Introduction
Spitz tumors are a distinct subtype of melanocytic lesions that represent a challenging entity due to their clinical, dermoscopic, and histopathologic features that may overlap with melanoma, making accurate diagnosis and management difficult. They also share a rapidly evolving behavior, characterized by an abrupt presentation and fast-growing phase followed by stabilization in the benign counterpart. The current classification of Spitz tumors includes benign Spitz nevi, atypical Spitz tumor (AST) or melanocytoma as a lesion of intermediate malignant potential, STUMP/MELTUMP, and Spitz melanoma [1].
Transepidermal elimination (TEE) is a phenomenon in which altered structures of the dermis and foreign material induce an inflammatory response causing the release of collagenases, elastases, and proteases. This inflammatory response generates alteration of the matrix with necrosis, pseudoepitheliomatous hyperplasia of the epidermis, and formation of transepidermal perforating canals with elimination of the dermal material [2].
TEE is thought to be rarely observed in Spitz nevus [3] and has been described in other entities: acquired perforating dermatosis, pseudoxanthoma elasticum, cutaneous metastasis, malignant melanoma, and eccrine poroma, among others [4–6].
Case Presentation
A 35-year-old man presented with an asymptomatic rapidly evolving lesion on his right thigh, which he had noticed three weeks earlier. On physical examination, we observed a well-defined pink tumor measuring 6 mm. Dermoscopy revealed a cobblestone pattern with comedo-like openings, along with regularly distributed dotted and coiled vessels both at the periphery and within the polygonal central areas; a central yellow-to-brown structureless area with a coiled vessel was identified (Figure 1A).
Figure 1.
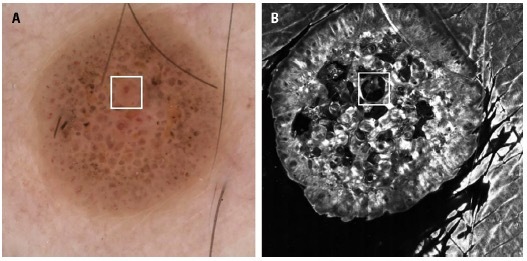
(A) Dermoscopy revealed a cobblestone pattern with comedo-like openings and some dotted and coiled vessels regularly distributed in the periphery and within the polygonal central areas. A central yellow-to-brown structureless area with a coiled vessel was observed. (B) Reflectance confocal microscopy of the same focal finding revealed a large round hyporeflective area simulating an epidermal invagination, with some cells and amorphous material inside.
Reflectance confocal microscopy (RCM) using the Vivascope 1500 (Vivascope GmbH, Munich, Germany) revealed a regular honeycomb pattern at the level of the stratum spinosum, accompanied by epidermal dark invaginations. Isolated dendritic and roundish cells and dense nests were identified at different levels of the epidermal layer; some of the nests were located within the dark spaces. At the center of the lesion, RCM revealed a large round hyporeflective structure simulating an epidermal invagination with some nevus cells and amorphous material inside (Figure 1B). At the dermoepidermal junction (DEJ) level, edge papilla and non-atypical cells were seen.
Histopathological examination revealed a symmetrical, sharply demarcated lesion with epidermal hyperplasia and rete ridges enclosing melanocytic nests. Additionally, clefts surrounding melanocytic nests exhibited a pagetoid distribution. TEE of nevus cell nests through the upper epidermis was identified (Figure 2A). Hyalin bodies were seen at the DEJ, and a lichenoid lymphocytic infiltrate was also present. After molecular ancillary techniques, the final diagnosis was compatible with AST. Only complete excision was required.
Figure 2.
