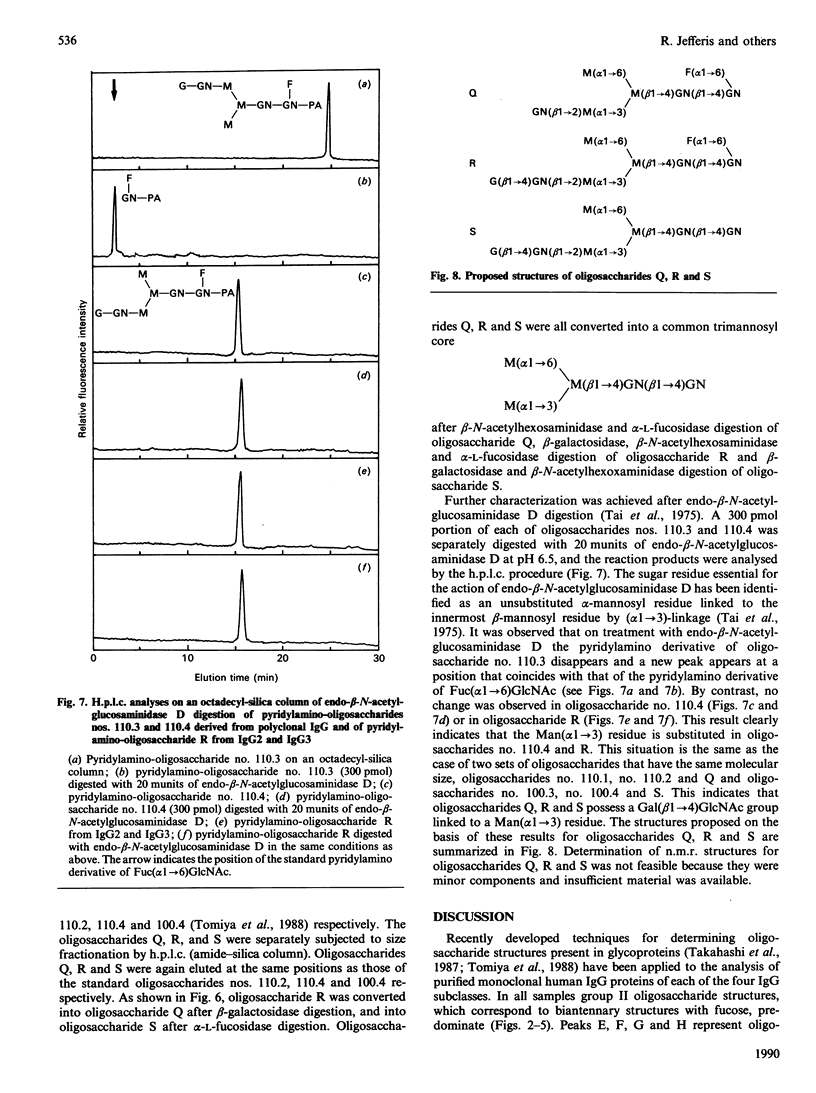
Quantitative oligosaccharide profiles were determined for each of 18 human IgG paraproteins representing the four subclasses. Each paraprotein exhibits a unique profile that may be substantially different from that observed for polyclonal IgG. The IgG2 and some IgG3 proteins analysed exhibit a predominance of oligosaccharide moieties having galactose on the Man(alpha 1----3) arm rather than the Man(alpha 1----6) arm; it was previously held that galactosylation of the Man(alpha 1----6) arm is preferred, as observed for IgG1, IgG4 and polyclonal IgG. An IgG4 protein is reported that has galactosylated Man(alpha 1----3) and Man(alpha 1----6) arms on both Fc-localized carbohydrate moieties; previous findings suggested that such fully glycosylated structures could not be accommodated within the internal space of the C gamma 2 domains. Unusual monoantennary oligosaccharides present in IgG2 and IgG3 proteins were isolated and their structures determined.
