Abstract
The detection of severe limb malformations in metamorphosing northern leopard frogs (Rana pipiens) from a Colorado pond in August 2022 prompted questions about the cause(s) and concern over the implications. Northern leopard frogs, which are a Tier 1 Species of Greatest Conservation Need in Colorado, have declined over much of their range in the state, particularly along the Front Range. Although malformations in amphibians have been reported in other parts of the USA, they are rare in Colorado, and the current case represents the most severe hotspot reported in the state for over 70 years. Across three survey events in late summer and early fall of 2022, approximately 68% of captured leopard frogs (late-stage larvae and metamorphic frogs) exhibited one or more malformations. Malformations exclusively affected the hind limbs and were dominated by skin webbings (51.7% of the total), bony triangles (32.2%), and extra limbs or digits (11%). Many animals had multiple malformations that limited the movement of one or both limbs (average of 2.3 malformations per malformed frog). Dissection of a subset of animals coupled with 28S rDNA genetic sequencing revealed the occurrence of the trematode Ribeiroia ondatrae at an average of 75.2 trematode cysts (metacercariae) per frog. The parasite was also detected in 2.6% of dissected snails (Helisoma trivolvis), which function as the trematode's first intermediate host. The relatively high loads of infection detected here – coupled with the similarity of observed malformations to those previously linked to R. ondatrae in experimental studies and from other malformation hotspots in the USA – offer compelling evidence that the current case is the result of parasite infection. Unresolved questions include why malformation prevalence was so high in 2022 and the degree to which such abnormalities will affect population persistence for local leopard frogs, particularly if malformations continue.
Keywords: Amphibian decline, Emerging infection, Aquatic conservation, Host-parasite interaction, Rocky Mountains
Graphical abstract
Highlights
-
•
We investigated a case of mass malformations in declining leopard frogs from Colorado.
-
•
Seventy percent of frogs exhibited skin webbings, bony triangles, or extra limbs.
-
•
Morphological and genetic analysis confirmed infection by Ribeiroia ondatrae.
-
•
Similarity of malformations with those from experiments suggest R. ondatrae as the cause.
-
•
We discuss the implications and potential contributing factors for the current case.
1. Introduction
In Fall (1946), the first recorded case of “mass malformations” in a US amphibian population was reported from Muskee Lake, twenty miles west of Boulder, Colorado (today the site is named Mud Lake; 39.97757, −105.50974). Nearly 90% of tiger salamanders (Ambystoma tigrinum) collected from the lake by David Bishop, Assistant Professor at the University of Colorado, Boulder, exhibited limb malformations, including extra digits and extra legs (Bishop, 1947; Hamilton, 1949). The phenomenon was initially hypothesized to be genetic in origin, with “environmental factors playing a relatively small part” (Bishop, 1947). A few years later, however, graduate student Willard Rosine discovered similar malformations in both tiger salamanders and leopard frogs (Rana pipiens) from the same site (Rosine, 1955). Observing such anomalies in two distinct orders of amphibians concurrently provided strong evidence that the etiological agent was instead environmental, although its specific identity would remain elusive for nearly 50 years (Johnson et al., 2003).
In the mid-1990s, malformed amphibians gained international attention following the discovery of a leopard frog population in Minnesota in which >50% of the emerging animals exhibited extra or misshapen hind limbs (see Souder, 2000). Additional malformation hotspots, in which statistically >5% of animals exhibited malformations, were reported from wetlands in the Upper Midwest, the northeastern USA, and the western USA (Helgen et al., 1998; Johnson et al., 1999; Converse et al., 2000; Hoppe, 2000; Johnson et al., 2002; Kiesecker, 2002; Eaton-Poole et al., 2003; Vandenlangenberg et al., 2003; Hoppe, 2005). The causes as well as the implications of the phenomena were the subject of considerable debate and scientific controversy (see reviews by Ankley et al., 2004; Johnson et al., 2010). Proposed causes have included UV-B radiation, chemical pollution, predation attempts, and parasite infection, among others (see Sessions and Ruth, 1990; Ouellet, 2000; Ankley et al., 2004). Affected individuals rarely survive to maturity (see Lunde and Johnson, 2012), raising additional concerns over the long-term consequences for amphibians, which are considered the most threatened group of vertebrates worldwide (e.g. Stuart et al., 2004; Wake and Vredenburg, 2008). Unraveling the mystery of deformed frogs has been complicated by the fact that (1) the relative importance of these causes likely varies among wetlands and geographic regions and (2) proposed causes may interact through complex mechanisms (Kiesecker, 2002; Johnson et al., 2006, 2010). The current study investigated a severe outbreak of malformations in leopard frogs from Boulder, Colorado, USA, in 2022.
