ABSTRACT
Urinary tract infections (UTIs) cause a substantial health care burden. UTIs (i) are most often caused by uropathogenic Escherichia coli (UPEC), (ii) primarily affect otherwise healthy females (50% of women will have a UTI), (iii) are associated with significant morbidity and economic impact, (iv) can become chronic, and (v) are highly recurrent. A history of UTI is a significant risk factor for a recurrent UTI (rUTI). In otherwise healthy women, an acute UTI leads to a 25 to 50% chance of rUTI within months of the initial infection. Interestingly, rUTIs are commonly caused by the same strain of E. coli that led to the initial infection, arguing that there exist host-associated reservoirs, like the gastrointestinal tract and underlying bladder tissue, that can seed rUTIs. Additionally, catheter-associated UTIs (CAUTI), caused by Enterococcus and Staphylococcus as well as UPEC, represent a major health care concern. The host’s response of depositing fibrinogen at the site of infection has been found to be critical to establishing CAUTI. The Drug Resistance Index, an evaluation of antibiotic resistance, indicates that UTIs have become increasingly difficult to treat since the mid-2000s. Thus, UTIs are a “canary in the coal mine,” warning of the possibility of a return to the preantibiotic era, where some common infections are untreatable with available antibiotics. Numerous alternative strategies for both the prevention and treatment of UTIs are being pursued, with a focus on the development of vaccines and small-molecule inhibitors targeting virulence factors, in the hopes of reducing the burden of urogenital tract infections in an antibiotic-sparing manner.
INTRODUCTION
Urinary tract infections (UTIs) refer to bacterial colonization of the urinary tract and are one of the most common bacterial infections, infecting an estimated 150 million people worldwide annually. In the United States alone, nearly 11 million cases are reported each year, resulting in approximately $5 billion in indirect and direct costs annually (1, 2). More than 50% of women will experience at least one UTI in their lifetime, and, despite antibiotic intervention, 20 to 30% of women with an initial UTI will experience a recurrent UTI (rUTI) within 3 to 4 months of the initial infection (2, 3). Such infections therefore represent a great health care burden and, as such, demand further research to advance treatment options and improve patient care. This article outlines what is currently known about the determinants and features of Escherichia coli pathogenesis in UTIs and highlights how such knowledge is now being translated into tools for alleviating that burden clinically.
UROGENITAL TRACT
The principal function of the urinary tract is to collect, transport, store, and eliminate urine, which is composed of excreted metabolic products and waste generated in the kidneys (4, 5). From its proximal to distal end, the urinary tract is composed of the kidneys, ureters, bladder, and urethra, and each of these organs plays a critical role in maintaining the homeostasis of this system. The upper urinary tract consists of the kidneys, which filter blood to produce urine, and the ureters, bilateral fibromuscular tubes that carry urine from the kidneys to the bladder. The bladder is a hollow, distensible organ composed of smooth muscle, collagen, and elastin (6). When devoid of urine, it adopts a tetrahedral shape; upon being filled, it becomes ovoid (7). Finally, the urethra connects to the neck of the bladder, begins at the distal end of the urethral sphincter, and serves as a duct by which urine is eliminated out of the body from the bladder (7).
In both males and females, the luminal surface of the urinary tract is lined with specialized epithelial tissue broadly known as the urothelium. The urothelium serves as a distensible and effective permeability barrier to accommodate urine flow and volume while preventing the unregulated exchange of metabolic products between the blood and urine (8). The superficial urothelium comprises a single layer of large polyhedral, multinucleated, highly differentiated umbrella cells, also termed superficial facet cells (8). Umbrella cells are decorated with a crystalline array of uroplakin proteins that form urothelial plaques. Importantly, uroplakins play a critical role in the maintenance of the superficial urothelium’s permeability barrier (9–13). The intermediate and basal layers of the urothelium are significantly smaller and less differentiated, and they are believed to contain urothelial stem cells required for umbrella cell regeneration (7, 14–16).
The urinary tract is thought to be relatively sterile (17), although recently, evidence for a urinary microbiota was presented (18). As is discussed in the following sections, upon accessing the urinary tract, bacteria can exploit tissue-specific receptors to establish infection.
INFECTION OF THE URINARY TRACT
The majority of uncomplicated UTIs manifest as infections of the lower urinary tract: infection and inflammation of the urethra (urethritis) or urinary bladder (cystitis) (2). If bacteria ascend the ureters to the upper urinary tract, this results in pyelonephritis (2). This is particularly concerning, as bacteria in the kidneys may enter the bloodstream, causing sepsis (2). Asymptomatic bacteriuria (ASB) is marked by positive urine cultures in the laboratory without urinary symptoms (2). Cystitis is typically diagnosed based on symptomology, such as frequency and urgency of urination, burning pain and sensation during urination, abdominal discomfort, and/or turbid, odorous urine paired with high levels of bacteria in the urine (bacteriuria) (2). Pyelonephritis typically presents with bacteriuria, pyuria (white blood cells in the urine), flank pain, or fever and may or may not present with symptoms associated with cystitis (2). The majority (85%) of uncomplicated, community-acquired UTIs are caused by uropathogenic E. coli (UPEC), and the remaining 15% are caused by other Gram-negative bacilli like Klebsiella or Gram-positive cocci such as Enterococcus or Staphylococcus (19). Risk factors for uncomplicated UTI include sexual activity, history of UTI, contraception, and host genetics and immune responses (2, 20). E. coli can also exist in the urinary tract asymptomatically in a condition known as ASB (21).
In the health care setting, catheterization increases the risk of complicated UTIs (22). Catheter-associated UTIs (CAUTI) account for 30 to 40% of all health care-associated infections in the United States (23). The majority of CAUTI are asymptomatic, but these infections can present with fever, chills, malaise, and/or generalizable discomfort or as cystitis or pyelonephritis once the catheter is removed (2). The two major causative agents of CAUTI are UPEC (65%) and Enterococcus spp. (11%) (24). CAUTI are particularly threatening, as they have the potential to disseminate in the health care setting.
Due to their prevalence and the high rate of recurrence, UTIs are a significant cause of morbidity in women throughout their lifetime. It is estimated that one in three women will be prescribed antibiotics to treat a UTI before the age of 24 (2). In the outpatient setting, 15% of antibiotic prescriptions have been reported to be for UTI treatment (25, 26). Frequent antibiotic usage coupled with antibiotic resistance among uropathogens (27) highlights the urgent need to develop new and improved treatment and prevention options.
UTI PATHOGENESIS
Uropathogenic Escherichia coli
UPEC (Fig. 1) lacks a “genetic signature” (28) that distinguishes it from non-UPEC. This is likely due to the broad definition of UPEC as any strain that is recovered from the urine of a patient with a symptomatic UTI. Recently, a high-resolution, comparative genomic study performed on E. coli isolates from women with recurrent UTIs revealed that the isolates were diverse and represented five major E. coli clades: A, B1, B2, D, and E (28). Two-thirds of these strains belonged to the clade B2, which comprises the majority of UPEC strains isolated in the United States and Europe (28). Interestingly, the strain’s phylogenetic background and carriage of virulence factors are not entirely predictive of its urovirulence (28). Instead, the expression of certain genes, such as those involved in motility and transport of sugars, is a better predictor of the virulence of a given strain in mice. Lending support to this is the fact that in some women suffering from recurrent UTIs with a strain different from that which caused the previous event, the new strain can actually encode fewer putative urovirulence factors than the strain that was replaced. Thus, work in multiple mouse models of UTIs has defined a “lock-and-key” mechanism of UTI pathogenesis in which the disease outcome is not completely fixed based on the pathogen or the host but, rather, is determined in part by how the fitness level of the introduced pathogen is matched against the resistance or susceptibility level of the host, which is influenced by history of infection and the presence of foreign bodies (28–30).
FIGURE 1.
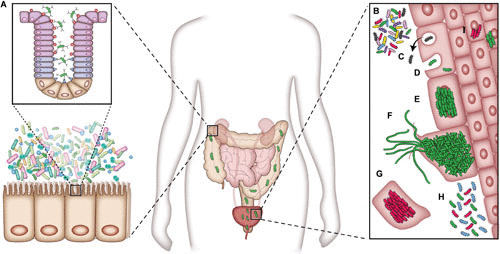
UPEC pathogenesis. (A) UPEC is housed in a reservoir in the gastrointestinal system. The bacteria are able to colonize the urinary tract from this reservoir. (B) Bacteria are able to adhere to and invade the bladder epithelial cells. (C) Bacterial cells can be evicted from the host cell in response. Bacterial cells can also enter the cytoplasm (D) and initiate IBC formation (E). (F) UPEC can, upon fluxing out of the host cells, filament and reinfect other urothelial cells. (G) To counteract intracellular pathogens, the host can initiate a program of host cell exfoliation. (H) Chronic cystitis in mice can occur with persistent high titers of bacteriuria. (I) QIRs can be established, in mice with resolved infections, in layers below superficial urothelial cells. Image and caption are adapted from reference 31.
The type 1 pilus is an important mediator of bladder colonization (31, 32). The adhesive tip protein or adhesin (33) of the type 1 pilus FimH binds to mannose (31, 34). This ligand is present on uroplakin 1a and on β1 and α3 integrin molecules on the surface of bladder urothelial cells (31, 35, 36). Changes in host cell cytoskeletal elements, thought to be mediated through Rho GTPases, allow type 1 facilitated invasion into urothelial cells (31, 37, 38). During infection, pathogen-associated molecular patterns (PAMPs) can stimulate the pattern recognition receptor Toll-like receptor 4 to activate host responses. One example of a PAMP is bacterial lipopolysaccharide (39). Cytokine production (39), the influx of inflammatory monocytes and neutrophils (40), bacterial eviction from host cells (39, 41), and the exfoliation of urothelial cells (39, 42) are all innate host responses encountered by UPEC (39) (Fig. 1C). Further, work has demonstrated that the role of urothelial exfoliation is to eliminate infected bladder cells from the body, thus reducing the UPEC burden in the bladder (43, 146). UPEC-induced exfoliation results in dead or dying shed epithelial cells, rather than the predominantly living host cells shed by chemical exfoliation (43). This exfoliation may occur by multiple pathways, including interleukin 1β (IL-1β) signaling and the NLRP3 inflammasome (42).
Exfoliation can be a double-edged sword, as it leads to the exposure of the underlying transitional epithelium, where bacteria can invade and persist in small quiescent intracellular reservoirs (QIRs) even after resolution of bacteriuria. The bacteria localized in QIRs can subsequently reactivate to seed recurrent UTI (44). Evidence suggests that chymase, from mast cells, activates procaspase to initiate this cytolysis (43). Interestingly, mast cells have been shown to induce an anti-inflammatory response in the bladder as well. In C57BL/6 mice infected with a UPEC isolate, IL-10 expression spikes at 6 hours postinfection in the bladder and remains elevated for at least 72 hours. Mast cells, which have been shown to increase in numbers in the bladder upon UPEC infection, can secrete IL-10, which also functions to reduce the number of mature dendritic cells and possibly other immune cells (45).
