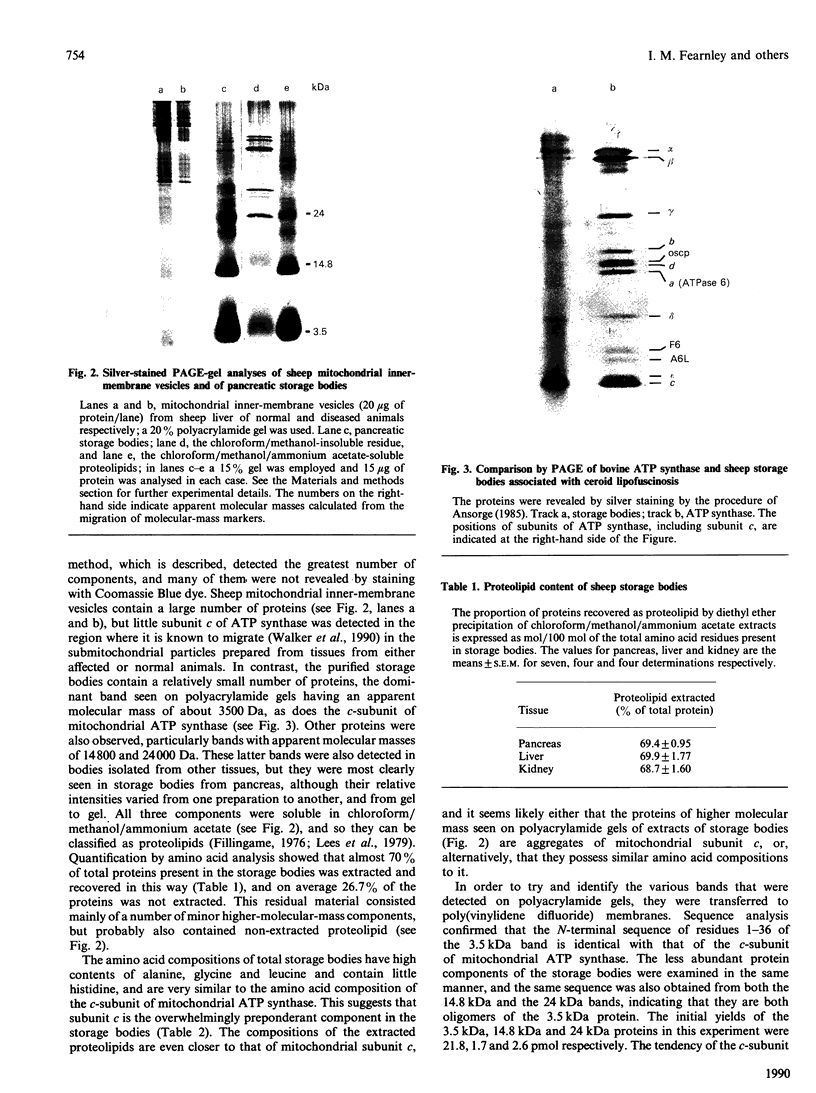
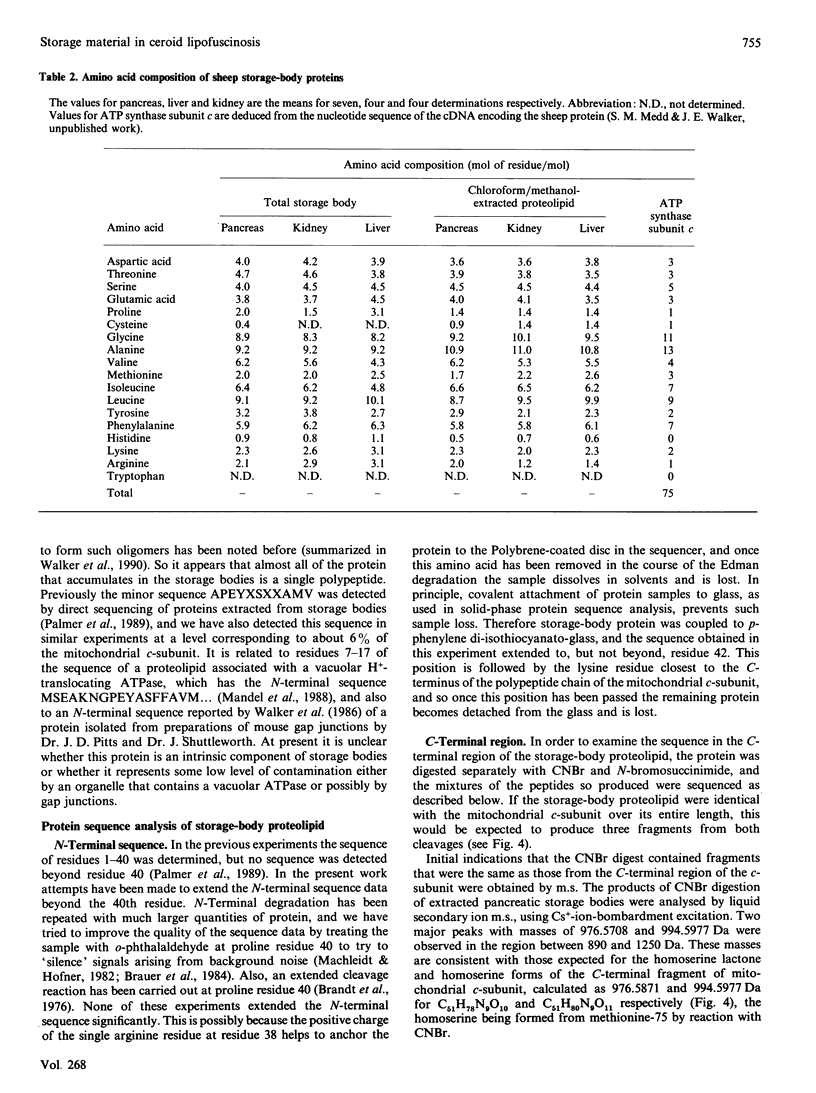
Abstract
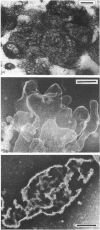
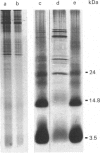
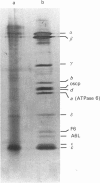
The ceroid lipofuscinoses are a group of neurodegenerative lysosomal storage diseases of children and animals that are recessively inherited. In diseased individuals fluorescent storage bodies accumulate in a wide variety of cells, including neurons. Previous studies of these bodies isolated from tissues of affected sheep confirmed that the storage occurs in lysosomes, and showed that the storage body is mostly made of a single protein with an apparent molecular mass of 3500 Da with an N-terminal amino acid sequence that is the same as residues 1-40 of the c-subunit (or dicyclohexylcarbodi-imide-reactive proteolipid) of mitochondrial ATP synthase. In the present work we have shown by direct analysis that the stored protein is identical in sequence with the entire c-subunit of mitochondrial ATP synthase, a very hydrophobic protein of 75 amino acid residues. As far as can be detected by the Edman degradation, the stored protein appears not to have been subject to any post-translational modification other than the correct removal of the mitochondrial import sequences that have been shown in other experiments to be present at the N-terminal of its two different precursors. No other protein accumulates in the storage bodies to any significant extent. Taken with studies of the cDNAs for the c-subunit in normal and diseased sheep, these results indicate that the material that is stored in lysosomes of diseased animals has probably entered mitochondria and has been subjected to the proteolytic processing that is associated with mitochondrial import. This implies that the defect that leads to the lysosomal accumulation concerns the degradative pathway of the c-subunit of ATP synthase. An alternative, but less likely, hypothesis is that for some unknown reason the precursors of subunit c are being directly mis-targeted to lysosomes, where they become processed to yield a protein identical with the protein that is normally found in the mitochondrial ATP synthase assembly, and which then accumulates.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amons R. Vapor-phase modification of sulfhydryl groups in proteins. FEBS Lett. 1987 Feb 9;212(1):68–72. doi: 10.1016/0014-5793(87)81558-2. [DOI] [PubMed] [Google Scholar]
- Ansorge W. Fast and sensitive detection of protein and DNA bands by treatment with potassium permanganate. J Biochem Biophys Methods. 1985 May;11(1):13–20. doi: 10.1016/0165-022x(85)90037-5. [DOI] [PubMed] [Google Scholar]
- Bowman B. J., Allen R., Wechser M. A., Bowman E. J. Isolation of genes encoding the Neurospora vacuolar ATPase. Analysis of vma-2 encoding the 57-kDa polypeptide and comparison to vma-1. J Biol Chem. 1988 Oct 5;263(28):14002–14007. [PubMed] [Google Scholar]
