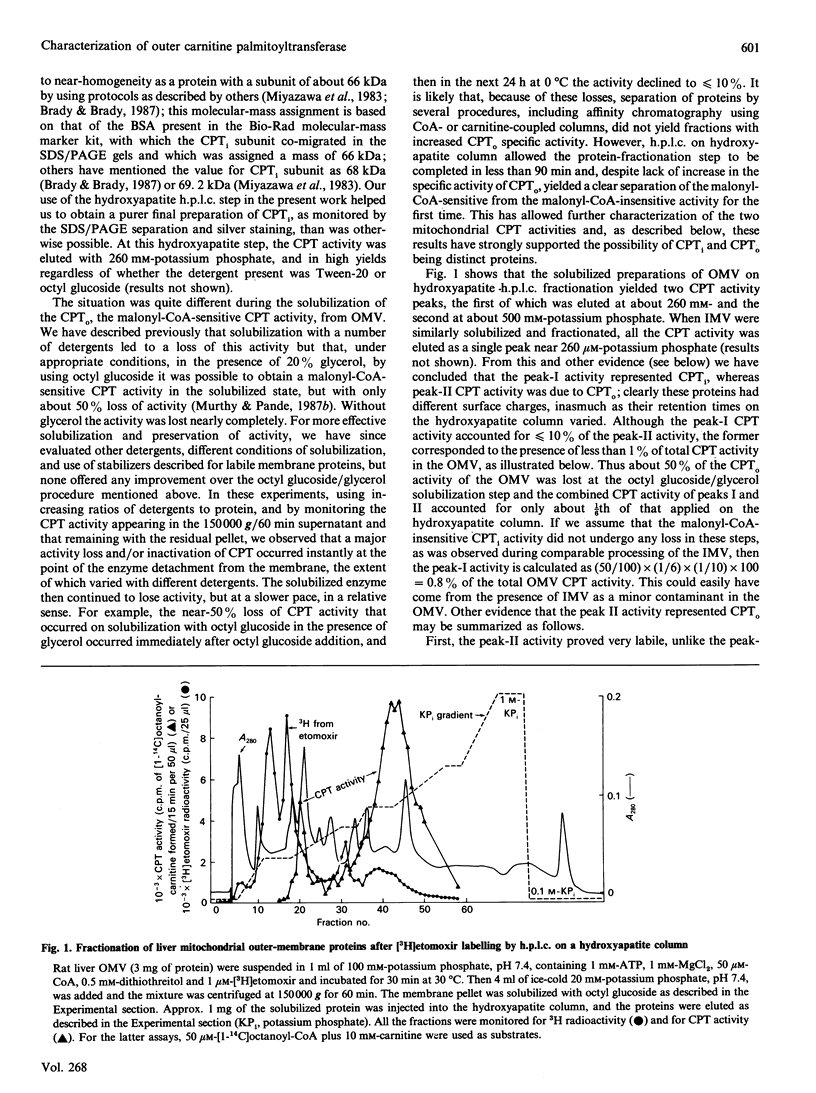
Abstract
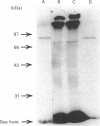
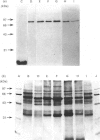
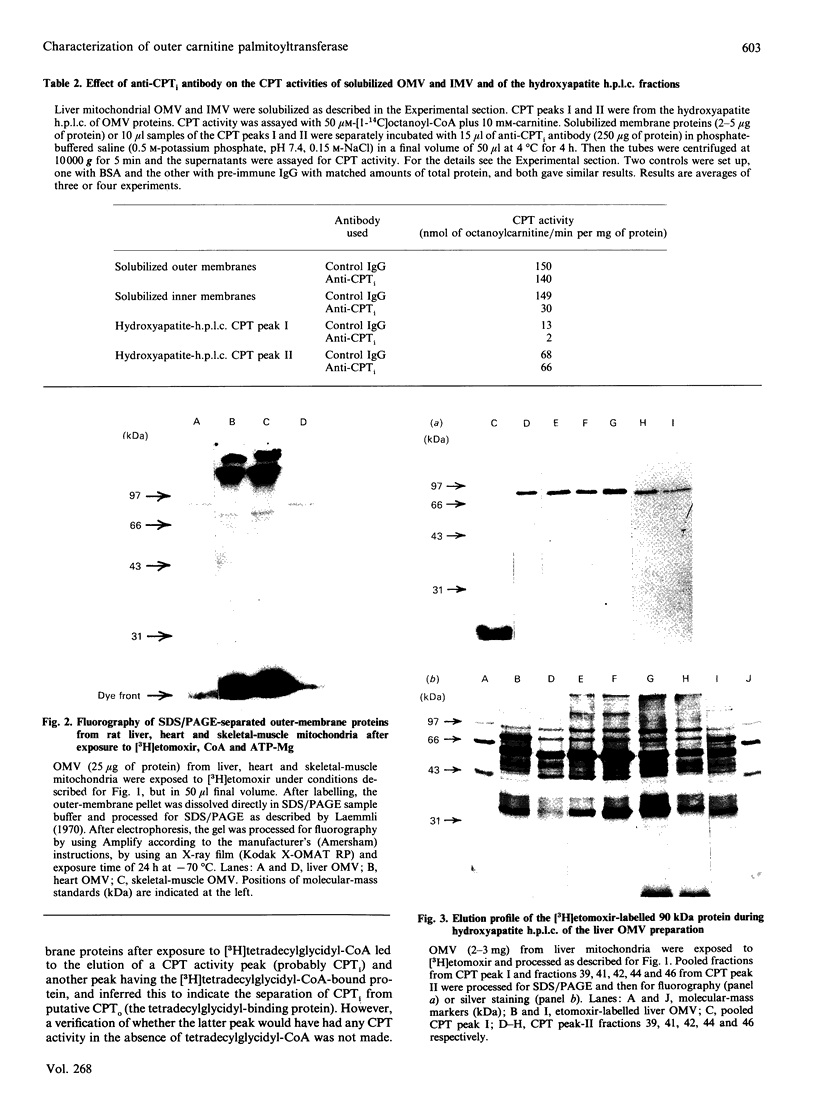
By using octyl glucoside in the presence of glycerol, it is possible to obtain a solubilized malonyl-CoA-sensitive carnitine palmitoyltransferase (CPTo) from the outer membranes of rat liver mitochondria. H.p.l.c. on hydroxyapatite column has now allowed a clear separation of the CPTo from the malonyl-CoA-insensitive CPT activity of the inner membranes (CPTi). The separated CPTo activity showed inhibition by low micromolar concentrations of malonyl-CoA, 2-tetradecylglycidyl-CoA and etomoxir-CoA. On solubilization and fractionation, the CPTo rapidly lost activity, unlike the relatively stable CPTi activity. Reconstitution into asolectin liposomes enhanced the activity and the malonyl-CoA-sensitivity of the CPTo fractions, whereas it had no such effect on the activity or malonyl-CoA insensitivity of the CPTi fractions. A polyclonal antibody raised against the malonyl-CoA-insensitive enzyme, purified from the inner membranes, precipitated the CPTi activity, but showed no reactivity with the CPTo fractions. In Western blots, the above antibody did not react with any polypeptide of the CPTo fractions. Incubation of the outer-membrane preparations with [3H]etomoxir, in the presence of ATP and CoA, led to labelling of a 90 kDa polypeptide that in the above hydroxyapatite chromatography was eluted in the same region as the CPTo. No such polypeptide labelling was seen in the CPTi fractions. With heart and skeletal-muscle mitochondria, the correspondingly labelled polypeptide was of about 86 kDa. These results show that the CPTo and CPTi are distinct proteins, that a subunit of 90 kDa for liver and 86 kDa for muscle constitutes a component of their respective CPTo systems, and that the 66 kDa subunit of the CPTi does not constitute a part of the CPTo system.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bieber L. L. Carnitine. Annu Rev Biochem. 1988;57:261–283. doi: 10.1146/annurev.bi.57.070188.001401. [DOI] [PubMed] [Google Scholar]
