Abstract
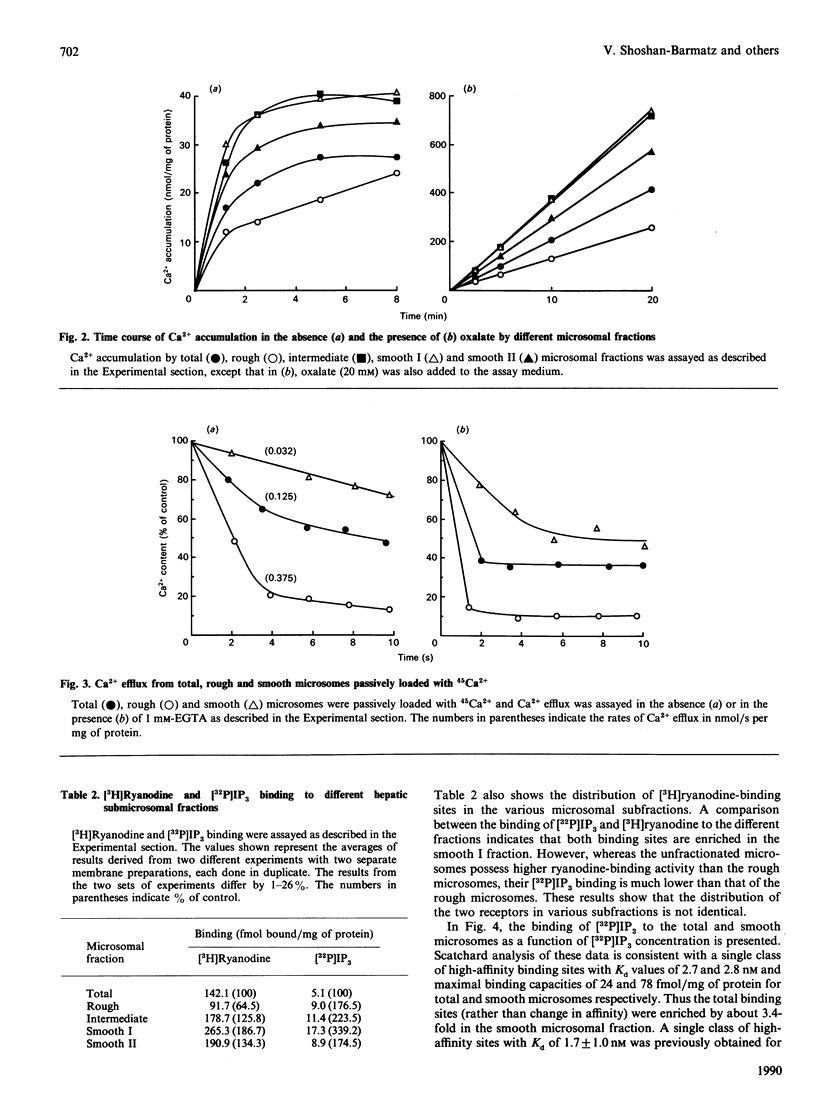
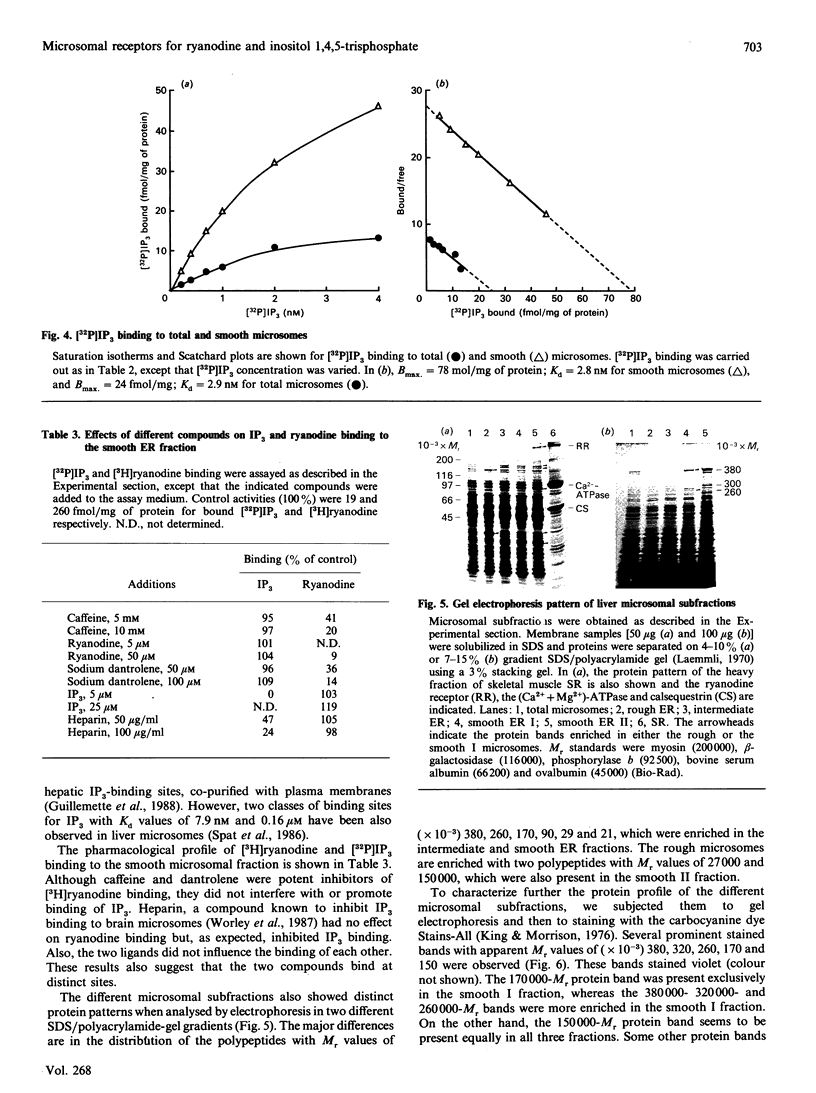
A light hepatic microsomal preparation was fractionated by sucrose-density centrifugation into one rough, one intermediate and two smooth fractions. The four fractions were characterized with respect to parameters relevant to Ca2+ sequestration. Ca2(+)-ATPase activity was similar in the rough, intermediate and smooth I fractions, but lower in the smooth II fraction. Ca2+ accumulation was the highest in the smooth I and intermediate fractions. On the other hand, Ca2+ efflux from the rough fraction was several-fold faster than from the smooth I fraction. All four subfractions exhibited specific binding sites for inositol 1,4,5-trisphosphate (IP3) and ryanodine; however, the receptors were especially enriched in the smooth I fraction. The total binding sites for ryanodine in that fraction exceeded the number of binding sites for IP3 by about 10-fold. The two receptors responded differently to pharmacological agents; caffeine and dantrolene strongly inhibited ryanodine binding but not IP3 binding, whereas heparin inhibited IP3 binding only. Thus the two receptors are distinct entities. The four fractions also showed distinct gel electrophoretic patterns. The use of two different SDS/polyacrylamide-gel gradients and two protein-staining methods revealed major differences in the distribution of the bands corresponding to Mr values of (x 10(-3) 380, 320, 260, 170, 90, 29 and 21. These proteins were enriched in the smooth fraction. The results indicate that the smooth I fraction might have special importance in stimulus-evoked Ca2(+)-release processes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beaufay H., Amar-Costesec A., Thinès-Sempoux D., Wibo M., Robbi M., Berthet J. Analytical study of microsomes and isolated subcellular membranes from rat liver. 3. Subfractionation of the microsomal fraction by isopycnic and differential centrifugation in density gradients. J Cell Biol. 1974 Apr;61(1):213–231. doi: 10.1083/jcb.61.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti A., Fulceri R., Romani A., Comporti M. MgATP-dependent glucose 6-phosphate-stimulated Ca2+ accumulation in liver microsomal fractions. Effects of inositol 1,4,5-trisphosphate and GTP. J Biol Chem. 1988 Mar 5;263(7):3466–3473. [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Bond M., Vadasz G., Somlyo A. V., Somlyo A. P. Subcellular calcium and magnesium mobilization in rat liver stimulated in vivo with vasopressin and glucagon. J Biol Chem. 1987 Nov 15;262(32):15630–15636. [PubMed] [Google Scholar]