One major cause of such malformations is infection by the digenetic trematode Ribeiroia ondatrae, which invades the developing limbs of amphibian larvae and can induce extra limbs, missing limbs, skin webbings, and bony triangles (Johnson et al., 1999, 2001a, 2012; Lunde and Johnson, 2012). Evidence from both large-scale field surveys and extensive experiments has implicated parasite infection as a likely cause of malformation hotspots across multiple species and regions, particularly in the Upper Midwest and western North America (Johnson et al., 2002, 2003, 2013; Sutherland, 2005; Johnson and Hartson, 2009; Lunde et al., 2012; Roberts and Dickinson, 2012; Haas et al., 2018; Strasburg and Boone, 2021). Ribeiroia ondatrae has a complex life cycle involving freshwater snails in the family Planorbidae, amphibians, and frog-eating birds (Beaver, 1939; Johnson et al., 2004). Infective free-swimming cercariae emerge from snails and penetrate into larval amphibians, often forming cysts (metacercariae) around the limb or gill regions, and only mature into adult parasites when infected amphibians are consumed by a suitable avian predator. Adult parasites reproduce sexually within the bird and release eggs in their hosts’ feces, helping them colonize aquatic habitats across the landscape. Both the presence of R. ondatrae and its average abundance within amphibian hosts are positive predictors of malformations, although not all amphibian species are equally susceptible to infection or subsequent pathology (e.g. Johnson and Hartson, 2009; Johnson et al., 2012). Infection by large numbers of cercariae can also kill larval amphibians directly (e.g., Johnson et al., 1999; Wilber et al., 2020), further underscoring the potential impacts of R. ondatrae for host populations.
Despite hosting the earliest recorded case of mass malformations in the USA, Colorado has had few subsequent reports of amphibians with morphological abnormalities reported by scientists or members of the public. Re-examination of vouchered leopard frogs collected from Muskee Lake in the 1950s identified R. ondatrae infection as the likely cause (Johnson et al., 2003), yet resurveys of the site in later years yielded no further malformations. Geographically, most reports of malformed amphibians in the USA have come from the western states (e.g., California, Oregon and Washington), the Upper Midwest, and the Northeast (e.g. Converse et al., 2000; Johnson et al., 2002; Kiesecker, 2002; Vandenlangenberg et al., 2003; Johnson and Hartson, 2009; Bowerman et al., 2010; Johnson et al., 2013; Reeves et al., 2013; Haas et al., 2018). Both local and large-scale field surveys of amphibian populations conducted across Colorado have found few malformations among studied amphibian populations. For instance, as part of a continental-scale survey for malformed amphibians conducted by US Fish and Wildlife Service, the Mountain Prairie Region (Region 6), which includes Colorado, had among the lowest reported frequency of abnormalities – despite considerable sampling effort across its National Wildlife Refuges (Reeves et al., 2013; Haas et al., 2018). This also corresponded with few detections of R. ondatrae infection (Haas et al., 2018). Unpublished surveys of ponds and wetlands across Colorado's Front Range, including those on Boulder Open Space & Mountain Parks lands, have also indicated that malformations are typically low in all surveyed species and sites (Johnson and Keeley, unpublished).
In the current study, we report on a malformation hotspot recently detected in Colorado. The case involves hind limb abnormalities in a large percentage of metamorphosing northern leopard frogs (R. pipiens) from a pond near Boulder, Colorado. Leopard frogs have historically declined across much of Colorado and are currently considered a Tier 1 State Species of Special Concern (Lambert, 2006; Johnson et al., 2011; Colorado Parks and Wildlife, 2015). Thus, observations of mass malformations and their potential consequences for affected leopard frog populations warrant additional investigation. We present information on the frequency and characteristics of observed malformations and investigate possible cause(s) of the outbreak. Specifically, we quantified the presence and abundance of infections by trematode parasites – including R. ondatrae – in amphibians and freshwater snails (the first intermediate hosts of most trematodes), and compared observed malformations to those in leopard frogs from other USA hotspots associated with R. ondatrae infection.