- Bowman E. J., Tenney K., Bowman B. J. Isolation of genes encoding the Neurospora vacuolar ATPase. Analysis of vma-1 encoding the 67-kDa subunit reveals homology to other ATPases. J Biol Chem. 1988 Oct 5;263(28):13994–14001. [PubMed] [Google Scholar]
- Brandt W. F., Edman P., Henschen A., Von Holt C. Abnormal behaviour of proline in the isothiocyanate degradation. Hoppe Seylers Z Physiol Chem. 1976 Nov;357(11):1505–1508. doi: 10.1515/bchm2.1976.357.2.1505. [DOI] [PubMed] [Google Scholar]
- Brauer A. W., Oman C. L., Margolies M. N. Use of o-phthalaldehyde to reduce background during automated Edman degradation. Anal Biochem. 1984 Feb;137(1):134–142. doi: 10.1016/0003-2697(84)90359-2. [DOI] [PubMed] [Google Scholar]
- Chio K. S., Reiss U., Fletcher B., Tappel A. L. Peroxidation of subcellular organelles: formation of lipofuscinlike fluorescent pigments. Science. 1969 Dec 19;166(3912):1535–1536. doi: 10.1126/science.166.3912.1535. [DOI] [PubMed] [Google Scholar]
- Dyer M. R., Gay N. J., Walker J. E. DNA sequences of a bovine gene and of two related pseudogenes for the proteolipid subunit of mitochondrial ATP synthase. Biochem J. 1989 May 15;260(1):249–258. doi: 10.1042/bj2600249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M. Proteolipides, a new type of tissue lipoproteins; their isolation from brain. J Biol Chem. 1951 Aug;191(2):807–817. [PubMed] [Google Scholar]
- Farrell L. B., Nagley P. Human liver cDNA clones encoding proteolipid subunit 9 of the mitochondrial ATPase complex. Biochem Biophys Res Commun. 1987 May 14;144(3):1257–1264. doi: 10.1016/0006-291x(87)91446-x. [DOI] [PubMed] [Google Scholar]
- Fearnley I. M., Walker J. E. Initiation codons in mammalian mitochondria: differences in genetic code in the organelle. Biochemistry. 1987 Dec 15;26(25):8247–8251. doi: 10.1021/bi00399a034. [DOI] [PubMed] [Google Scholar]
- Fearnley I. M., Walker J. E. Two overlapping genes in bovine mitochondrial DNA encode membrane components of ATP synthase. EMBO J. 1986 Aug;5(8):2003–2008. doi: 10.1002/j.1460-2075.1986.tb04456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillingame R. H. Purification of the carbodiimide-reactive protein component of the ATP energy-transducing system of Escherichia coli. J Biol Chem. 1976 Nov 10;251(21):6630–6637. [PubMed] [Google Scholar]
- Gay N. J., Walker J. E. Two genes encoding the bovine mitochondrial ATP synthase proteolipid specify precursors with different import sequences and are expressed in a tissue-specific manner. EMBO J. 1985 Dec 16;4(13A):3519–3524. doi: 10.1002/j.1460-2075.1985.tb04111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graydon R. J., Jolly R. D. Ceroid-lipofuscinosis (Batten's disease). Sequential electrophysiologic and pathologic changes in the retina of the ovine model. Invest Ophthalmol Vis Sci. 1984 Mar;25(3):294–301. [PubMed] [Google Scholar]