In humans, UPEC infections have numerous outcomes, including ASB, acute and self-resolving UTIs, chronic UTIs, and/or recurrent UTIs (20) (Fig. 1H and I). Murine models of cystitis have been developed that are capable of mimicking these clinical outcomes. For example, C3H/HeN mouse models have recapitulated two disease courses. Mice experience (i) acute infection followed by spontaneous resolution within 1 to 4 weeks of infection, or (ii) acute infection that then progresses to a long-lasting persistent infection termed chronic cystitis (39).
The fate of infection is determined in part by whether a host-pathogen checkpoint is activated. Activation of the checkpoint leads to elevated levels of COX2 expression (see below), which licenses the transmigration of neutrophils across the bladder epithelium, leading to the associated mucosal damage that ensues (40). Thus, activation of the host-pathogen checkpoint leads to persistent high-titer bacteriuria, which is accompanied by severe immunopathology and ablation of the terminally differentiated superficial umbrella cells in a condition we have termed chronic cystitis. Chronic and recurrent cystitis can be predicted 24 hours postinfection by increased levels of the serum biomarkers IL-5, IL-6, neutrophil cytokine CXCL1, and granulocyte colony-stimulating factor (29, 40). Similarly, in the sera of young women with acute UTI, UTI recurrence was predicted by increased levels of soluble biomarkers involved in myeloid cell development and chemotaxis (40).
While type 1 pili are important for the progression of acute and chronic cystitis, another pilus type, Fim-like (Fml) or F9, is also important for UPEC persistence in the inflamed bladder. Bladder inflammation leads to the exposure of the galactose β1-3 N-acetylgalactosamine receptor recognized by the Fml adhesin FmlH which facilitates binding to the inflamed tissue and enables persistent bacteriuria and high bladder bacterial burdens throughout chronic cystitis (46).
Furthermore, clinically, a history of cystitis is one of the key risk factors for the development of recurrent infections (rUTIs), specifically, an incidence of UTI at a young age or two or more previous incidences of UTIs (29). Mechanistically, a possible explanation for this phenomenon was recently proposed. The remodeling of the urothelium during chronic infection permanently alters its architecture, even after antibiotic therapy and convalescence from infection, resulting in hundreds of differentially expressed genes and proteins in the remodeled bladder compared to an age-matched naïve bladder (29). Thus, mice with a history of chronic infection are left with a molecular imprint on the bladder defined by a defect in terminal differentiation of the bladder epithelium, resulting in significantly smaller luminal cells and an altered transcriptome (29). Importantly, bladder remodeling changes host-pathogen interactions during acute pathogenesis by conferring resistance to early colonization events. However, mice with a history of chronic infection succumb to severe bladder infection, a process that is COX-2 dependent and leads to the transmigration of neutrophils across the bladder epithelium, mucosal wounding, and unchecked bacterial replication (29). In support of this, treatment with a COX-2 inhibitor leads to a significant reduction in both chronic and recurrent cystitis (29, 40). Thus, bladder mucosal remodeling can occur as a consequence of persistent infection, and this reprogramming of the bladder predisposes the host to more severe rUTI upon subsequent bacterial exposure, even with less pathogenic strains.
UPEC in the gastrointestinal tract
The major source of UPEC is thought to be the gastrointestinal tract, where UPEC can reside transiently or as a commensal member of the gut microbiota (3, 47–49). UPEC is then shed in the feces, inoculating the periurethral area or vagina, and subsequently introduced into the urinary tract during periods of physical manipulation, such as during sexual activity or catheterization (20). Several recent studies identified chaperone usher pathway (CUP) pilus types that promote the establishment and/or maintenance of the UPEC intestinal reservoir. Interestingly, a role for type 1 pili in UPEC intestinal colonization in mice has been reported by several groups (47, 50, 51). Additionally, a previously uncharacterized pilus, the F17-like pilus, has also been implicated in UPEC intestinal colonization in mice (47). Purified lectin domains of the type 1 and F17-like adhesins (FimH and UclD, respectively) were shown to bind within the colonic crypt, suggesting that type 1 and F17-like pili facilitate colonization within that niche (Fig. 1A). However, further studies examining the localization of whole bacteria expressing type 1 or F17-like pili within the mouse gut are required to determine if UPEC binds within the crypts during intestinal colonization in vivo. Phylogenomic and structural analyses suggest that UPEC acquired F17-like pili from intestinal pathogens, and B2 UPEC strains causing same-strain recurrences were found to be significantly enriched for the carriage of the F17-like pilus gene cluster (47). These analyses reveal that F17-like pili might have evolved to enable maintenance of a UPEC intestinal reservoir by promoting UPEC persistence in women with rUTIs. Thus, the identification of UPEC genes involved in gastrointestinal colonization provides the framework for future studies elucidating the mechanisms that underlie UPEC persistence in the gut.
Intracellular bacterial communities
Intracellular bacterial communities (IBCs) are clonal collections of bacterial cells housed within the cytoplasm of superficial facet cells of the bladder (52, 53). IBCs are encased within a biofilm-like matrix (31, 52) and are replicative, metabolically active communities (52, 54). IBCs provide a mechanism for UPEC replication in the bladder while being protected from immune responses and possible antibiotic treatment (31, 52). Studies have found that the IBCs, although studied extensively in mice (39, 55, 56), are a feature of human infection (31, 57, 58). IBC formation has also been documented in a number of bacterial species in the family Enterobacteriaceae (52). IBC formation occurs during acute infection and is restricted to the superficial umbrella cells. Exfoliation of these cells is part of an innate defense, and the ablation of the luminal epithelium restricts further IBC formation during chronic infection (39, 52).
A number of factors have been found to be critical in IBC formation. FimH, the type 1 pilus adhesin known to mediate binding to and invasion of bladder urothelium (53, 59), also plays a role in bacterial association within the IBC biomass (60). The K1 capsule also allows clumping of cells within the host cell (52). LacZ and GalK, factors involved in metabolism, have been found to be important for the establishment of IBCs. In a murine model, strains with individual deletions of the genes encoding these proteins were found to lack fitness in competitive infections against the wild type (54). YeaR, a recently described protein involved in the oxidative stress response, is critical to IBC formation in a type 1-dependent manner (54). Iron uptake systems, including siderophore biosynthetic genes, are highly upregulated in IBCs, as are reciprocal iron responses in neighboring host cells. Thus, a competition for iron occurs at the interface between the IBC and neighboring epithelial cells (61). For example, ChuA, a hemin receptor, is highly upregulated in IBCs, and neighboring epithelial cells respond by upregulating the transferrin receptor, an iron-scavenging factor (52, 61). Developmentally, bacterial cells within IBCs progress from a coccoid shape to a rod shape. Bacteria then generally take a filamentous form, mediated by SulA, a cell division regulator, as they exit host cells to the extracellular environment (52). This development and exit are of note, as they provide a mechanism of infection of neighboring cells, allowing the infection to spread in the bladder (52). It is clear that the formation of IBCs is a hallmark of UPEC pathogenesis (52) and represents a critical topic for future study.
Quiescent intracellular reservoirs
QIRs are small communities of bacterial cells contained within Lamp1+ vesicles in host cells (44, 52). These communities contain 4 to 10 bacterial cells, oriented in a rosette-like fashion (44), and are nonreplicating (52), in contrast to IBCs (52). QIRs can be present in both superficial epithelial and transitional bladder cells (44) and can persist for 12 weeks (44). Beyond being protected from antibiotics (62), such reservoirs are thought to be able to initiate a recurrent infection (52), as work has demonstrated that in mice possessing bladder QIRs, exfoliation of the superficial bladder epithelial cells can result in an activation of the bacteria within the QIR to cause pyuria, bacteriuria, and increased bacterial bladder titers (44). Interestingly, there may exist an interplay between the vaginal microbiome and rUTI, as it has been shown that in bladders containing QIRs, exposure to Gardnerella vaginalis can result in activation of the reservoir, leading to rUTI (63). Additional work has examined the contribution of host cytoskeletal elements to QIR behavior. Interrupting the host actin network causes QIRs to replicate and then exit the vesicle into the cytosolic space (64). Considering the substantial burden of recurrent UTI, QIRs represent a rich area of study to understand the mechanisms of recurrence.
Virulence and Bacterial Colonization
The determinants by which UPEC causes UTIs (Fig. 2) have been extensively studied. To facilitate survival within human urine, an environment rich in amino acids and peptides, UPEC relies on amino acid biosynthesis and amino acid and carbohydrate metabolism (65, 66). As described above, to fulfill nutritional metal requirements to survive within host cells, UPEC utilizes iron acquisition molecules called siderophores to chelate iron from the host environment. Iron-siderophore complexes are then recognized by cognate outer membrane (OM) receptors on the bacterium for their reuptake into the bacterial cell. In particular, enterobactin, yersiniabactin, and salmochelin are important siderophores in the context of UTIs (67). UPEC also utilizes certain toxins that play important roles in pathogenesis (68, 69). Finally, surface-localized structures, such as flagella, pili, capsule, and OM adhesins, and the regulation of these factors are important for the motility, colonization, and biofilm formation of UPEC during infection (Fig. 2) (70).
FIGURE 2.
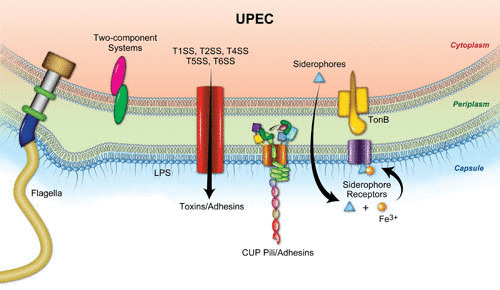
Overview of UPEC fitness and virulence factors. Surface-associated structures that play a role in UTI pathogenesis include lipopolysaccharide, polysaccharide capsule, flagella, pili, toxins, secretion systems (SS), and siderophore receptors. Image and caption are adapted from reference 68.