2. Materials and methods
2.1. Study site and history
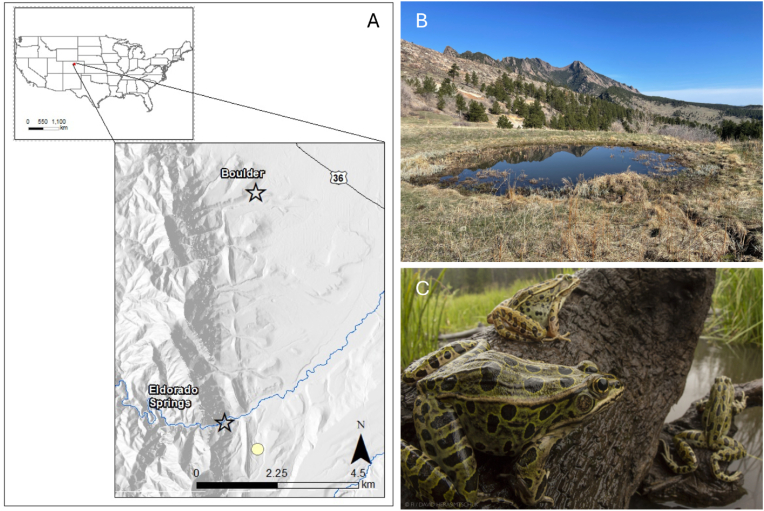
On 25 August 2022, staff biologists with City of Boulder Open Space & Mountain Parks (OSMP) noted that recently metamorphosed northern leopard frogs from a small pond (Spring Brook North [SBN]; Fig. 1) were exhibiting abnormal swimming patterns. Affected individuals were unable to adequately extend one or both of their hind limbs. Of the 24 captured, all showed evidence of some kind of abnormality, although careful inspections and malformation descriptions were not undertaken until subsequent visits. SBN is a small, spring-fed pond (ca 0.02 ha; 39.92456, −105.27197) located in mixed native grassland and Ponderosa pine (Pinus ponderosa) forest near Boulder, Colorado, USA. The surrounding land is managed by OSMP to conserve natural habitat and provide outdoor recreational opportunities. In 2013, a floating mat of cattails (Typha spp.) approximately 1 m thick was mechanically removed to increase open water conditions from <5% to >95%. During subsequent years, non-native vegetation was removed annually and native wetland vegetation restored (e.g., Juncus spp., Schoenoplectus spp., Carex spp.). Historically, the pond was used to water livestock; however, cattle have not been present in this area since OSMP purchased the property in 1990 and the pond is currently managed for wildlife, including amphibians. During annual monitoring conducted by OSMP (∼5 visits per summer) following restoration to the pond, leopard frog egg masses or larvae were observed each year between 2015 and 2023 (Keeley, unpublished data). However, throughout these surveys there has been no evidence of malformations noted in opportunistically captured frogs, or observations of erratic movements of malformed individuals prior to 2022.
Fig. 1.
(A) Spring Brook North (SBN) Pond is located in southern Boulder County near Eldorado Springs, Colorado, USA. (B) Image of the pond in spring. (C) Northern leopard frogs (Rana pipiens) use the pond as breeding habitat (image copyright David Herasimtschuk).
2.2. Field surveys for malformations
To collect detailed information on malformation frequency and composition, we surveyed the pond three times in late summer and early fall of 2022 (27 August, 2 September, 7 October). During each visit, late-stage larvae with well-developed hind limbs (Gosner [1960] stage 38 or later) and metamorphic frogs were captured by hand or using a long-handled dipnet in the water or in shoreline vegetation. Earlier-staged tadpoles were not included because not all features of the limbs could be adequately assessed. Frogs were kept shaded and cool in a container with natural vegetation and high humidity until no new frogs were captured for 20 min. Each captured frog was carefully examined for morphological abnormalities using standard protocols (Meteyer et al., 2000; Johnson et al., 2001b; Lunde and Johnson, 2012). Limbs were gently extended to check for skin webbings (i.e., a thin layer of skin connecting the back of the thigh to the back of the calf, which can vary in severity from barely noticeable to complete restriction of limb extension; see Johnson et al., 2010) (Fig. 2). Abnormalities were described initially in the field and characterized in additional detail using photographs of each animal's dorsal and ventral side. A water sample was analyzed for pH, alkalinity, color, conductivity, dissolved organic carbon, ammonium, nitrate, nitrite, phosphorus, arsenic, and various metals and ions (e.g. aluminum, lead, zinc, calcium, cadmium, mercury, silver, copper, and magnesium) (City of Boulder Water Quality Office; see Environmental Protection Agency method 200.8: https://www.epa.gov/sites/default/files/2015-06/documents/epa-200.8.pdf).
Fig. 2.