- Horn M. J., Laursen R. A. Solid-phase edman degradation: attachment of carboxyl-terminal homoserine peptides to an insoluble resin. FEBS Lett. 1973 Nov 1;36(3):285–288. doi: 10.1016/0014-5793(73)80392-8. [DOI] [PubMed] [Google Scholar]
- Jolly R. D., Shimada A., Craig A. S., Kirkland K. B., Palmer D. N. Ovine ceroid-lipofuscinosis II: Pathologic changes interpreted in light of biochemical observations. Am J Med Genet Suppl. 1988;5:159–170. doi: 10.1002/ajmg.1320310619. [DOI] [PubMed] [Google Scholar]
- Jolly R. D., Shimada A., Dopfmer I., Slack P. M., Birtles M. J., Palmer D. N. Ceroid-lipofuscinosis (Batten's disease): pathogenesis and sequential neuropathological changes in the ovine model. Neuropathol Appl Neurobiol. 1989 Jul-Aug;15(4):371–383. doi: 10.1111/j.1365-2990.1989.tb01236.x. [DOI] [PubMed] [Google Scholar]
- Koobs D. H., Schultz R. L., Jutzy R. V. The origin of lipofuscin and possible consequences to the myocardium. Arch Pathol Lab Med. 1978 Feb;102(2):66–68. [PubMed] [Google Scholar]
- Lees M. B., Sakura J. D., Sapirstein V. S., Curatolo W. Structure and function of proteolipids in myelin and non-myelin membranes. Biochim Biophys Acta. 1979 Aug 20;559(2-3):209–230. doi: 10.1016/0304-4157(79)90002-9. [DOI] [PubMed] [Google Scholar]
- Mandel M., Moriyama Y., Hulmes J. D., Pan Y. C., Nelson H., Nelson N. cDNA sequence encoding the 16-kDa proteolipid of chromaffin granules implies gene duplication in the evolution of H+-ATPases. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5521–5524. doi: 10.1073/pnas.85.15.5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolson M. F., Ouellette B. F., Filion M., Poole R. J. cDNA sequence and homologies of the "57-kDa" nucleotide-binding subunit of the vacuolar ATPase from Arabidopsis. J Biol Chem. 1988 Dec 5;263(34):17987–17994. [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Mayhew I. G., Jolly R. D., Pickett B. T., Slack P. M. Ceroid-lipofuscinosis (Batten's disease): pathogenesis of blindness in the ovine model. Neuropathol Appl Neurobiol. 1985 Jul-Aug;11(4):273–290. doi: 10.1111/j.1365-2990.1985.tb00025.x. [DOI] [PubMed] [Google Scholar]
- Nedergaard J., Cannon B. Overview--preparation and properties of mitochondria from different sources. Methods Enzymol. 1979;55:3–28. doi: 10.1016/0076-6879(79)55003-4. [DOI] [PubMed] [Google Scholar]
- Palmer D. N., Barns G., Husbands D. R., Jolly R. D. Ceroid lipofuscinosis in sheep. II. The major component of the lipopigment in liver, kidney, pancreas, and brain is low molecular weight protein. J Biol Chem. 1986 Feb 5;261(4):1773–1777. [PubMed] [Google Scholar]