CUP pilus assembly
Pili and adhesins are particularly important, as they are critical for all stages of the UPEC pathogenic cascade, except for growth in urine (71, 72). To facilitate adhesion to host- and tissue-specific niches, UPEC encodes CUP pili. E. coli carries genes for at least 38 CUP pili in its pangenome, and UPEC utilizes CUP pili, such as type 1 and P pili, to mediate adherence critical in cystitis and pyelonephritis, respectively (Fig. 3) (19, 73–76). Gram-negative bacteria assemble CUP pili to mediate adhesion to host and environmental surfaces, facilitate invasion into host tissues, and promote formation of intra- and extracellular biofilm communities (77). Further, as discussed above, recent work suggests that the type 1 and F17-like pilus types promote UPEC colonization within the mouse colon (47, 50, 51). Expression of type 1 pili is under the control of an invertible promoter, fimS, that oscillates between ON and OFF (72). Interestingly, there exist factors in the urine that promote fimS, in planktonic UPEC in the urine, to adopt a phase OFF orientation; however, bacteria bound to bladder cells shed into the urine remain in phase ON (72). Microarray and RNA-Seq studies of bacteria isolated from the urine of UTI patients have revealed patients with different patterns (both high and low) of fim expression (71, 78). Based on these and other human studies and from work in a murine model, one hypothesis is that planktonic bacteria in urine are (or become) nonpiliated, while bacteria colonizing the bladder tissue or bound to shed epithelial cells express type 1 pili (71, 79–81). Additionally, it has been postulated that exponential growth in human urine suppresses type 1 pilus expression (82). Taken together, these results indicate that type 1 pili are temporally and spatially regulated and are required for colonization of host tissues.
FIGURE 3.
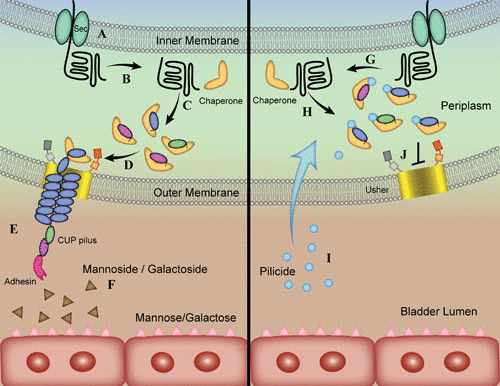
Overview of CUP of pilus assembly and mode of action of antivirulence compounds. (A) Sec transports unfolded subunits of the pilus structure into the periplasmic space. (B, C, G, H) Pilus subunits interact with the pilus type-specific chaperone and fold. (D) Chaperone-subunit complexes interact with the N terminus of the pilus usher. (E) Secreted subunits, bound together through donor strand exchange, form the pilus. (F) Small-molecule inhibitors, mannosides and galactosides, antagonize interactions between the adhesive tip of the pilus and its ligand. Pilicides bind to the chaperone (I) and interrupt the interaction between chaperone and the N terminus of the usher (J). Image and caption are adapted from reference 31.
CUP pili are assembled by dedicated chaperone-usher machinery, which is encoded by operons that contain the genetic determinants required to assemble a mature pilus: a periplasmic chaperone protein, an OM usher protein, major and minor pilus subunits, and, in most cases, a tip adhesin protein (77). Adhesins are two-domain proteins, with an N-terminal lectin domain that binds to receptors with stereochemical specificity, while the C-terminal pilin domain joins the adhesin to the pilus rod (59). In CUP pilus assembly, individual pilus subunits or pilins are first exported across the inner membrane to the periplasm, where they are guided to the OM usher via the chaperone (59) (Fig. 3A). Each pilin comprises a single domain having an immunoglobulin (Ig)-like structure (59) that is incomplete because it lacks a seventh C-terminal β-strand.
In a process termed donor-strand complementation, the chaperone, a boomerang-shaped protein composed of two complete Ig-like domains, provides in trans its G1 β-strand to transiently complete the pilin’s Ig-like fold, thus catalyzing folding directly on the chaperone template (59) (Fig. 3B, C, G, and H). Chaperone-pilin complexes are then targeted to the OM usher, a β-barrel channel that catalyzes subunit-subunit interactions through a reaction called donor-strand exchange, wherein every pilin subunit has an N-terminal extension that completes the Ig fold of its neighboring subunit (59) (Fig. 3D and E). The OM usher is composed of five functional domains: a 24-stranded integral β-barrel translocation domain (TD), a β-sandwich plug domain that gates the pore of the TD, a periplasmic amino-terminal domain (NTD), and two carboxy-terminal domains (CTD1 and CTD2) (83, 84). The concerted coordination of the usher’s domains ensures that the subunits interact productively during fiber polymerization. The molecular mechanisms that drive this cooperative coordination of the different domains of the usher have been studied, and the studies have demonstrated that conformational flexibility and allostery drive this bacterial nanomachine in the absence of cellular energy at the OM (83–91). In particular, it has been shown that upon initiation of CUP pilus assembly, the TD and PD undergo marked rearrangements to accommodate transit of the growing fiber, while the periplasmic NTD and CTDs participate in substrate recruitment, catalysis of donor strand exchange, and translocation through the TD pore (83, 84).
While the OM usher serves as the assembly platform for the growing pilus fiber and anchors it to the OM, the majority of the pilus is composed of homopolymers of the major rod subunit (59). Once the pilus rod extrudes into the extracellular milieu, it coils into a right-handed helical fiber that has the ability to unwind into a linear structure (59). Recent structural studies on the type 1 and P pilus rods have identified the molecular determinants for the formation of the helical rod (92–94). Disruption of critical subunit-subunit interactions within the rod resulted in pili that were more prone to helical unwinding in the presence of shear force and displayed attenuation in murine models of cystitis and intestinal colonization by UPEC (92–94). Taken together, these studies suggest that the dynamics of rod coiling and uncoiling play a critical role in UPEC pathogenesis.
Beyond its own role in pathogenesis, the pilus rod also serves as a scaffold to present the pilus tip adhesin at the host-pathogen interface. In addition to the OM usher and pilus rod displaying conformational flexibility, recent work has shown that the two-domain adhesin protein, FimH, exists in equilibrium between conformational states as well (95). One study focusing on the type 1 pilus adhesin FimH demonstrated that it adopts two-state conformational ensembles (95). Remarkably, it appears that positively selected residues within the protein modulate the equilibrium between these two states, and this equilibrium is crucial to bacterial persistence within the bladder during the progression of UTI (95). In summary, conformational dynamics play a significant role at every level of pilus assembly.
Virulence factors
A variety of virulence factors have been the subject of study in UPEC pathogenesis. Work has revealed that surface-associated structures such as capsules are also critical for immune evasion and for the successful development of IBCs during infection (96–99) (Fig. 2). Among such virulence factors is antigen 43 (Ag43), an autotransporter protein of the AIDA-I type, which functions in the formation of biofilms and aggregation (100, 101). Ag43 is thought to be important to bladder colonization, as evidence suggests that deletion of one Ag43, Ag43a, in CFT073 causes attenuation of bladder colonization 5 days postinfection (101). Structurally, Ag43 exhibits a functionally significant L-shaped secondary structure. Along an interface of this structure, Ag43a autoaggregates in an interaction mediated by hydrogen and electrostatic bonding. As such, a “Velcro-like” mechanism has been proposed for cellular adhesion mediated by Ag43 (100). Additionally, curli, secreted amyloids which contribute to the formation of biofilm extracellular matrix (102), have been found to improve bacterial adherence to kidney epithelial cells. Curli also improve relative growth of bacteria when exposed to human antimicrobial peptide LL-37 and mouse antimicrobial peptide mCRAMP (103). A number of other characterized virulence factors are briefly described in Table 1.
TABLE 1.
Virulence factors in UPEC pathogenesis
| Virulence factor(s) | Type of factor | Role in pathogenesisa | Reference(s) |
|---|---|---|---|
| QseBC, Cpx, and PhoPQ | Two-component regulatory systems | Regulation of virulence factor expression | 130–135 |
| SAT (secreted autotransporter toxin) | Toxin | Induction of vacuolation within the bladder and kidney cells in vitro; UPEC proliferation | 69 |
| CNF-1 | Toxin | Activates Rho GTPases; enhances UPEC invasion of urothelial cells in vitro | 136 |
| HlyA (alpha-hemolysin) | Toxin | Exfoliation of urothelium; partially regulated by Cof phosphatase | 42, 137, 138 |
| YefM-YeoB | Toxin-antitoxin system | Bladder colonization in competitive infection | 139 |
| YbaJ-Hha | Toxin-antitoxin system | Bladder colonization in competitive infection | 139 |
| PasT-PasI | Toxin-antitoxin system | Kidney colonization in both competitive and noncompetitive infections; PasT promotes formation of persister cells | 139 |
| RqiL | Component of toxin-antitoxin system | GIT colonization | 140 |
| GlpG | Protease | Deficient growth in mucus medium (recapitulating the GIT mucus) | 141 |
| UpaB | Autotransporter | Bladder colonization 1 dpi | 142 |
| TosA | RTX factor | Upper urinary tract, liver, and spleen adherence | 143 |
| UpaH | Autotransporter | Bladder infection (from competitive studies); biofilm formation (CFT073) | 144 |
| neaT | Acyltransferase gene | Bacteremia | 145 |
GIT, gastrointestinal tract; dpi, day postinfection.
Gender and UPEC UTIs
Women are more likely to experience uncomplicated, community-acquired UTIs than men. This is thought to be due to higher rates of bacterial colonization of the urethral and periurethral body sites (2). This, paired with shorter urethral lengths in women, makes it more likely for bacteria to ascend the urethra and access the bladder for colonization and to establish an infection in this population (2). However, there is a significant male patient population that experiences complicated UTIs due to risk factors that include spinal cord injuries, anatomical and physiological abnormalities in the urinary system (such as vesicoureteral reflux), diabetes, and urethral instrumentation (3).
Demographic data suggest that beyond simple anatomical differences between the male and female urinary tracts, hormones could play a role in pathogenesis (104). Cell culture work has found that estrogen aids the host defense against UTIs, increasing the expression of genes for antimicrobial peptides and proteins involved in forming cellular junctions while reducing intracellular bacterial titers in vivo (105). On the other hand, while community-acquired UTIs are more common among females, the rate of mortality from complicated UTI and pyelonephritis is higher in males (106). In order to study how sex influences UTI pathogenesis, surgical and nonsurgical male models for studying UTIs have been developed (106, 107). The surgical model of infection using the C3H/HeN strain of mice found that male C3H/HeN mouse bladders are colonized with UTI89 at higher levels at 6 hours postinfection than female bladders and that males were more likely to develop chronic cystitis (106). Furthermore, male C57BL/6 and C3H/HeN mice exhibited higher kidney titers with UTI89 than their female counterparts, and all of the UTI89-infected C3H/HeN mice developed renal abscesses, while less than 10% of their female counterparts developed them (106). Beyond developing a male model of UTI, the same study demonstrated that testosterone plays a role in the observed higher kidney and bladder colonization in C3H/HeN male mice (106). Discrepancies in the characteristics of UTIs between males and females represent an opportunity to further probe the features which define the natural history of UTI.