Limb malformations in leopard frogs from this study include (A–D) skin webbings, which can affect one (A–B) or both (C–D) hind limbs; (E–H) bony triangles, which entail a triangular folding in a longbone and an associated shortening of the limb; and (H–K) extra limbs, feet, and digits, which were typically ventral. Frog in (H) exhibits a double bony triangle and severe truncation in the limb with a duplicated foot. Many animals exhibited multiple malformations and severe structural limb deformities, such as (L).
Malformation terminology and classification followed Johnson et al. (2001b, 2010), with a particular emphasis on limb and skin disruptions associated with infection by R. ondatrae. Note that we counted only ‘independent’ malformations. Thus, abnormalities located on an abnormal appendage (distal to the first malformation) were not quantified to avoid double counting (see Johnson et al., 2001b). For example, in a frog with a bony triangle in the femur or tibiofibula, the foot is nearly always also malformed (extra digits or duplicated foot). In such cases, only the more proximal bony triangle is counted while the malformed foot is ignored. We calculated both the prevalence of abnormalities (number of malformed frogs divided by the total number inspected) and the number of abnormalities per abnormal frog (abnormality severity, see Johnson et al., 2001b). A subset of 18 frogs was retained to examine for trematode parasite infection and to radiograph; remaining frogs were released into the pond near the point of capture.
2.3. Laboratory examinations and parasite quantification
A haphazard subset of 15 frogs was systematically necropsied to detect and quantify macroparasite infections. This number was kept low to minimize any additional impacts to the frog population. Following established protocols, each frog was euthanized using an overdose of neutral-buffered MS-222 (Gentz, 2007), measured using digital calipers (snout-vent length), and immediately dissected to examine still-living parasites from the skin and major organs (Johnson et al., 2018; Riepe et al., 2019; Calhoun et al., 2020). The location and count of parasites was recorded.
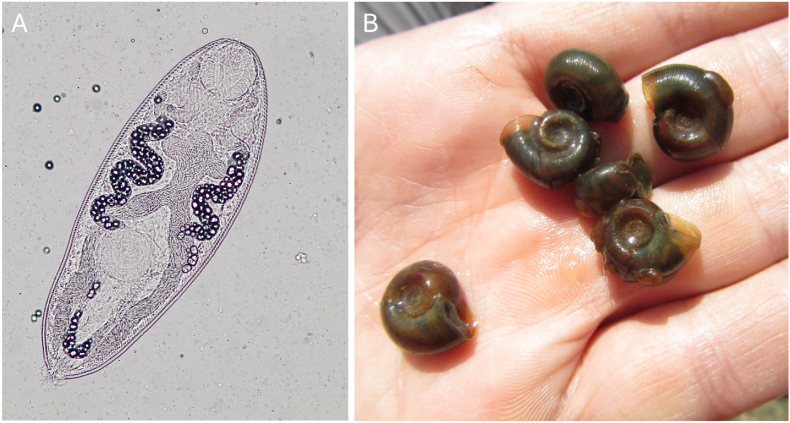
Parasites were identified using morphological structures with the aid of taxonomic keys (Schell 1985; Gibson et al., 2002). Metacercariae of R. ondatrae were mechanically excysted using gentle pressure from surgical forceps or a cover slip and then examined at 100-200x magnification on a stereomicroscope Olympus SZX16 (Olympus, Tokyo) to observe the esophageal diverticula diagnostic of the genus (Fig. 4; Johnson et al., 2004). Other parasites were similarly identified based on key structural characteristics. Voucher samples of each parasite were preserved in 95% ethanol for genomic analysis (see below).
Fig. 4.
(A) Excysted metacercaria of Ribeiroia ondatrae from an infected frog; (B) Rams horn snails (Helisoma trivolvis) function as first intermediate hosts for multiple trematode species, including Ribeiroia ondatrae. Several of these snails have egg masses on their shells, which can be common in the spring.
