Abstract
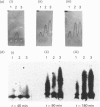
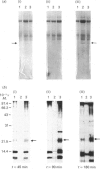
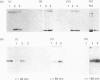
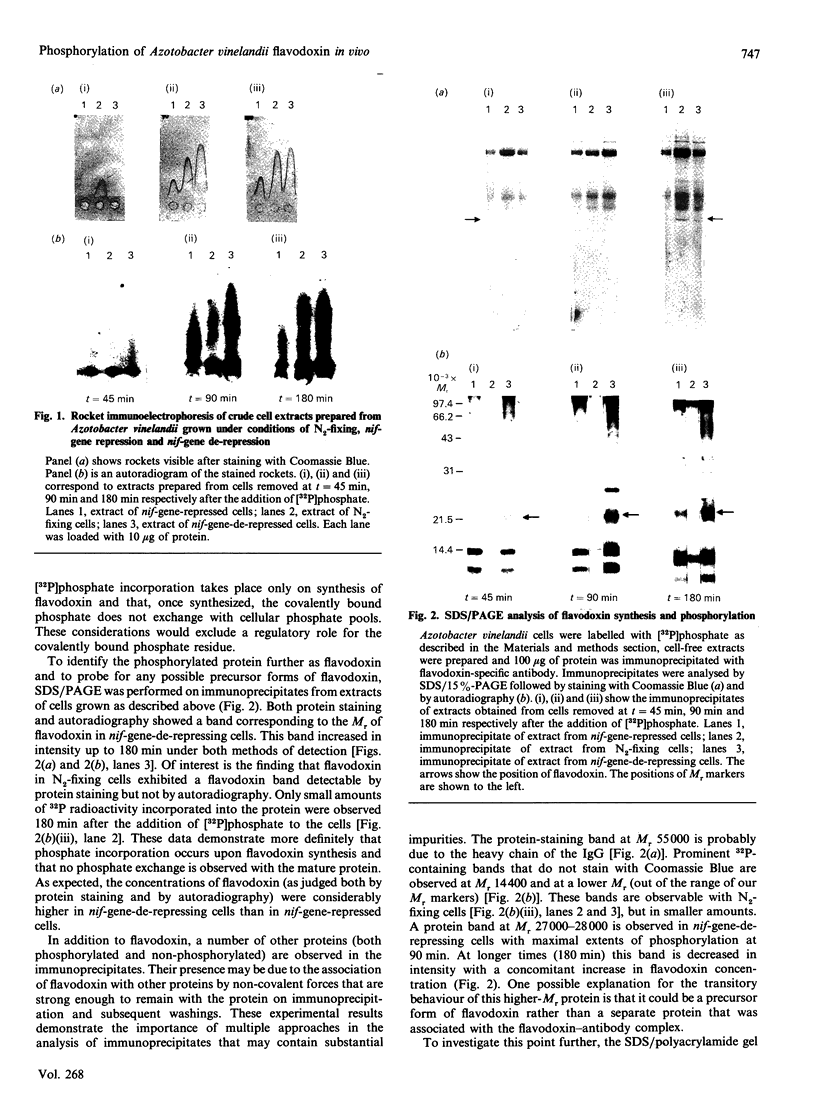
Previous studies have shown the flavodoxin from Azotobacter vinelandii (strain OP, Berkeley) to contain a covalently bound disubstituted phosphate residue [Edmondson & James (1979) Proc. Natl. Acad. Sci. U.S.A. 76, 3786-3789]. Phosphorylation of the protein in vivo was investigated by the addition of [32P]phosphate to cells grown under N2-fixing conditions, under conditions of nif-gene repression and under conditions of nif-gene de-repression. Rocket immunoelectrophoresis of cell extracts showed an approx. 5-fold decrease in the concentration of flavodoxin expressed in cells grown in the presence of NH4+ as compared with those grown under N2-fixing conditions. A similar increase in flavodoxin concentration was observed on nif-gene de-repression. Incorporation of [32P]phosphate occurs only into newly synthesized flavodoxin, as observed on SDS/PAGE of immunoprecipitates of cell extracts. Western blots demonstrated no observable precursor forms of flavodoxin. These data provide conclusive evidence for the phosphorylation of Azotobacter strain OP flavodoxin in vivo and suggest that the covalently bound phosphate residue does not exchange with cellular phosphate pools. Thus the role of this phosphodiester cross-link is proposed to be structural rather than regulatory.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benemann J. R., Yoch D. C., Valentine R. C., Arnon D. I. The electron transport system in nitrogen fixation by Azotobacter. I. Azotoflavin as an electron carrier. Proc Natl Acad Sci U S A. 1969 Nov;64(3):1079–1086. doi: 10.1073/pnas.64.3.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett L. T., Jacobson M. R., Dean D. R. Isolation, sequencing, and mutagenesis of the nifF gene encoding flavodoxin from Azotobacter vinelandii. J Biol Chem. 1988 Jan 25;263(3):1364–1369. [PubMed] [Google Scholar]
- Bulen W. A., LeComte J. R. Nitrogenase complex and its components. Methods Enzymol. 1972;24:456–470. doi: 10.1016/0076-6879(72)24091-5. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Cusanovich M. A., Edmondson D. E. The isolation and characterization of Rhodospirillum rubrum flavodoxin. Biochem Biophys Res Commun. 1971 Oct 15;45(2):327–336. doi: 10.1016/0006-291x(71)90822-9. [DOI] [PubMed] [Google Scholar]