Catheter-associated UTIs caused by UPEC
Catheterization is a common phenomenon in inpatient settings, where 20 to 50% of patients can be catheterized (108). Catheterization is responsible for a substantial majority, 70 to 80%, of complicated UTIs (24), and about one-half of all CAUTI are caused by UPEC (109). Catheter-associated infections can also be caused by a number of other microorganisms, including Enterococcus, Staphylococcus, and Proteus (108, 110, 111). The use of urinary catheters has been shown to have an effect on the pathobiology of UPEC UTIs (30). Catheterization induces bladder inflammation and edema (30), and UPEC thus enters and colonizes a different environment than it would normally (30). For example, it has been shown that timing of infection postcatheterization can affect initial UPEC colonization (109). Furthermore, work has shown that implanted bladders exhibit lower IBC burdens than nonimplanted bladders while remaining morphologically similar. Nonetheless, implanted bladders exhibit greater exfoliation than the nonimplanted bladders, suggesting that the reduction of IBC burden results from this exfoliation phenomenon. Additionally, the mere presence of an implant activates bacteria in QIRs of previously infected mice, resulting in recurrence of infection.
This study also identified FimH as a virulence factor in CAUTI (30). Deletion of FimH reduced infectious burden in implanted mouse bladders and correspondingly affected biofilm formation in vitro and bacterial colonization of the implant itself. However, this study suggests that factors other than FimH could play a role in this pathogenesis (30).
TREATMENT STRATEGIES FOR UROGENITAL INFECTIONS
Antibiotic Resistance
Antibiotic resistance of UTI-causing bacteria has increased in recent years. UTIs became more difficult to treat from 1999 to 2010 according to the Drug Resistance Index, which evaluates the degree of difficulty in the treatment of infections, with this trend being attributed to increasing antibiotic resistance (112). In the United Kingdom, resistance to trimethoprim specifically has become prevalent in uropathogens (27). One example of a general antibiotic resistance phenotype is ST131. ST131 represents a group of strains of extraintestinal E. coli exhibiting multidrug resistance (51, 113, 114). These strains exhibit resistance to beta-lactams and fluoroquinolones and, indeed, seem to be driving antibiotic resistance globally (113). Such strains have become pandemic, with isolates being identified across the globe (51, 113–115), causing, among other infections, UTIs and bacteremia (115).
Novel Lines of Treatment
Vaccines
With the substantial health care burden UTIs cause, vaccine development has become an important pillar in the effort to reduce and prevent the disease burden. Such work has leveraged current understandings of virulence factors. One group of factors targeted in vaccine development is bacterial adhesins, including FimH, FmlH, pilus, PapG, and EbpA. IgG antibodies to FimH, generated in response to vaccination with FimCH in mice and cynomolgus monkeys, were shown to protect against UTI (116, 117). It has been postulated that the antibodies’ effect is based on its ability to prevent bacterial colonization by FimH-tipped type 1 pili (116). Additionally, a FimCH experimental vaccine recently completed a phase 1a/1b trial. Vaccination of two different cohorts, whose members had a 24-month history of rUTI upon enrollment, resulted in 74% and 70% reductions in total UTI once FimH immunity was achieved. For UTIs caused specifically by E. coli and Klebsiella, 70% and 87% reductions, respectively, were observed (Gary Eldridge, personal communication). Based on these promising results, the FDA has allowed compassionate use of the vaccine for patients suffering from infections caused by multidrug-resistant UPEC strains.
Vaccination of mice with FmlHAD (the lectin domain of the two-domain FmlH adhesin protein) prior to infection with CFT073 significantly decreased bladder and kidney bacterial burden 2 and 3 days after infection in mice (46). P pili, tipped with the PapG adhesin, have been shown to play a critical role in pyelonephritis in cynomolgus monkeys (75). IgG antibodies are produced when cynomolgus monkeys are vaccinated with PapDG (118). No difference in bacteriuria between vaccinated and nonvaccinated monkeys was observed, but histologically, with the exception of mononuclear cells, vaccinated monkeys exhibited none of the other recorded signs of kidney pathology, with a subset of these categories proving statistically significant, while each of these signs of pathology was found in a proportion of nonvaccinated control monkeys (118).
Enterococci express Ebp pili that are tipped with EbpA, which is a fibrinogen-binding adhesin. Urinary catheterization results in the release of fibrinogen, which subsequently coats the catheter. Enterococcus uses EbpA to bind to and form biofilms on the fibrinogen-coated catheter. Recent evidence has shown that antibodies to the N-terminal domain of EbpA can prevent and treat Enterococcus-mediated CAUTI (108). However, in a mouse model, a history of Enterococcus infection is not sufficient to reduce future Enterococcal infection (108). Beyond harnessing structural components of adhesion for vaccine development, siderophores have been found to be a promising lead. Yersiniabactin and aerobactin conjugated to bovine serum albumin and administered together to immunize mice exhibited reduced kidney colonization and pathology 48 hours postinfection compared to a nonconjugated bovine serum albumin mock vaccination, while bladder colonization and pathology remained similar (119). Vaccination and subsequent boosting with factors involved in the iron uptake are able to reduce murine bladder (LutA and IreA) and kidney (FyuA) colonization 48 hours postinfection, and further thought has been given to generating multivalent vaccines from these iron uptake proteins (120).
Small-molecule inhibitors
The critical nature of host-pathogen interactions during the course of UPEC pathogenesis has warranted the development of ligand mimetics designed to inhibit adhesion to host tissues or block the biogenesis of CUP pili. The ultimate goal of these compounds is to create novel antibiotic-sparing therapies that selectively deplete UPEC from their various habitats in the host.
Mannosides
Mannosides are compounds developed to be ligand mimetics to the FimH adhesin that tips the type 1 pilus, which is important for establishing bladder infections (34, 121, 122). Built on a biphenyl scaffold linked to a mannose moiety, early iterations of mannosides with various substituent groups were developed and evaluated for the ability to inhibit type 1-mediated biofilm formation (122). Early iterations of mannosides demonstrated greatly improved inhibitory activity relative to methyl-α-mannose, with an increase in potency of hemagglutination inhibition on the order of approximately 105- to 107-fold (31, 34). An initially optimized compound showed, in a murine model, the ability to (i) reduce bacterial titers in bladders, both luminally and intracellularly, when administered prophylactically; (ii) efficaciously treat UTIs after oral delivery; and (iii) improve the ability of trimethoprim-sulfamethoxazole (TMP-SMX), an antibiotic, to reduce bladder bacterial load (122). Mannoside ZFH-04269 was able to render a TMP-SMX-resistant ST131 strain, EC958, sensitive to TMP-SMX treatment by preventing invasion of UPEC into the bladder epithelium, thus exposing the luminal UPEC to concentrations of TMP-SMX above the MIC (123).
Structural data and inhibitory assays suggest that interactions with the tyrosine gate associated with the binding pocket on the FimH lectin domain, composed of Tyr48 and Tyr137 and a hydrophobic region, Ile13, would generate more potent inhibitory compounds (121, 124). Continued optimization has looked to improve the stability of originally O-linked mannosides, by replacing the O linkage located between the biphenyl scaffold and alpha-d-mannose moiety with a C linkage (121). Iterations of C-linked mannosides showed improved ability to prevent and treat infections in mice (121). Moreover, in mice, oral mannoside treatment reduces intestinal colonization of genetically diverse UPEC isolates, while simultaneously treating UTI, without significantly disrupting the structural configuration of the gut microbiota. By selectively depleting the intestinal UPEC reservoir, mannosides could significantly reduce the rate of UTI and rUTI by eradicating the reservoir (47). Recently, a small-molecule compound, which is orally available, has been identified for the prevention and treatment of UTIs (125).
Galactosides
In line with this mannoside work, recent structure-based drug design efforts have resulted in the development of glycomimetic inhibitors of the FmlH adhesin from the Fml/F9 pilus involved in UPEC persistence during bladder inflammation (Fig. 3F) (33, 46). These high-affinity aryl galactosides are able to competitively block binding of the FmlH to its endogenous ligand in vitro, in in vivo murine models of UTI, and in ex vivo binding assays using healthy human kidney tissues (33). This study provides further evidence for the utility of the development of ligand mimetics for efficacious antivirulence strategies.
Pilicides
Pilicides are compounds capable of disrupting pilus biogenesis (126). A pipeline of pilicide development on a bicyclic 2-pyridone base structure has been established, and pilicides have been shown to reduce type 1, P, S, and Dr pilus biogenesis (126–128). Mechanistically, structural studies demonstrate that the pilicide interrupts pilus biogenesis by blocking the targeting of chaperone-subunit complexes to the usher’s N terminus (126, 129). Characterization of the effect of pilicides, specifically ec240, found altered gene expression of non-CUP pilus genes, including those involved in motility and iron homeostasis, suggesting a broader antivirulence effect beyond simply pilus biogenesis (127). Such compounds could work in concert with ligand mimetics like mannosides or galactosides and prove efficacious by targeting the formation of the pilus (126) as a whole while also targeting the specific function of the pilus type.
CONCLUSION
UTIs, encompassing a variety of infectious etiologies, represent a significant threat to human health, and work in the field has broadened our understanding of the multifactorial set of determinants that contribute to colonization, pathogenesis, and morbidity. Of great significance to human health is the burden of antibiotic resistance in urinary tract-colonizing microorganisms, which dictates that the field place an increased emphasis on the development of antivirulence strategies. This has led to the targeting of the bacterial machinery necessary for establishing colonization and infection and competitive inhibition of bacterial adhesins critical in host-pathogen interactions. Such work has harnessed the field’s knowledge of UTI pathogenesis and promises to deliver relief to those affected.
ACKNOWLEDGMENTS
We thank Tom Hannan, Karen Dodson, and Gary Eldridge for their critical feedback on this work, and Roger Klein for his contribution.
Contributor Information
Kevin O. Tamadonfar, Department of Molecular Microbiology, Washington University School of Medicine, St. Louis, MO 63110
Natalie S. Omattage, Department of Molecular Microbiology, Washington University School of Medicine, St. Louis, MO 63110
Caitlin N. Spaulding, Department of Molecular Microbiology, Washington University School of Medicine, St. Louis, MO 63110 Harvard University School of Public Health, Boston, MA 02115.
Scott J. Hultgren, Department of Molecular Microbiology, Washington University School of Medicine, St. Louis, MO 63110 Center for Women’s Infectious Disease Research, Washington University, School of Medicine, St. Louis, MO 63110.
Pascale Cossart, Institut Pasteur, Paris, France.
Craig R. Roy, Yale University School of Medicine, New Haven, Connecticut
Philippe Sansonetti, Institut Pasteur, Paris, France.