Full text
PDF
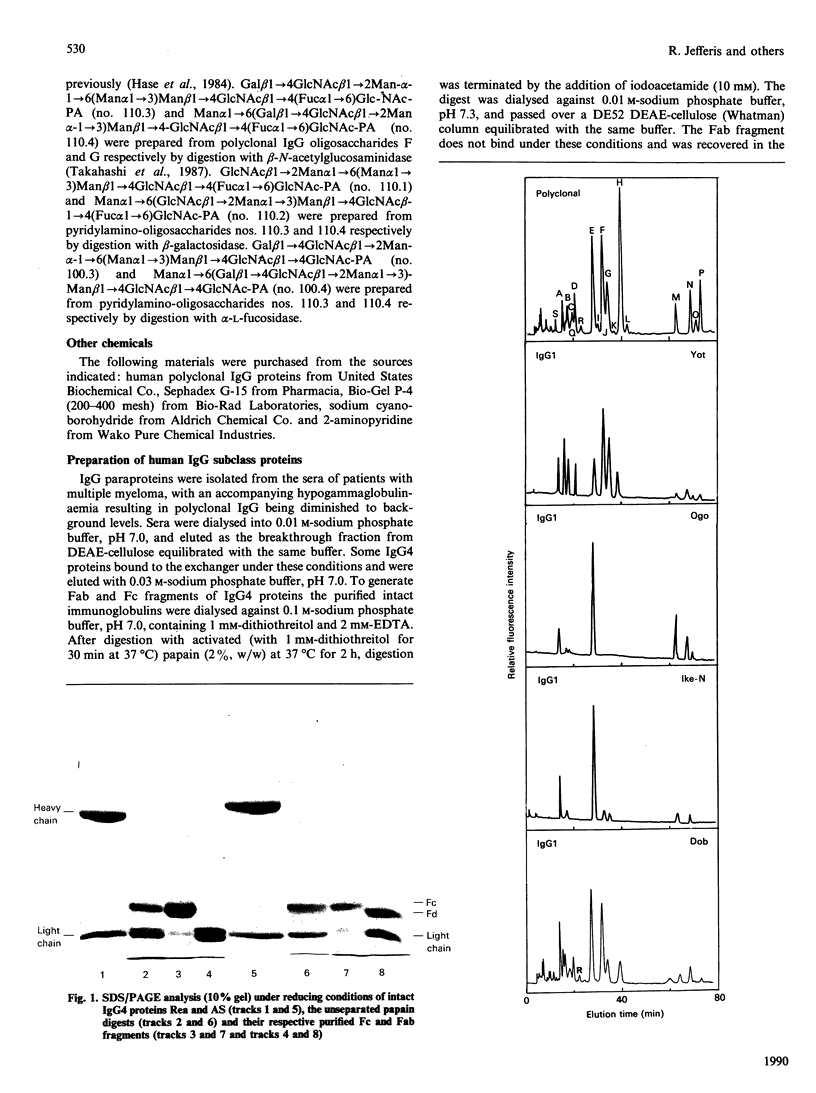
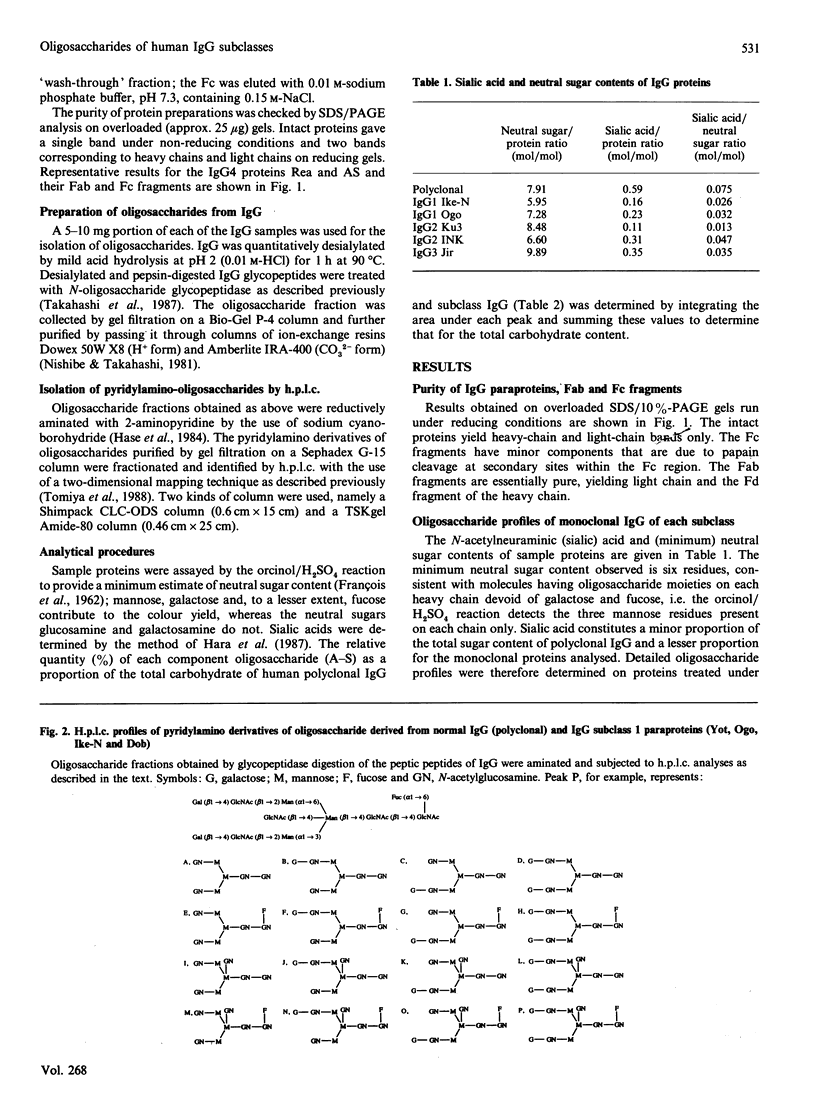
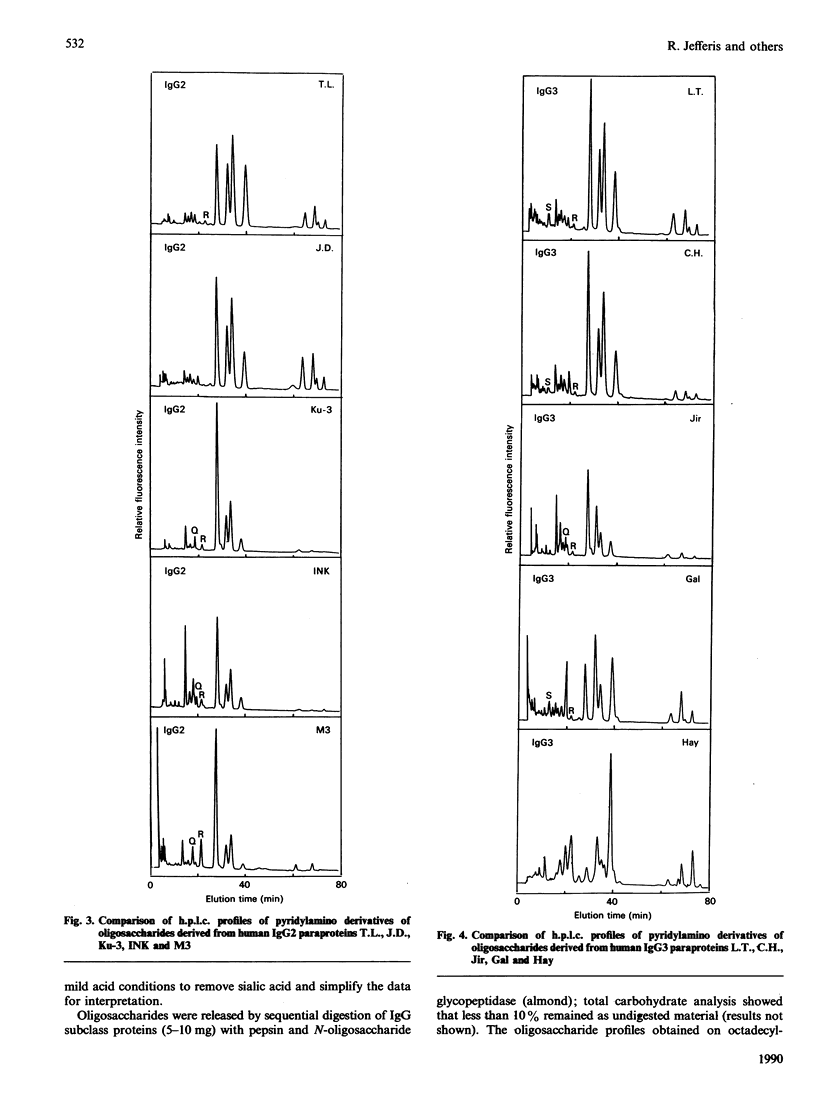

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axford J. S., Mackenzie L., Lydyard P. M., Hay F. C., Isenberg D. A., Roitt I. M. Reduced B-cell galactosyltransferase activity in rheumatoid arthritis. Lancet. 1987 Dec 26;2(8574):1486–1488. doi: 10.1016/s0140-6736(87)92621-3. [DOI] [PubMed] [Google Scholar]
- Deisenhofer J. Crystallographic refinement and atomic models of a human Fc fragment and its complex with fragment B of protein A from Staphylococcus aureus at 2.9- and 2.8-A resolution. Biochemistry. 1981 Apr 28;20(9):2361–2370. [PubMed] [Google Scholar]
- FRANCOIS C., MARSHALL R. D., NEUBERGER A. Carbohydrates in protein. 4. The determination of mannose in hen's-egg albumin by radioisotope dilution. Biochem J. 1962 May;83:335–341. doi: 10.1042/bj0830335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara S., Takemori Y., Yamaguchi M., Nakamura M., Ohkura Y. Fluorometric high-performance liquid chromatography of N-acetyl- and N-glycolylneuraminic acids and its application to their microdetermination in human and animal sera, glycoproteins, and glycolipids. Anal Biochem. 1987 Jul;164(1):138–145. doi: 10.1016/0003-2697(87)90377-0. [DOI] [PubMed] [Google Scholar]
- Hase S., Ibuki T., Ikenaka T. Reexamination of the pyridylamination used for fluorescence labeling of oligosaccharides and its application to glycoproteins. J Biochem. 1984 Jan;95(1):197–203. doi: 10.1093/oxfordjournals.jbchem.a134585. [DOI] [PubMed] [Google Scholar]