- Brady P. S., Brady L. J. Hepatic carnitine palmitoyltransferase turnover and translation rates in fed, starved, streptozotocin-diabetic and diethylhexyl phthalate-treated rats. Biochem J. 1987 Sep 15;246(3):641–649. doi: 10.1042/bj2460641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady P. S., Brady L. J. Regulation of carnitine palmitoyltransferase in vivo by glucagon and insulin. Biochem J. 1989 Mar 15;258(3):677–682. doi: 10.1042/bj2580677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer J. Carnitine--metabolism and functions. Physiol Rev. 1983 Oct;63(4):1420–1480. doi: 10.1152/physrev.1983.63.4.1420. [DOI] [PubMed] [Google Scholar]
- Brosnan J. T., Kopec B., Fritz I. B. The localization of carnitine palmitoyltransferase on the inner membrane of bovine liver mitochondria. J Biol Chem. 1973 Jun 10;248(11):4075–4082. [PubMed] [Google Scholar]
- Declercq P. E., Falck J. R., Kuwajima M., Tyminski H., Foster D. W., McGarry J. D. Characterization of the mitochondrial carnitine palmitoyltransferase enzyme system. I. Use of inhibitors. J Biol Chem. 1987 Jul 15;262(20):9812–9821. [PubMed] [Google Scholar]
- Hajra A. K., Bishop J. E. Preparation of radioactive acyl coenzyme A. Methods Enzymol. 1986;122:50–53. doi: 10.1016/0076-6879(86)22147-3. [DOI] [PubMed] [Google Scholar]
- Healy M. J., Kerner J., Bieber L. L. Enzymes of carnitine acylation. Is overt carnitine palmitoyltransferase of liver peroxisomal carnitine octanoyltransferase? Biochem J. 1988 Jan 1;249(1):231–237. doi: 10.1042/bj2490231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiorpes T. C., Hoerr D., Ho W., Weaner L. E., Inman M. G., Tutwiler G. F. Identification of 2-tetradecylglycidyl coenzyme A as the active form of methyl 2-tetradecylglycidate (methyl palmoxirate) and its characterization as an irreversible, active site-directed inhibitor of carnitine palmitoyltransferase A in isolated rat liver mitochondria. J Biol Chem. 1984 Aug 10;259(15):9750–9755. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lund H. Carnitine palmitoyltransferase: characterization of a labile detergent-extracted malonyl-CoA-sensitive enzyme from rat liver mitochondria. Biochim Biophys Acta. 1987 Mar 13;918(1):67–75. doi: 10.1016/0005-2760(87)90010-5. [DOI] [PubMed] [Google Scholar]
- McGarry J. D., Woeltje K. F., Kuwajima M., Foster D. W. Regulation of ketogenesis and the renaissance of carnitine palmitoyltransferase. Diabetes Metab Rev. 1989 May;5(3):271–284. doi: 10.1002/dmr.5610050305. [DOI] [PubMed] [Google Scholar]
- Miyazawa S., Ozasa H., Osumi T., Hashimoto T. Purification and properties of carnitine octanoyltransferase and carnitine palmitoyltransferase from rat liver. J Biochem. 1983 Aug;94(2):529–542. doi: 10.1093/oxfordjournals.jbchem.a134384. [DOI] [PubMed] [Google Scholar]