- Palmer D. N., Husbands D. R., Winter P. J., Blunt J. W., Jolly R. D. Ceroid lipofuscinosis in sheep. I. Bis(monoacylglycero)phosphate, dolichol, ubiquinone, phospholipids, fatty acids, and fluorescence in liver lipopigment lipids. J Biol Chem. 1986 Feb 5;261(4):1766–1772. [PubMed] [Google Scholar]
- Palmer D. N., Martinus R. D., Barns G., Reeves R. D., Jolly R. D. Ovine ceroid-lipofuscinosis. I: Lipopigment composition is indicative of a lysosomal proteinosis. Am J Med Genet Suppl. 1988;5:141–158. doi: 10.1002/ajmg.1320310618. [DOI] [PubMed] [Google Scholar]
- Palmer D. N., Martinus R. D., Cooper S. M., Midwinter G. G., Reid J. C., Jolly R. D. Ovine ceroid lipofuscinosis. The major lipopigment protein and the lipid-binding subunit of mitochondrial ATP synthase have the same NH2-terminal sequence. J Biol Chem. 1989 Apr 5;264(10):5736–5740. [PubMed] [Google Scholar]
- Pongor S., Ulrich P. C., Bencsath F. A., Cerami A. Aging of proteins: isolation and identification of a fluorescent chromophore from the reaction of polypeptides with glucose. Proc Natl Acad Sci U S A. 1984 May;81(9):2684–2688. doi: 10.1073/pnas.81.9.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebald W., Wachter E., Tzagoloff A. Identification of amino acid substitutions in the dicyclohexylcarbodiimide-binding subunit of the mitochondrial ATPase complex from oligomycin-resistant mutants of Saccharomyces cerevisiae. Eur J Biochem. 1979 Oct 15;100(2):599–607. doi: 10.1111/j.1432-1033.1979.tb04207.x. [DOI] [PubMed] [Google Scholar]
- Wachter E., Machleidt W., Hofner H., Otto J. Aminopropyl glass and its p-phenylene diisothiocyanate derivative, a new support in solid-phase Edman degradation of peptides and proteins. FEBS Lett. 1973 Sep 1;35(1):97–102. doi: 10.1016/0014-5793(73)80585-x. [DOI] [PubMed] [Google Scholar]
- Wachter E., Werhahn R. Attachment of tryptophanyl peptides to 3-aminopropyl-glass suited for subsequent solid-phase Edman degradation. Anal Biochem. 1979 Aug;97(1):56–64. doi: 10.1016/0003-2697(79)90327-0. [DOI] [PubMed] [Google Scholar]
- Walker J. E., Fearnley I. M., Blows R. A. A rapid solid-phase protein microsequencer. Biochem J. 1986 Jul 1;237(1):73–84. doi: 10.1042/bj2370073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrle J. P., Cintrón N. M., Pedersen P. L. Phosphate transport in rat liver mitochondria. Energy-dependent accumulation of phosphate by inverted inner membrane vesicles. J Biol Chem. 1978 Dec 10;253(23):8598–8603. [PubMed] [Google Scholar]
- Zeman W., Dyken P. Neuronal ceroid-lipofuscinosis (Batten's disease): relationship to amaurotic family idiocy? Pediatrics. 1969 Oct;44(4):570–583. [PubMed] [Google Scholar]
- Zimniak L., Dittrich P., Gogarten J. P., Kibak H., Taiz L. The cDNA sequence of the 69-kDa subunit of the carrot vacuolar H+-ATPase. Homology to the beta-chain of F0F1-ATPases. J Biol Chem. 1988 Jul 5;263(19):9102–9112. [PubMed] [Google Scholar]