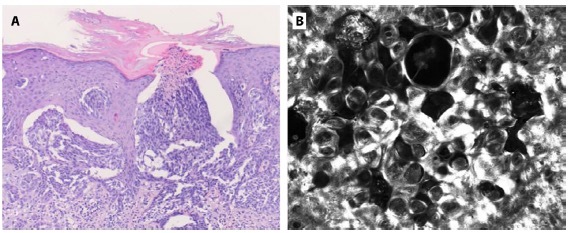
(A) H&E staining. Histopathology showed epidermal hyperplasia and rete ridges clutching some melanocytic nests. Transepidermal elimination of whole nests of nevocytes in the upper epidermis was observed. (B) Reflectance confocal microscopy identified multiple dense nests within dark spaces at different levels of the epidermal layer.
Conclusion
We observed a yellow-to-brown area in dermoscopy that correlated with the hyporeflective structure found in RCM with an amorphous material inside. This structure was identified in the histopathological study proving the TEE of cell nests through the epidermis. In addition, the dense nests within dark spaces at different levels of the epidermis observed with RCM correlate with the clefts around melanocytic nests in a pagetoid distribution observed in histopathology. In-vivo, real-time RCM examination offers the unique opportunity to understand and prove some phenomena present in these rapidly evolving cutaneous tumors.
Footnotes
Funding: The research at the Melanoma Unit in Hospital Clinic of Barcelona is partially financed by grants PI15/00716, PI15/00956, PI18/0959 and PI18/00419, from Fondo de Investigaciones Sanitarias (Spain), by the CIBERER of the Instituto de Salud Carlos III, Spain, co-funded by ISCIII-Subdireccion General de Evaluacion and European Regional Development Fund (ERDF), “a way to make Europe”; AGAUR 2017_SGR_1134 and CERCA Programme by Generalitat de Catalunya, Spain; European Commission under the 6th Framework Programme, Contract No. LSHC-CT-2006-018702 (GenoMEL), by the European Commission under the 7th Framework Programme, Diagnoptics, and the European Commision under the HORIZON2020 Framework Programme, iTobos, Qualitop, MELCAYA; The National Cancer Institute (NCI) of the US National Institute of Health (NIH) (CA83115); a grant from “Fundació La Marató de TV3” 201331-30, Catalonia, Spain; and a grant from “Fundación Científica de la Asociación Española Contra el Cáncer” GCB15152978SOEN, Spain.
Role of the Sponsors: The sponsors had no role in the design and conduct of the study; in the collection, analysis, and interpretation of data; or in the preparation, review, or approval of the manuscript.
Competing Interests: None.
Authorship: All authors have contributed significantly to this publication.
References
- 1.Cheng TW, Ahern MC, Giubellino A. The Spectrum of Spitz Melanocytic Lesions: From Morphologic Diagnosis to Molecular Classification. Front Oncol. 2022;12:889223. doi: 10.3389/fonc.2022.889223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shah H, Tiwary A, Kumar P. Transepidermal elimination: Historical evolution, pathogenesis and nosology. Indian J Dermatol Venereol Leprol. 2018;84(6):753. doi: 10.4103/ijdvl.IJDVL_396_17. [DOI] [PubMed] [Google Scholar]
- 3.Kobayashi H, Oishi K, Miyake M, et al. Spitz nevus on the sole of a foot presenting with transepidermal elimination. Dermatol Pract Concept. doi: 10.5826/dpc.0402a08. Published online April 30, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luna A, Merino ME, Alberdi CG, Abba M, Segal-Eiras A, Croce M. MUC1 positive cutaneous metastasis with transepidermal elimination from a breast carcinoma. IMCRJ. doi: 10.2147/IMCRJ.S52614. Published online November 2013:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abbas O, Salem Z, Haddad F, Kibbi AG. Perforating cutaneous metastasis from an ovarian adenocarcinoma. Journal of Cutaneous Pathology. 2010;37(9):e53–e56. doi: 10.1111/j.1600-0560.2009.01357.x. [DOI] [PubMed] [Google Scholar]
- 6.Arai K, Fujita H, Suzuki M, Iwasaki K. A Case of Eccrine Poroma with Multiple Transepidermal Elimination. The Journal of Dermatology. 1997;24(8):539–542. doi: 10.1111/j.1346-8138.1997.tb02836.x. [DOI] [PubMed] [Google Scholar]