- Cala S. E., Jones L. R. Rapid purification of calsequestrin from cardiac and skeletal muscle sarcoplasmic reticulum vesicles by Ca2+-dependent elution from phenyl-sepharose. J Biol Chem. 1983 Oct 10;258(19):11932–11936. [PubMed] [Google Scholar]
- Codina J., Kimura S., Kraus-Friedmann N. Demonstration of the presence of G-proteins in hepatic microsomal fraction. Biochem Biophys Res Commun. 1988 Jan 29;150(2):848–852. doi: 10.1016/0006-291x(88)90469-x. [DOI] [PubMed] [Google Scholar]
- Dallner G., Ernster L. Subfractionation and composition of microsomal membranes: a review. J Histochem Cytochem. 1968 Oct;16(10):611–632. doi: 10.1177/16.10.611. [DOI] [PubMed] [Google Scholar]
- Depierre J. W., Dallner G. Structural aspects of the membrane of the endoplasmic reticulum. Biochim Biophys Acta. 1975 Dec 29;415(4):411–472. doi: 10.1016/0304-4157(75)90006-4. [DOI] [PubMed] [Google Scholar]
- Fleischer S., Kervina M. Subcellular fractionation of rat liver. Methods Enzymol. 1974;31:6–41. doi: 10.1016/0076-6879(74)31005-1. [DOI] [PubMed] [Google Scholar]
- Fleschner C. R., Kraus-Friedmann N. The effect of Mg2+ on hepatic microsomal Ca2+ and Sr2+ transport. Eur J Biochem. 1986 Jan 15;154(2):313–320. doi: 10.1111/j.1432-1033.1986.tb09399.x. [DOI] [PubMed] [Google Scholar]
- Furuichi T., Yoshikawa S., Miyawaki A., Wada K., Maeda N., Mikoshiba K. Primary structure and functional expression of the inositol 1,4,5-trisphosphate-binding protein P400. Nature. 1989 Nov 2;342(6245):32–38. doi: 10.1038/342032a0. [DOI] [PubMed] [Google Scholar]
- Gierow P., Jergil B. Heterogeneity of smooth endoplasmic reticulum from rat liver studied by two-phase partitioning. Biochem J. 1989 Aug 15;262(1):55–61. doi: 10.1042/bj2620055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godelaine D., Beaufay H., Wibo M., Ravoet A. M. Alteration of membrane barrier in stripped rough microsomes from rat liver on incubation with GTP: its relevance to the stimulation by this nucleotide of the dolichol pathway for protein glycosylation. J Cell Biol. 1983 Aug;97(2):340–350. doi: 10.1083/jcb.97.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemette G., Balla T., Baukal A. J., Catt K. J. Characterization of inositol 1,4,5-trisphosphate receptors and calcium mobilization in a hepatic plasma membrane fraction. J Biol Chem. 1988 Apr 5;263(10):4541–4548. [PubMed] [Google Scholar]