REFERENCES
- 1.Griebling TL. 2005. Urologic diseases in America project: trends in resource use for urinary tract infections in women. J Urol 173:1281–1287 10.1097/01.ju.0000155596.98780.82. [PubMed] [DOI] [PubMed] [Google Scholar]
- 2.Foxman B. 2014. Urinary tract infection syndromes: occurrence, recurrence, bacteriology, risk factors, and disease burden. Infect Dis Clin North Am 28:1–13 10.1016/j.idc.2013.09.003. [PubMed] [DOI] [PubMed] [Google Scholar]
- 3.Foxman B. 2002. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Am J Med 113(Suppl 1A):5–13 10.1016/S0002-9343(02)01054-9. [DOI] [PubMed] [Google Scholar]
- 4.Fowler CJ, Griffiths D, de Groat WC. 2008. The neural control of micturition. Nat Rev Neurosci 9:453–466 10.1038/nrn2401. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elbadawi A. 1996. Functional anatomy of the organs of micturition. Urol Clin North Am 23:177–210 10.1016/S0094-0143(05)70304-9. [DOI] [PubMed] [Google Scholar]
- 6.Macarak EJ, Howard PS. 1999. The role of collagen in bladder filling. Adv Exp Med Biol 462:215–223, 225–233 10.1007/978-1-4615-4737-2_17. [PubMed] [DOI] [PubMed] [Google Scholar]
- 7.Hickling DR, Sun TT, Wu XR. 2015. Anatomy and physiology of the urinary tract: relation to host defense and microbial infection. Microbiol Spectr 3:UTI-0016-2012 10.1128/microbiolspec.UTI-0016-2012. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khandelwal P, Abraham SN, Apodaca G. 2009. Cell biology and physiology of the uroepithelium. Am J Physiol Renal Physiol 297:F1477–F1501 10.1152/ajprenal.00327.2009. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kachar B, Liang F, Lins U, Ding M, Wu XR, Stoffler D, Aebi U, Sun TT. 1999. Three-dimensional analysis of the 16 nm urothelial plaque particle: luminal surface exposure, preferential head-to-head interaction, and hinge formation. J Mol Biol 285:595–608 10.1006/jmbi.1998.2304. [PubMed] [DOI] [PubMed] [Google Scholar]
- 10.Vergara J, Longley W, Robertson JD. 1969. A hexagonal arrangement of subunits in membrane of mouse urinary bladder. J Mol Biol 46:593–596 10.1016/0022-2836(69)90200-9. [DOI] [PubMed] [Google Scholar]
- 11.Hicks RM, Ketterer B. 1969. Hexagonal lattice of subunits in the thick luminal membrane of the rat urinary bladder. Nature 224:1304–1305 10.1038/2241304a0. [PubMed] [DOI] [PubMed] [Google Scholar]
- 12.Taylor KA, Robertson JD. 1984. Analysis of the three-dimensional structure of the urinary bladder epithelial cell membranes. J Ultrastruct Res 87:23–30 10.1016/S0022-5320(84)90113-8. [DOI] [PubMed] [Google Scholar]
- 13.Walz T, Häner M, Wu XR, Henn C, Engel A, Sun TT, Aebi U. 1995. Towards the molecular architecture of the asymmetric unit membrane of the mammalian urinary bladder epithelium: a closed “twisted ribbon” structure. J Mol Biol 248:887–900 10.1006/jmbi.1995.0269. [PubMed] [DOI] [PubMed] [Google Scholar]
- 14.Wu XR, Kong XP, Pellicer A, Kreibich G, Sun TT. 2009. Uroplakins in urothelial biology, function, and disease. Kidney Int 75:1153–1165 10.1038/ki.2009.73. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ho PL, Kurtova A, Chan KS. 2012. Normal and neoplastic urothelial stem cells: getting to the root of the problem. Nat Rev Urol 9:583–594 10.1038/nrurol.2012.142. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shin K, Lee J, Guo N, Kim J, Lim A, Qu L, Mysorekar IU, Beachy PA. 2011. Hedgehog/Wnt feedback supports regenerative proliferation of epithelial stem cells in bladder. Nature 472:110–114 10.1038/nature09851. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Grady F, Cattell WR. 1966. Kinetics of urinary tract infection. II. The bladder. Br J Urol 38:156–162 10.1111/j.1464-410X.1966.tb09694.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 18.Thomas-White K, Forster SC, Kumar N, Van Kuiken M, Putonti C, Stares MD, Hilt EE, Price TK, Wolfe AJ, Lawley TD. 2018. Culturing of female bladder bacteria reveals an interconnected urogenital microbiota. Nat Commun 9:1557 10.1038/s41467-018-03968-5. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ronald A. 2003. The etiology of urinary tract infection: traditional and emerging pathogens. Dis Mon 49:71–82 10.1067/mda.2003.8. [DOI] [PubMed] [Google Scholar]
- 20.Scholes D, Hooton TM, Roberts PL, Stapleton AE, Gupta K, Stamm WE. 2000. Risk factors for recurrent urinary tract infection in young women. J Infect Dis 182:1177–1182 10.1086/315827. [PubMed] [DOI] [PubMed] [Google Scholar]
- 21.Nicolle LE. 2015. Asymptomatic bacteriuria and bacterial interference. Microbiol Spectr 3:UTI-0001-2012 10.1128/microbiolspec.UTI-0001-2012. [PubMed] [DOI] [PubMed] [Google Scholar]
- 22.Sedor J, Mulholland SG. 1999. Hospital-acquired urinary tract infections associated with the indwelling catheter. Urol Clin North Am 26:821–828 10.1016/S0094-0143(05)70222-6. [DOI] [PubMed] [Google Scholar]
- 23.Edwards JR, Peterson KD, Mu Y, Banerjee S, Allen-Bridson K, Morrell G, Dudeck MA, Pollock DA, Horan TC. 2009. National Healthcare Safety Network (NHSN) report: data summary for 2006 through 2008, issued December 2009. Am J Infect Control 37:783–805 10.1016/j.ajic.2009.10.001. [PubMed] [DOI] [PubMed] [Google Scholar]
- 24.Flores-Mireles AL, Walker JN, Caparon M, Hultgren SJ. 2015. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol 13:269–284 10.1038/nrmicro3432. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kang CI, Kim J, Park DW, Kim BN, Ha US, Lee SJ, Yeo JK, Min SK, Lee H, Wie SH. 2018. Clinical practice guidelines for the antibiotic treatment of community-acquired urinary tract infections. Infect Chemother 50:67–100 10.3947/ic.2018.50.1.67. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mazzulli T. 2002. Resistance trends in urinary tract pathogens and impact on management. J Urol 168:1720–1722 10.1016/S0022-5347(05)64397-2. [PubMed] [DOI] [PubMed] [Google Scholar]
- 27.Public Health England. 2017. English Surveillance Programme for Antimicrobial Utilisation and Resistance (ESPAUR): Report 2017. Public Health England, London, United Kingdom. [Google Scholar]
- 28.Schreiber HL IV, Conover MS, Chou WC, Hibbing ME, Manson AL, Dodson KW, Hannan TJ, Roberts PL, Stapleton AE, Hooton TM, Livny J, Earl AM, Hultgren SJ. 2017. Bacterial virulence phenotypes of Escherichia coli and host susceptibility determine risk for urinary tract infections. Sci Transl Med 9:eaaf1283 10.1126/scitranslmed.aaf1283. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Brien VP, Hannan TJ, Yu L, Livny J, Roberson ED, Schwartz DJ, Souza S, Mendelsohn CL, Colonna M, Lewis AL, Hultgren SJ. 2016. A mucosal imprint left by prior Escherichia coli bladder infection sensitizes to recurrent disease. Nat Microbiol 2:16196 10.1038/nmicrobiol.2016.196. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guiton PS, Cusumano CK, Kline KA, Dodson KW, Han Z, Janetka JW, Henderson JP, Caparon MG, Hultgren SJ. 2012. Combinatorial small-molecule therapy prevents uropathogenic Escherichia coli catheter-associated urinary tract infections in mice. Antimicrob Agents Chemother 56:4738–4745 10.1128/AAC.00447-12. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spaulding CN, Hultgren SJ. 2016. Adhesive pili in UTI pathogenesis and drug development. Pathogens 5:30 10.3390/pathogens5010030. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwartz DJ, Kalas V, Pinkner JS, Chen SL, Spaulding CN, Dodson KW, Hultgren SJ. 2013. Positively selected FimH residues enhance virulence during urinary tract infection by altering FimH conformation. Proc Natl Acad Sci USA 110:15530–15537 10.1073/pnas.1315203110. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalas V, Hibbing ME, Maddirala AR, Chugani R, Pinkner JS, Mydock-McGrane LK, Conover MS, Janetka JW, Hultgren SJ. 2018. Structure-based discovery of glycomimetic FmlH ligands as inhibitors of bacterial adhesion during urinary tract infection. Proc Natl Acad Sci USA 115:E2819–E2828 10.1073/pnas.1720140115. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Han Z, Pinkner JS, Ford B, Obermann R, Nolan W, Wildman SA, Hobbs D, Ellenberger T, Cusumano CK, Hultgren SJ, Janetka JW. 2010. Structure-based drug design and optimization of mannoside bacterial FimH antagonists. J Med Chem 53:4779–4792 10.1021/jm100438s. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eto DS, Jones TA, Sundsbak JL, Mulvey MA. 2007. Integrin-mediated host cell invasion by type 1-piliated uropathogenic Escherichia coli. PLoS Pathog 3:e100 10.1371/journal.ppat.0030100. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou G, Mo WJ, Sebbel P, Min G, Neubert TA, Glockshuber R, Wu XR, Sun TT, Kong XP. 2001. Uroplakin Ia is the urothelial receptor for uropathogenic Escherichia coli: evidence from in vitro FimH binding. J Cell Sci 114:4095–4103. [DOI] [PubMed] [Google Scholar]