- Leatherbarrow R. J., Dwek R. A. The effect of aglycosylation on the binding of mouse IgG to staphylococcal protein A. FEBS Lett. 1983 Dec 12;164(2):227–230. doi: 10.1016/0014-5793(83)80290-7. [DOI] [PubMed] [Google Scholar]
- Leatherbarrow R. J., Rademacher T. W., Dwek R. A., Woof J. M., Clark A., Burton D. R., Richardson N., Feinstein A. Effector functions of a monoclonal aglycosylated mouse IgG2a: binding and activation of complement component C1 and interaction with human monocyte Fc receptor. Mol Immunol. 1985 Apr;22(4):407–415. doi: 10.1016/0161-5890(85)90125-7. [DOI] [PubMed] [Google Scholar]
- Mizuochi T., Taniguchi T., Shimizu A., Kobata A. Structural and numerical variations of the carbohydrate moiety of immunoglobulin G. J Immunol. 1982 Nov;129(5):2016–2020. [PubMed] [Google Scholar]
- Nishibe H., Takahashi N. The release of carbohydrate moieties from human fibrinogen by almond glycopeptidase without alteration in fibrinogen clottability. Biochim Biophys Acta. 1981 Oct 13;661(2):274–279. doi: 10.1016/0005-2744(81)90015-2. [DOI] [PubMed] [Google Scholar]
- Nose M., Wigzell H. Biological significance of carbohydrate chains on monoclonal antibodies. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6632–6636. doi: 10.1073/pnas.80.21.6632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh R. B., Dwek R. A., Sutton B. J., Fernandes D. L., Leung A., Stanworth D., Rademacher T. W., Mizuochi T., Taniguchi T., Matsuta K. Association of rheumatoid arthritis and primary osteoarthritis with changes in the glycosylation pattern of total serum IgG. Nature. 1985 Aug 1;316(6027):452–457. doi: 10.1038/316452a0. [DOI] [PubMed] [Google Scholar]