- Inui M., Saito A., Fleischer S. Isolation of the ryanodine receptor from cardiac sarcoplasmic reticulum and identity with the feet structures. J Biol Chem. 1987 Nov 15;262(32):15637–15642. [PubMed] [Google Scholar]
- Jungermann K., Katz N. Functional hepatocellular heterogeneity. Hepatology. 1982 May-Jun;2(3):385–395. doi: 10.1002/hep.1840020316. [DOI] [PubMed] [Google Scholar]
- King L. E., Jr, Morrison M. The visualization of human erythrocyte membrane proteins and glycoproteins in SDS polyacrylamide gels employing a single staining procedure. Anal Biochem. 1976 Mar;71(1):223–230. doi: 10.1016/0003-2697(76)90031-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lai F. A., Meissner G. The muscle ryanodine receptor and its intrinsic Ca2+ channel activity. J Bioenerg Biomembr. 1989 Apr;21(2):227–246. doi: 10.1007/BF00812070. [DOI] [PubMed] [Google Scholar]
- Lewis J. A., Tata J. R. A rapidly sedimenting fraction of rat liver endoplasmic reticulum. J Cell Sci. 1973 Sep;13(2):447–459. doi: 10.1242/jcs.13.2.447. [DOI] [PubMed] [Google Scholar]
- Lindros K. O., Penttilä K. E. Digitonin-collagenase perfusion for efficient separation of periportal or perivenous hepatocytes. Biochem J. 1985 Jun 15;228(3):757–760. doi: 10.1042/bj2280757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks A. R., Tempst P., Hwang K. S., Taubman M. B., Inui M., Chadwick C., Fleischer S., Nadal-Ginard B. Molecular cloning and characterization of the ryanodine receptor/junctional channel complex cDNA from skeletal muscle sarcoplasmic reticulum. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8683–8687. doi: 10.1073/pnas.86.22.8683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignery G. A., Südhof T. C., Takei K., De Camilli P. Putative receptor for inositol 1,4,5-trisphosphate similar to ryanodine receptor. Nature. 1989 Nov 9;342(6246):192–195. doi: 10.1038/342192a0. [DOI] [PubMed] [Google Scholar]
- Moore L., Chen T., Knapp H. R., Jr, Landon E. J. Energy-dependent calcium sequestration activity in rat liver microsomes. J Biol Chem. 1975 Jun 25;250(12):4562–4568. [PubMed] [Google Scholar]