- 37.Martinez JJ, Mulvey MA, Schilling JD, Pinkner JS, Hultgren SJ. 2000. Type 1 pilus-mediated bacterial invasion of bladder epithelial cells. EMBO J 19:2803–2812 10.1093/emboj/19.12.2803. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martinez JJ, Hultgren SJ. 2002. Requirement of Rho-family GTPases in the invasion of type 1-piliated uropathogenic Escherichia coli. Cell Microbiol 4:19–28 10.1046/j.1462-5822.2002.00166.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 39.Hannan TJ, Mysorekar IU, Hung CS, Isaacson-Schmid ML, Hultgren SJ. 2010. Early severe inflammatory responses to uropathogenic E. coli predispose to chronic and recurrent urinary tract infection. PLoS Pathog 6:e1001042 10.1371/journal.ppat.1001042. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hannan TJ, Roberts PL, Riehl TE, van der Post S, Binkley JM, Schwartz DJ, Miyoshi H, Mack M, Schwendener RA, Hooton TM, Stappenbeck TS, Hansson GC, Stenson WF, Colonna M, Stapleton AE, Hultgren SJ. 2014. Inhibition of cyclooxygenase-2 prevents chronic and recurrent cystitis. EBioMedicine 1:46–57 10.1016/j.ebiom.2014.10.011. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Song J, Bishop BL, Li G, Grady R, Stapleton A, Abraham SN. 2009. TLR4-mediated expulsion of bacteria from infected bladder epithelial cells. Proc Natl Acad Sci USA 106:14966–14971 10.1073/pnas.0900527106. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nagamatsu K, Hannan TJ, Guest RL, Kostakioti M, Hadjifrangiskou M, Binkley J, Dodson K, Raivio TL, Hultgren SJ. 2015. Dysregulation of Escherichia coli α-hemolysin expression alters the course of acute and persistent urinary tract infection. Proc Natl Acad Sci USA 112:E871–E880 10.1073/pnas.1500374112. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Choi HW, Bowen SE, Miao Y, Chan CY, Miao EA, Abrink M, Moeser AJ, Abraham SN. 2016. Loss of bladder epithelium induced by cytolytic mast cell granules. Immunity 45:1258–1269 10.1016/j.immuni.2016.11.003. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mysorekar IU, Hultgren SJ. 2006. Mechanisms of uropathogenic Escherichia coli persistence and eradication from the urinary tract. Proc Natl Acad Sci USA 103:14170–14175 10.1073/pnas.0602136103. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chan CY, St John AL, Abraham SN. 2013. Mast cell interleukin-10 drives localized tolerance in chronic bladder infection. Immunity 38:349–359 10.1016/j.immuni.2012.10.019. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Conover MS, Ruer S, Taganna J, Kalas V, De Greve H, Pinkner JS, Dodson KW, Remaut H, Hultgren SJ. 2016. Inflammation-induced adhesin-receptor interaction provides a fitness advantage to uropathogenic E. coli during chronic infection. Cell Host Microbe 20:482–492 10.1016/j.chom.2016.08.013. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spaulding CN, Klein RD, Ruer S, Kau AL, Schreiber HL, Cusumano ZT, Dodson KW, Pinkner JS, Fremont DH, Janetka JW, Remaut H, Gordon JI, Hultgren SJ. 2017. Selective depletion of uropathogenic E. coli from the gut by a FimH antagonist. Nature 546:528–532 10.1038/nature22972. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moreno E, Andreu A, Pigrau C, Kuskowski MA, Johnson JR, Prats G. 2008. Relationship between Escherichia coli strains causing acute cystitis in women and the fecal E. coli population of the host. J Clin Microbiol 46:2529–2534 10.1128/JCM.00813-08. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen SL, Wu M, Henderson JP, Hooton TM, Hibbing ME, Hultgren SJ, Gordon JI. 2013. Genomic diversity and fitness of E. coli strains recovered from the intestinal and urinary tracts of women with recurrent urinary tract infection. Sci Transl Med 5:184ra60 10.1126/scitranslmed.3005497. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Russell CW, Fleming BA, Jost CA, Tran A, Stenquist AT, Wambaugh MA, Bronner MP, Mulvey MA. 2018. Context-dependent requirements for FimH and other canonical virulence factors in gut colonization by extraintestinal pathogenic Escherichia coli. Infect Immun 86:e00746-17 10.1128/IAI.00746-17. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sarkar S, Hutton ML, Vagenas D, Ruter R, Schüller S, Lyras D, Schembri MA, Totsika M. 2018. Intestinal colonization traits of pandemic multidrug-resistant Escherichia coli ST131. J Infect Dis 218:979–990 10.1093/infdis/jiy031. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hannan TJ, Totsika M, Mansfield KJ, Moore KH, Schembri MA, Hultgren SJ. 2012. Host-pathogen checkpoints and population bottlenecks in persistent and intracellular uropathogenic Escherichia coli bladder infection. FEMS Microbiol Rev 36:616–648 10.1111/j.1574-6976.2012.00339.x. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen SL, Hung CS, Pinkner JS, Walker JN, Cusumano CK, Li Z, Bouckaert J, Gordon JI, Hultgren SJ. 2009. Positive selection identifies an in vivo role for FimH during urinary tract infection in addition to mannose binding. Proc Natl Acad Sci USA 106:22439–22444 10.1073/pnas.0902179106. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Conover MS, Hadjifrangiskou M, Palermo JJ, Hibbing ME, Dodson KW, Hultgren SJ. 2016. Metabolic requirements of Escherichia coli in intracellular bacterial communities during urinary tract infection pathogenesis. mBio 7:e00104-16 10.1128/mBio.00104-16. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schwartz DJ, Chen SL, Hultgren SJ, Seed PC. 2011. Population dynamics and niche distribution of uropathogenic Escherichia coli during acute and chronic urinary tract infection. Infect Immun 79:4250–4259 10.1128/IAI.05339-11. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Duraiswamy S, Chee JLY, Chen S, Yang E, Lees K, Chen SL. 2018. Purification of intracellular bacterial communities during experimental urinary tract infection reveals an abundant and viable bacterial reservoir. Infect Immun 86:e00740-17 10.1128/IAI.00740-17. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Robino L, Scavone P, Araujo L, Algorta G, Zunino P, Vignoli R. 2013. Detection of intracellular bacterial communities in a child with Escherichia coli recurrent urinary tract infections. Pathog Dis 68:78–81 10.1111/2049-632X.12047. [PubMed] [DOI] [PubMed] [Google Scholar]
- 58.Rosen DA, Hooton TM, Stamm WE, Humphrey PA, Hultgren SJ. 2007. Detection of intracellular bacterial communities in human urinary tract infection. PLoS Med 4:e329 10.1371/journal.pmed.0040329. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Waksman G, Hultgren SJ. 2009. Structural biology of the chaperone-usher pathway of pilus biogenesis. Nat Rev Microbiol 7:765–774 10.1038/nrmicro2220. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wright KJ, Seed PC, Hultgren SJ. 2007. Development of intracellular bacterial communities of uropathogenic Escherichia coli depends on type 1 pili. Cell Microbiol 9:2230–2241 10.1111/j.1462-5822.2007.00952.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 61.Reigstad CS, Hultgren SJ, Gordon JI. 2007. Functional genomic studies of uropathogenic Escherichia coli and host urothelial cells when intracellular bacterial communities are assembled. J Biol Chem 282:21259–21267 10.1074/jbc.M611502200. [PubMed] [DOI] [PubMed] [Google Scholar]
- 62.Blango MG, Ott EM, Erman A, Veranic P, Mulvey MA. 2014. Forced resurgence and targeting of intracellular uropathogenic Escherichia coli reservoirs. PLoS One 9:e93327 10.1371/journal.pone.0093327. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gilbert NM, O’Brien VP, Lewis AL. 2017. Transient microbiota exposures activate dormant Escherichia coli infection in the bladder and drive severe outcomes of recurrent disease. PLoS Pathog 13:e1006238 10.1371/journal.ppat.1006238. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Eto DS, Sundsbak JL, Mulvey MA. 2006. Actin-gated intracellular growth and resurgence of uropathogenic Escherichia coli. Cell Microbiol 8:704–717 10.1111/j.1462-5822.2006.00691.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 65.Alteri CJ, Smith SN, Mobley HL. 2009. Fitness of Escherichia coli during urinary tract infection requires gluconeogenesis and the TCA cycle. PLoS Pathog 5:e1000448 10.1371/journal.ppat.1000448. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hull RA, Hull SI. 1997. Nutritional requirements for growth of uropathogenic Escherichia coli in human urine. Infect Immun 65:1960–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Henderson JP, Crowley JR, Pinkner JS, Walker JN, Tsukayama P, Stamm WE, Hooton TM, Hultgren SJ. 2009. Quantitative metabolomics reveals an epigenetic blueprint for iron acquisition in uropathogenic Escherichia coli. PLoS Pathog 5:e1000305 10.1371/journal.ppat.1000305. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.O’Brien VP, Hannan TJ, Nielsen HV, Hultgren SJ. 2016. Drug and vaccine development for the treatment and prevention of urinary tract infections. Microbiol Spectr 4:UTI-0013-2012. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Guyer DM, Radulovic S, Jones FE, Mobley HL. 2002. Sat, the secreted autotransporter toxin of uropathogenic Escherichia coli, is a vacuolating cytotoxin for bladder and kidney epithelial cells. Infect Immun 70:4539–4546 10.1128/IAI.70.8.4539-4546.2002. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lane MC, Alteri CJ, Smith SN, Mobley HL. 2007. Expression of flagella is coincident with uropathogenic Escherichia coli ascension to the upper urinary tract. Proc Natl Acad Sci USA 104:16669–16674 10.1073/pnas.0607898104. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hagan EC, Lloyd AL, Rasko DA, Faerber GJ, Mobley HL. 2010. Escherichia coli global gene expression in urine from women with urinary tract infection. PLoS Pathog 6:e1001187 10.1371/journal.ppat.1001187. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Greene SE, Hibbing ME, Janetka J, Chen SL, Hultgren SJ. 2015. Human urine decreases function and expression of type 1 pili in uropathogenic Escherichia coli. mBio 6:e00820-15 10.1128/mBio.00820-15. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ronald AR, Nicolle LE, Stamm E, Krieger J, Warren J, Schaeffer A, Naber KG, Hooton TM, Johnson J, Chambers S, Andriole V. 2001. Urinary tract infection in adults: research priorities and strategies. Int J Antimicrob Agents 17:343–348 10.1016/S0924-8579(01)00303-X. [DOI] [PubMed] [Google Scholar]
- 74.Melican K, Sandoval RM, Kader A, Josefsson L, Tanner GA, Molitoris BA, Richter-Dahlfors A. 2011. Uropathogenic Escherichia coli P and type 1 fimbriae act in synergy in a living host to facilitate renal colonization leading to nephron obstruction. PLoS Pathog 7:e1001298 10.1371/journal.ppat.1001298. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Roberts JA, Marklund BI, Ilver D, Haslam D, Kaack MB, Baskin G, Louis M, Möllby R, Winberg J, Normark S. 1994. The Gal(alpha 1-4)Gal-specific tip adhesin of Escherichia coli P-fimbriae is needed for pyelonephritis to occur in the normal urinary tract. Proc Natl Acad Sci USA 91:11889–11893 10.1073/pnas.91.25.11889. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Abraham SN, Sun D, Dale JB, Beachey EH. 1988. Conservation of the d-mannose-adhesion protein among type 1 fimbriated members of the family Enterobacteriaceae. Nature 336:682–684 10.1038/336682a0. [PubMed] [DOI] [PubMed] [Google Scholar]