- Edmondson D. E., James T. L. Covalently bound non-coenzyme phosphorus residues in flavoproteins: 31P nuclear magnetic resonance studies of Azotobacter flavodoxin. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3786–3789. doi: 10.1073/pnas.76.8.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. L., London R. E., Rajagopalan K. V. Covalently bound phosphate residues in bovine milk xanthine oxidase and in glucose oxidase from Aspergillus niger: a reevaluation. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6493–6497. doi: 10.1073/pnas.86.17.6493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klugkist J., Haaker H., Veeger C. Studies on the mechanism of electron transport to nitrogenase in Azotobacter vinelandii. Eur J Biochem. 1986 Feb 17;155(1):41–46. doi: 10.1111/j.1432-1033.1986.tb09456.x. [DOI] [PubMed] [Google Scholar]
- Klugkist J., Haaker H., Wassink H., Veeger C. The catalytic activity of nitrogenase in intact Azotobacter vinelandii cells. Eur J Biochem. 1985 Feb 1;146(3):509–515. doi: 10.1111/j.1432-1033.1985.tb08681.x. [DOI] [PubMed] [Google Scholar]
- Klugkist J., Voorberg J., Haaker H., Veeger C. Characterization of three different flavodoxins from Azotobacter vinelandii. Eur J Biochem. 1986 Feb 17;155(1):33–40. doi: 10.1111/j.1432-1033.1986.tb09455.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mayhew S. G., Strating M. J. Properties of immobilized flavodoxin from Peptostreptococcus elsdenii. An affinity ligand for the purification of riboflavin 5'-phosphate (FMN) and its analogues. Eur J Biochem. 1975 Nov 15;59(2):539–544. doi: 10.1111/j.1432-1033.1975.tb02480.x. [DOI] [PubMed] [Google Scholar]
- Nieva-Gómez D., Roberts G. P., Klevickis S., Brill W. J. Electron transport to nitrogenase in Klebsiella pneumoniae. Proc Natl Acad Sci U S A. 1980 May;77(5):2555–2558. doi: 10.1073/pnas.77.5.2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordlund S., Ludden P. W. Incorporation of adenine into the modifying group of inactive iron protein of nitrogenase from Rhodospirillum rubrum. Biochem J. 1983 Mar 1;209(3):881–884. doi: 10.1042/bj2090881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simondsen R. P., Tollin G. Structure-function relations in flavodoxins. Mol Cell Biochem. 1980 Dec 10;33(1-2):13–24. doi: 10.1007/BF00224568. [DOI] [PubMed] [Google Scholar]
- Tollin G., Brown K., De Francesco R., Edmondson D. E. Flavodoxin-cytochrome c interactions: circular dichroism and nuclear magnetic resonance studies. Biochemistry. 1987 Aug 11;26(16):5042–5048. doi: 10.1021/bi00390a024. [DOI] [PubMed] [Google Scholar]
- Yoch D. C. Electron transport carriers involved in nitrogen fixation by the coliform, Klebsiella pneumoniae. J Gen Microbiol. 1974 Jul;83(0):153–164. doi: 10.1099/00221287-83-1-153. [DOI] [PubMed] [Google Scholar]