- Nilsson R., Peterson E., Dallner G. Permeability of microsomal membranes isolated from rat liver. J Cell Biol. 1973 Mar;56(3):762–776. doi: 10.1083/jcb.56.3.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paiement J., Rindress D., Smith C. E., Poliquin L., Bergeron J. J. Properties of a GTP sensitive microdomain in rough microsomes. Biochim Biophys Acta. 1987 Mar 26;898(1):6–22. doi: 10.1016/0005-2736(87)90105-2. [DOI] [PubMed] [Google Scholar]
- Pryme I. F. Compartmentation of the rough endoplasmic reticulum. Mol Cell Biochem. 1986 Jun;71(1):3–18. doi: 10.1007/BF00219323. [DOI] [PubMed] [Google Scholar]
- Scharschmidt B. F., Keeffe E. B., Blankenship N. M., Ockner R. K. Validation of a recording spectrophotometric method for measurement of membrane-associated Mg- and NaK-ATPase activity. J Lab Clin Med. 1979 May;93(5):790–799. [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Spät A., Fabiato A., Rubin R. P. Binding of inositol trisphosphate by a liver microsomal fraction. Biochem J. 1986 Feb 1;233(3):929–932. doi: 10.1042/bj2330929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streb H., Irvine R. F., Berridge M. J., Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983 Nov 3;306(5938):67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- Supattapone S., Worley P. F., Baraban J. M., Snyder S. H. Solubilization, purification, and characterization of an inositol trisphosphate receptor. J Biol Chem. 1988 Jan 25;263(3):1530–1534. [PubMed] [Google Scholar]
- Takeshima H., Nishimura S., Matsumoto T., Ishida H., Kangawa K., Minamino N., Matsuo H., Ueda M., Hanaoka M., Hirose T. Primary structure and expression from complementary DNA of skeletal muscle ryanodine receptor. Nature. 1989 Jun 8;339(6224):439–445. doi: 10.1038/339439a0. [DOI] [PubMed] [Google Scholar]
- Taylor C. W., Putney J. W., Jr Size of the inositol 1,4,5-trisphosphate-sensitive calcium pool in guinea-pig hepatocytes. Biochem J. 1985 Dec 1;232(2):435–438. doi: 10.1042/bj2320435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanstapel F., Pua K., Blanckaert N. Assay of mannose-6-phosphatase in untreated and detergent-disrupted rat-liver microsomes for assessment of integrity of microsomal preparations. Eur J Biochem. 1986 Apr 1;156(1):73–77. doi: 10.1111/j.1432-1033.1986.tb09550.x. [DOI] [PubMed] [Google Scholar]
- Worley P. F., Baraban J. M., Supattapone S., Wilson V. S., Snyder S. H. Characterization of inositol trisphosphate receptor binding in brain. Regulation by pH and calcium. J Biol Chem. 1987 Sep 5;262(25):12132–12136. [PubMed] [Google Scholar]