- Sutton B. J., Phillips D. C. The three-dimensional structure of the carbohydrate within the Fc fragment of immunoglobulin G. Biochem Soc Trans. 1983 Apr;11(2):130–132. [PubMed] [Google Scholar]
- Tai T., Yamashita K., Ogata-Arakawa M., Koide N., Muramatsu T., Iwashita S., Inoue Y., Kobata A. Structural studies of two ovalbumin glycopeptides in relation to the endo-beta-N-acetylglucosaminidase specificity. J Biol Chem. 1975 Nov 10;250(21):8569–8575. [PubMed] [Google Scholar]
- Takahashi N., Ishii I., Ishihara H., Mori M., Tejima S., Jefferis R., Endo S., Arata Y. Comparative structural study of the N-linked oligosaccharides of human normal and pathological immunoglobulin G. Biochemistry. 1987 Feb 24;26(4):1137–1144. doi: 10.1021/bi00378a023. [DOI] [PubMed] [Google Scholar]
- Tomana M., Schrohenloher R. E., Koopman W. J., Alarcón G. S., Paul W. A. Abnormal glycosylation of serum IgG from patients with chronic inflammatory diseases. Arthritis Rheum. 1988 Mar;31(3):333–338. doi: 10.1002/art.1780310304. [DOI] [PubMed] [Google Scholar]
- Tomiya N., Awaya J., Kurono M., Endo S., Arata Y., Takahashi N. Analyses of N-linked oligosaccharides using a two-dimensional mapping technique. Anal Biochem. 1988 May 15;171(1):73–90. doi: 10.1016/0003-2697(88)90126-1. [DOI] [PubMed] [Google Scholar]
- Walker M. R., Lund J., Thompson K. M., Jefferis R. Aglycosylation of human IgG1 and IgG3 monoclonal antibodies can eliminate recognition by human cells expressing Fc gamma RI and/or Fc gamma RII receptors. Biochem J. 1989 Apr 15;259(2):347–353. doi: 10.1042/bj2590347. [DOI] [PMC free article] [PubMed] [Google Scholar]