- 77.Nuccio SP, Bäumler AJ. 2007. Evolution of the chaperone/usher assembly pathway: fimbrial classification goes Greek. Microbiol Mol Biol Rev 71:551–575 10.1128/MMBR.00014-07. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Subashchandrabose S, Hazen TH, Brumbaugh AR, Himpsl SD, Smith SN, Ernst RD, Rasko DA, Mobley HL. 2014. Host-specific induction of Escherichia coli fitness genes during human urinary tract infection. Proc Natl Acad Sci USA 111:18327–18332 10.1073/pnas.1415959112. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lim JK, Gunther NW IV, Zhao H, Johnson DE, Keay SK, Mobley HL. 1998. In vivo phase variation of Escherichia coli type 1 fimbrial genes in women with urinary tract infection. Infect Immun 66:3303–3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gunther NW IV, Lockatell V, Johnson DE, Mobley HL. 2001. In vivo dynamics of type 1 fimbria regulation in uropathogenic Escherichia coli during experimental urinary tract infection. Infect Immun 69:2838–2846 10.1128/IAI.69.5.2838-2846.2001. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hultgren SJ, Porter TN, Schaeffer AJ, Duncan JL. 1985. Role of type 1 pili and effects of phase variation on lower urinary tract infections produced by Escherichia coli. Infect Immun 50:370–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Forsyth VS, Armbruster CE, Smith SN, Pirani A, Springman AC, Walters MS, Nielubowicz GR, Himpsl SD, Snitkin ES, Mobley HLT. 2018. Rapid growth of uropathogenic Escherichia coli during human urinary tract infection. mBio 9:e00186-18 10.1128/mBio.00186-18. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Phan G, Remaut H, Wang T, Allen WJ, Pirker KF, Lebedev A, Henderson NS, Geibel S, Volkan E, Yan J, Kunze MB, Pinkner JS, Ford B, Kay CW, Li H, Hultgren SJ, Thanassi DG, Waksman G. 2011. Crystal structure of the FimD usher bound to its cognate FimC-FimH substrate. Nature 474:49–53 10.1038/nature10109. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Geibel S, Procko E, Hultgren SJ, Baker D, Waksman G. 2013. Structural and energetic basis of folded-protein transport by the FimD usher. Nature 496:243–246 10.1038/nature12007. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Farabella I, Pham T, Henderson NS, Geibel S, Phan G, Thanassi DG, Delcour AH, Waksman G, Topf M. 2014. Allosteric signalling in the outer membrane translocation domain of PapC usher. eLife 3:e03532 10.7554/eLife.03532. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mapingire OS, Henderson NS, Duret G, Thanassi DG, Delcour AH. 2009. Modulating effects of the plug, helix, and N- and C-terminal domains on channel properties of the PapC usher. J Biol Chem 284:36324–36333 10.1074/jbc.M109.055798. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pham T, Henderson NS, Werneburg GT, Thanassi DG, Delcour AH. 2015. Electrostatic networks control plug stabilization in the PapC usher. Mol Membr Biol 32:198–207 10.3109/09687688.2016.1160450. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Remaut H, Tang C, Henderson NS, Pinkner JS, Wang T, Hultgren SJ, Thanassi DG, Waksman G, Li H. 2008. Fiber formation across the bacterial outer membrane by the chaperone/usher pathway. Cell 133:640–652 10.1016/j.cell.2008.03.033. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Saulino ET, Thanassi DG, Pinkner JS, Hultgren SJ. 1998. Ramifications of kinetic partitioning on usher-mediated pilus biogenesis. EMBO J 17:2177–2185 10.1093/emboj/17.8.2177. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Volkan E, Ford BA, Pinkner JS, Dodson KW, Henderson NS, Thanassi DG, Waksman G, Hultgren SJ. 2012. Domain activities of PapC usher reveal the mechanism of action of an Escherichia coli molecular machine. Proc Natl Acad Sci USA 109:9563–9568 10.1073/pnas.1207085109. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Volkan E, Kalas V, Pinkner JS, Dodson KW, Henderson NS, Pham T, Waksman G, Delcour AH, Thanassi DG, Hultgren SJ. 2013. Molecular basis of usher pore gating in Escherichia coli pilus biogenesis. Proc Natl Acad Sci USA 110:20741–20746 10.1073/pnas.1320528110. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Spaulding CN, Schreiber HL IV, Zheng W, Dodson KW, Hazen JE, Conover MS, Wang F, Svenmarker P, Luna-Rico A, Francetic O, Andersson M, Hultgren S, Egelman EH. 2018. Functional role of the type 1 pilus rod structure in mediating host-pathogen interactions. eLife 7:e31662 10.7554/eLife.31662. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hospenthal MK, Zyla D, Costa TRD, Redzej A, Giese C, Lillington J, Glockshuber R, Waksman G. 2017. The cryoelectron microscopy structure of the type 1 chaperone-usher pilus rod. Structure 25:1829-1838.e4. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hospenthal MK, Redzej A, Dodson K, Ukleja M, Frenz B, Rodrigues C, Hultgren SJ, DiMaio F, Egelman EH, Waksman G. 2016. Structure of a chaperone-usher pilus reveals the molecular basis of rod uncoiling. Cell 164:269–278 10.1016/j.cell.2015.11.049. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kalas V, Pinkner JS, Hannan TJ, Hibbing ME, Dodson KW, Holehouse AS, Zhang H, Tolia NH, Gross ML, Pappu RV, Janetka J, Hultgren SJ. 2017. Evolutionary fine-tuning of conformational ensembles in FimH during host-pathogen interactions. Sci Adv 3:e1601944 10.1126/sciadv.1601944. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bahrani-Mougeot FK, Buckles EL, Lockatell CV, Hebel JR, Johnson DE, Tang CM, Donnenberg MS. 2002. Type 1 fimbriae and extracellular polysaccharides are preeminent uropathogenic Escherichia coli virulence determinants in the murine urinary tract. Mol Microbiol 45:1079–1093 10.1046/j.1365-2958.2002.03078.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 97.Buckles EL, Wang X, Lane MC, Lockatell CV, Johnson DE, Rasko DA, Mobley HL, Donnenberg MS. 2009. Role of the K2 capsule in Escherichia coli urinary tract infection and serum resistance. J Infect Dis 199:1689–1697 10.1086/598524. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Burns SM, Hull SI. 1999. Loss of resistance to ingestion and phagocytic killing by O– and K– mutants of a uropathogenic Escherichia coli O75:K5 strain. Infect Immun 67:3757–3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Anderson GG, Goller CC, Justice S, Hultgren SJ, Seed PC. 2010. Polysaccharide capsule and sialic acid-mediated regulation promote biofilm-like intracellular bacterial communities during cystitis. Infect Immun 78:963–975 10.1128/IAI.00925-09. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Heras B, Totsika M, Peters KM, Paxman JJ, Gee CL, Jarrott RJ, Perugini MA, Whitten AE, Schembri MA. 2014. The antigen 43 structure reveals a molecular Velcro-like mechanism of autotransporter-mediated bacterial clumping. Proc Natl Acad Sci USA 111:457–462 10.1073/pnas.1311592111. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ulett GC, Valle J, Beloin C, Sherlock O, Ghigo JM, Schembri MA. 2007. Functional analysis of antigen 43 in uropathogenic Escherichia coli reveals a role in long-term persistence in the urinary tract. Infect Immun 75:3233–3244 10.1128/IAI.01952-06. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Klein RD, Shu Q, Cusumano ZT, Nagamatsu K, Gualberto NC, Lynch AJL, Wu C, Wang W, Jain N, Pinkner JS, Amarasinghe GK, Hultgren SJ, Frieden C, Chapman MR. 2018. Structure-function analysis of the curli accessory protein CsgE defines surfaces essential for coordinating amyloid fiber formation. mBio 9:e01349-18 10.1128/mBio.01349-18. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kai-Larsen Y, Lüthje P, Chromek M, Peters V, Wang X, Holm A, Kádas L, Hedlund KO, Johansson J, Chapman MR, Jacobson SH, Römling U, Agerberth B, Brauner A. 2010. Uropathogenic Escherichia coli modulates immune responses and its curli fimbriae interact with the antimicrobial peptide LL-37. PLoS Pathog 6:e1001010 10.1371/journal.ppat.1001010. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ingersoll MA. 2017. Sex differences shape the response to infectious diseases. PLoS Pathog 13:e1006688 10.1371/journal.ppat.1006688. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lüthje P, Brauner H, Ramos NL, Ovregaard A, Gläser R, Hirschberg AL, Aspenström P, Brauner A. 2013. Estrogen supports urothelial defense mechanisms. Sci Transl Med 5:190ra80 10.1126/scitranslmed.3005574. [PubMed] [DOI] [PubMed] [Google Scholar]
- 106.Olson PD, Hruska KA, Hunstad DA. 2016. Androgens enhance male urinary tract infection severity in a new model. J Am Soc Nephrol 27:1625–1634 10.1681/ASN.2015030327. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zychlinsky Scharff A, Albert ML, Ingersoll MA. 2017. Urinary tract infection in a small animal model: transurethral catheterization of male and female mice. J Vis Exp 2017:e54432 10.3791/54432. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Flores-Mireles AL, Walker JN, Potretzke A, Schreiber HL IV, Pinkner JS, Bauman TM, Park AM, Desai A, Hultgren SJ, Caparon MG. 2016. Antibody-based therapy for enterococcal catheter-associated urinary tract infections. mBio 7:e01653-16 10.1128/mBio.01653-16. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rousseau M, Goh HMS, Holec S, Albert ML, Williams RB, Ingersoll MA, Kline KA. 2016. Bladder catheterization increases susceptibility to infection that can be prevented by prophylactic antibiotic treatment. JCI Insight 1:e88178 10.1172/jci.insight.88178. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Walker JN, Flores-Mireles AL, Pinkner CL, Schreiber HL IV, Joens MS, Park AM, Potretzke AM, Bauman TM, Pinkner JS, Fitzpatrick JAJ, Desai A, Caparon MG, Hultgren SJ. 2017. Catheterization alters bladder ecology to potentiate Staphylococcus aureus infection of the urinary tract. Proc Natl Acad Sci USA 114:E8721–E8730 10.1073/pnas.1707572114. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Armbruster CE, Forsyth-DeOrnellas V, Johnson AO, Smith SN, Zhao L, Wu W, Mobley HLT. 2017. Genome-wide transposon mutagenesis of Proteus mirabilis: essential genes, fitness factors for catheter-associated urinary tract infection, and the impact of polymicrobial infection on fitness requirements. PLoS Pathog 13:e1006434 10.1371/journal.ppat.1006434. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Center for Disease Dynamics, Economics & Policy. 2018. Drug Resistance Index. https://resistancemap.cddep.org/DRI.php. Accessed 26 July 2018.