- Murthy M. S., Pande S. V. Malonyl-CoA binding site and the overt carnitine palmitoyltransferase activity reside on the opposite sides of the outer mitochondrial membrane. Proc Natl Acad Sci U S A. 1987 Jan;84(2):378–382. doi: 10.1073/pnas.84.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy M. S., Pande S. V. Some differences in the properties of carnitine palmitoyltransferase activities of the mitochondrial outer and inner membranes. Biochem J. 1987 Dec 15;248(3):727–733. doi: 10.1042/bj2480727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pande S. V., Murthy M. S., Noël H. Differential effects of phosphatidylcholine and cardiolipin on carnitine palmitoyltransferase activity. Biochim Biophys Acta. 1986 Jun 27;877(2):223–230. doi: 10.1016/0005-2760(86)90298-5. [DOI] [PubMed] [Google Scholar]
- Ramsay R. R. The soluble carnitine palmitoyltransferase from bovine liver. A comparison with the enzymes from peroxisomes and from the mitochondrial inner membrane. Biochem J. 1988 Jan 1;249(1):239–245. doi: 10.1042/bj2490239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woeltje K. F., Kuwajima M., Foster D. W., McGarry J. D. Characterization of the mitochondrial carnitine palmitoyltransferase enzyme system. II. Use of detergents and antibodies. J Biol Chem. 1987 Jul 15;262(20):9822–9827. [PubMed] [Google Scholar]
- Wolf H. P., Engel D. W. Decrease of fatty acid oxidation, ketogenesis and gluconeogenesis in isolated perfused rat liver by phenylalkyl oxirane carboxylate (B 807-27) due to inhibition of CPT I (EC 2.3.1.21). Eur J Biochem. 1985 Jan 15;146(2):359–363. doi: 10.1111/j.1432-1033.1985.tb08661.x. [DOI] [PubMed] [Google Scholar]
- Zammit V. A., Corstorphine C. G., Kelliher M. G. Evidence for distinct functional molecular sizes of carnitine palmitoyltransferases I and II in rat liver mitochondria. Biochem J. 1988 Mar 1;250(2):415–420. doi: 10.1042/bj2500415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit V. A., Corstorphine C. G., Kolodziej M. P. Target size analysis by radiation inactivation of carnitine palmitoyltransferase activity and malonyl-CoA binding in outer membranes from rat liver mitochondria. Biochem J. 1989 Oct 1;263(1):89–95. doi: 10.1042/bj2630089. [DOI] [PMC free article] [PubMed] [Google Scholar]