- 113.Riley LW. 2014. Pandemic lineages of extraintestinal pathogenic Escherichia coli. Clin Microbiol Infect 20:380–390 10.1111/1469-0691.12646. [PubMed] [DOI] [PubMed] [Google Scholar]
- 114.Totsika M, Beatson SA, Sarkar S, Phan MD, Petty NK, Bachmann N, Szubert M, Sidjabat HE, Paterson DL, Upton M, Schembri MA. 2011. Insights into a multidrug resistant Escherichia coli pathogen of the globally disseminated ST131 lineage: genome analysis and virulence mechanisms. PLoS One 6:e26578 10.1371/journal.pone.0026578. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Schembri MA, Zakour NL, Phan MD, Forde BM, Stanton-Cook M, Beatson SA. 2015. Molecular characterization of the multidrug resistant Escherichia coli ST131 clone. Pathogens 4:422–430 10.3390/pathogens4030422. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Langermann S, Palaszynski S, Barnhart M, Auguste G, Pinkner JS, Burlein J, Barren P, Koenig S, Leath S, Jones CH, Hultgren SJ. 1997. Prevention of mucosal Escherichia coli infection by FimH-adhesin-based systemic vaccination. Science 276:607–611 10.1126/science.276.5312.607. [PubMed] [DOI] [PubMed] [Google Scholar]
- 117.Langermann S, Möllby R, Burlein JE, Palaszynski SR, Auguste CG, DeFusco A, Strouse R, Schenerman MA, Hultgren SJ, Pinkner JS, Winberg J, Guldevall L, Söderhäll M, Ishikawa K, Normark S, Koenig S. 2000. Vaccination with FimH adhesin protects cynomolgus monkeys from colonization and infection by uropathogenic Escherichia coli. J Infect Dis 181:774–778 10.1086/315258. [PubMed] [DOI] [PubMed] [Google Scholar]
- 118.Roberts JA, Kaack MB, Baskin G, Chapman MR, Hunstad DA, Pinkner JS, Hultgren SJ. 2004. Antibody responses and protection from pyelonephritis following vaccination with purified Escherichia coli PapDG protein. J Urol 171:1682–1685 10.1097/01.ju.0000116123.05160.43. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mike LA, Smith SN, Sumner CA, Eaton KA, Mobley HL. 2016. Siderophore vaccine conjugates protect against uropathogenic Escherichia coli urinary tract infection. Proc Natl Acad Sci USA 113:13468–13473 10.1073/pnas.1606324113. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mobley HL, Alteri CJ. 2015. Development of a vaccine against Escherichia coli urinary tract infections. Pathogens 5:1 10.3390/pathogens5010001. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mydock-McGrane L, Cusumano Z, Han Z, Binkley J, Kostakioti M, Hannan T, Pinkner JS, Klein R, Kalas V, Crowley J, Rath NP, Hultgren SJ, Janetka JW. 2016. Antivirulence C-mannosides as antibiotic-sparing, oral therapeutics for urinary tract infections. J Med Chem 59:9390–9408 10.1021/acs.jmedchem.6b00948. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cusumano CK, Pinkner JS, Han Z, Greene SE, Ford BA, Crowley JR, Henderson JP, Janetka JW, Hultgren SJ. 2011. Treatment and prevention of urinary tract infection with orally active FimH inhibitors. Sci Transl Med 3:109ra115 10.1126/scitranslmed.3003021. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Totsika M, Kostakioti M, Hannan TJ, Upton M, Beatson SA, Janetka JW, Hultgren SJ, Schembri MA. 2013. A FimH inhibitor prevents acute bladder infection and treats chronic cystitis caused by multidrug-resistant uropathogenic Escherichia coli ST131. J Infect Dis 208:921–928 10.1093/infdis/jit245. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Han Z, Pinkner JS, Ford B, Chorell E, Crowley JM, Cusumano CK, Campbell S, Henderson JP, Hultgren SJ, Janetka JW. 2012. Lead optimization studies on FimH antagonists: discovery of potent and orally bioavailable ortho-substituted biphenyl mannosides. J Med Chem 55:3945–3959 10.1021/jm300165m. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fimbrion Therapeutics, Inc. 6 December 2018. Fimbrion and GSK identify novel, antibiotic-sparing development candidate for urinary tract infections. PR Newswire.
- 126.Åberg V, Almqvist F. 2007. Pilicides-small molecules targeting bacterial virulence. Org Biomol Chem 5:1827–1834 10.1039/B702397A. [PubMed] [DOI] [PubMed] [Google Scholar]
- 127.Greene SE, Pinkner JS, Chorell E, Dodson KW, Shaffer CL, Conover MS, Livny J, Hadjifrangiskou M, Almqvist F, Hultgren SJ. 2014. Pilicide ec240 disrupts virulence circuits in uropathogenic Escherichia coli. mBio 5:e02038-14 10.1128/mBio.02038-14. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Piatek R, Zalewska-Piatek B, Dzierzbicka K, Makowiec S, Pilipczuk J, Szemiako K, Cyranka-Czaja A, Wojciechowski M. 2013. Pilicides inhibit the FGL chaperone/usher assisted biogenesis of the Dr fimbrial polyadhesin from uropathogenic Escherichia coli. BMC Microbiol 13:131 10.1186/1471-2180-13-131. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chorell E, Pinkner JS, Phan G, Edvinsson S, Buelens F, Remaut H, Waksman G, Hultgren SJ, Almqvist F. 2010. Design and synthesis of C-2 substituted thiazolo and dihydrothiazolo ring-fused 2-pyridones: pilicides with increased antivirulence activity. J Med Chem 53:5690–5695 10.1021/jm100470t. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sperandio V, Li CC, Kaper JB. 2002. Quorum-sensing Escherichia coli regulator A: a regulator of the LysR family involved in the regulation of the locus of enterocyte effacement pathogenicity island in enterohemorrhagic E. coli. Infect Immun 70:3085–3093 10.1128/IAI.70.6.3085-3093.2002. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Alteri CJ, Lindner JR, Reiss DJ, Smith SN, Mobley HL. 2011. The broadly conserved regulator PhoP links pathogen virulence and membrane potential in Escherichia coli. Mol Microbiol 82:145–163 10.1111/j.1365-2958.2011.07804.x. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Clarke MB, Hughes DT, Zhu C, Boedeker EC, Sperandio V. 2006. The QseC sensor kinase: a bacterial adrenergic receptor. Proc Natl Acad Sci USA 103:10420–10425 10.1073/pnas.0604343103. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Debnath I, Norton JP, Barber AE, Ott EM, Dhakal BK, Kulesus RR, Mulvey MA. 2013. The Cpx stress response system potentiates the fitness and virulence of uropathogenic Escherichia coli. Infect Immun 81:1450–1459 10.1128/IAI.01213-12. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Guckes KR, Kostakioti M, Breland EJ, Gu AP, Shaffer CL, Martinez CR III, Hultgren SJ, Hadjifrangiskou M. 2013. Strong cross-system interactions drive the activation of the QseB response regulator in the absence of its cognate sensor. Proc Natl Acad Sci USA 110:16592–16597 10.1073/pnas.1315320110. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kostakioti M, Hadjifrangiskou M, Cusumano CK, Hannan TJ, Janetka JW, Hultgren SJ. 2012. Distinguishing the contribution of type 1 pili from that of other QseB-misregulated factors when QseC is absent during urinary tract infection. Infect Immun 80:2826–2834 10.1128/IAI.00283-12. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Doye A, Mettouchi A, Bossis G, Clément R, Buisson-Touati C, Flatau G, Gagnoux L, Piechaczyk M, Boquet P, Lemichez E. 2002. CF1 exploits the ubiquitin-proteasome machinery to restrict Rho GTPase activation for bacterial host cell invasion. Cell 111:553–564 10.1016/S0092-8674(02)01132-7. [DOI] [PubMed] [Google Scholar]
- 137.Dhakal BK, Mulvey MA. 2012. The UPEC pore-forming toxin α-hemolysin triggers proteolysis of host proteins to disrupt cell adhesion, inflammatory, and survival pathways. Cell Host Microbe 11:58–69 10.1016/j.chom.2011.12.003. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Murthy AMV, Phan MD, Peters KM, Nhu NTK, Welch RA, Ulett GC, Schembri MA, Sweet MJ. 2018. Regulation of hemolysin in uropathogenic Escherichia coli fine-tunes killing of human macrophages. Virulence 9:967–980 10.1080/21505594.2018.1465786. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Norton JP, Mulvey MA. 2012. Toxin-antitoxin systems are important for niche-specific colonization and stress resistance of uropathogenic Escherichia coli. PLoS Pathog 8:e1002954 10.1371/journal.ppat.1002954. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Russell CW, Mulvey MA. 2015. The extraintestinal pathogenic Escherichia coli factor RqlI constrains the genotoxic effects of the RecQ-like helicase RqlH. PLoS Pathog 11:e1005317 10.1371/journal.ppat.1005317. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Russell CW, Richards AC, Chang AS, Mulvey MA. 2017. The rhomboid protease GlpG promotes the persistence of extraintestinal pathogenic Escherichia coli within the gut. Infect Immun 85:e00866-16 10.1128/IAI.00866-16. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Allsopp LP, Beloin C, Ulett GC, Valle J, Totsika M, Sherlock O, Ghigo JM, Schembri MA. 2012. Molecular characterization of UpaB and UpaC, two new autotransporter proteins of uropathogenic Escherichia coli CFT073. Infect Immun 80:321–332 10.1128/IAI.05322-11. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Vigil PD, Wiles TJ, Engstrom MD, Prasov L, Mulvey MA, Mobley HL. 2012. The repeat-in-toxin family member TosA mediates adherence of uropathogenic Escherichia coli and survival during bacteremia. Infect Immun 80:493–505 10.1128/IAI.05713-11. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Allsopp LP, Totsika M, Tree JJ, Ulett GC, Mabbett AN, Wells TJ, Kobe B, Beatson SA, Schembri MA. 2010. UpaH is a newly identified autotransporter protein that contributes to biofilm formation and bladder colonization by uropathogenic Escherichia coli CFT073. Infect Immun 78:1659–1669 10.1128/IAI.01010-09. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wiles TJ, Norton JP, Smith SN, Lewis AJ, Mobley HL, Casjens SR, Mulvey MA. 2013. A phyletically rare gene promotes the niche-specific fitness of an E. coli pathogen during bacteremia. PLoS Pathog 9:e1003175 10.1371/journal.ppat.1003175. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mulvey MA. 1998. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science 282:1494–1497. [PubMed] [DOI] [PubMed] [Google Scholar]