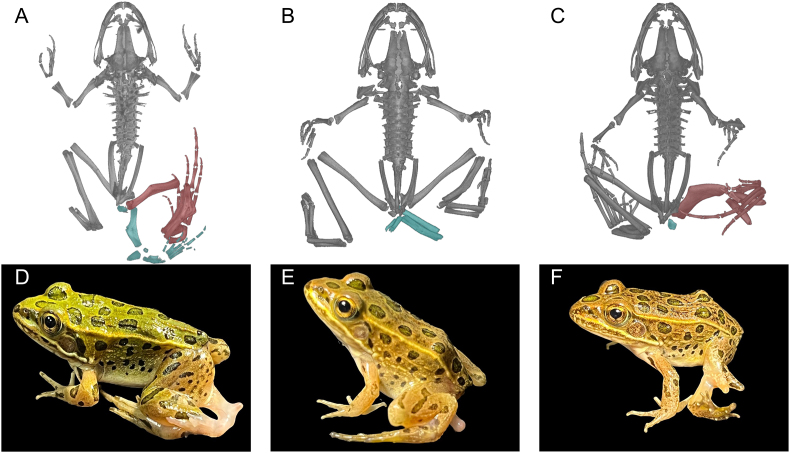
Three additional malformed frogs were fixed in 10% formalin and examined using Micro Computed-Tomography (Micro-CT) to visualize the 3D structures. Scans were performed with samples submerged in PBS and a 0.4x objective (100 kV, 23–24 μA, 1–1.8 s excitation, air filter, and 1601 projections) resulting in an approximate 4.3 × 4.3 mm field of view and voxel size of 4.27 μm3. Automated centering and beam hardening corrections were applied using Scout-and-Scan Control System Reconstructor software (version 14.0.14829.38124). Reconstructions were imported into Dragonfly Pro (version 4.1, Object Research Systems). These animals were subsequently deposited at the University of Colorado Museum of Natural History (UCM 69082 [Fig. 3A], UCM 69083 [Fig. 3B], and UCM 69084 [Fig. 3C]).
Fig. 3.
Whole-body ventrodorsal Micro-CT scans of three leopard frogs from SBN illustrating malformations caused by the trematode Ribeiroia ondatrae. Panels A–C present 3D reconstructions of the skeletons (ventral view) of each of the living frogs in the corresponding lower panels (D–F). For each, obviously abnormal portions of the skeleton are colored in red while supernumerary limb elements are colored in teal. (A) Frog with a severely rotated ilium on the left axis, a thickened femur, thickened tibiofibula (calcaneum) with a bony triangle, an extra bone at the base of the ischium, a supernumerary left hindlimb (polymelia) with a bony triangle in the tibiofibular; the right leg is missing all metatarsals and phalanges. (B) Frog with polymelia of the left leg, with two supernumerary femurs and two unidentified supernumerary bones near the ischium; both primary hind limbs appear to have reductions of the metatarsals and phalanges. (C) Frog with an extremely thickened femur (possibly fusion of multiple femurs) in the left hind limb, a double bony triangle in the tibiofibula, and an extra bone caudal to the ilium.
2.4. Genetic analysis
DNA of all encountered trematode taxa was extracted following the protocol provided by Tkach and Pawlowski (1999). For the collection of leopard frogs from SBN, this included: Ribeiroia ondatrae, Lechriorchis tygarti, Cephalogonimus americanus, Brachylaima sp., and Gorgoderina sp. In all cases, a fragment of the nuclear large ribosomal subunit (28S) rDNA was amplified by polymerase chain reaction (PCR) using forward primer digL2 (5’−AAG CAT ATC ACT AAG CGG−3′) and reverse primer 1500R (5’−GCT ATC CTG AGG GAA ACT TCG−3′) (Tkach et al., 2003). In addition, we amplified the ribosomal ITS region (ITS1 + 5.8S + ITS2) of C. americanus, G. attentuata, and L. tygarti using forward primer ITSf (5′–CGC CCG TCG CTA CTA133CCG ATT G–3′) and reverse primer 300R (5′–CAA CTT TCC CTC ACG GTA CTT G–3′) and the mitochondrial cytochrome c oxidase subunit 1 (cox1) mtDNA gene of C. americanus was amplified using forward primer JB3 (5′−TTT TTT GGG CAT CCT GAG GTT TAT−3′) and reverse primer JB5 (5′−AGC ACC TAA ACT TAA AAC ATA ATG AAA−3′) (Bowles et al., 1992; Derycke et al., 2005; Snyder and Tkach, 2007). PCR reactions were carried out in a total volume of 25 μl using a One-Taq quick load PCR mix from New England Biolabs (Ipswich, Massachusetts). The nuclear ribosomal reactions were run with an annealing temperature of 53°C, while cox1 reactions used an annealing temperature of 45°C. Positive PCR products were purified using an Illustra ExoProStar PCR clean-up enzymatic kit from Cytiva (Marlborough, Massachusetts) and cycle-sequenced using a Bright Dye Terminator Cycle Sequencing Kit (MCLAB, South San Francisco, California) using PCR primers. The internal primer d58F (5’−GCG GTG GAT CAC TCG GCT CGT G−3′) was also used for sequencing ITS2 (Kudlai et al., 2015). Subsequently, sequencing reactions were purified using a BigDye Sequencing Clean Up kit from MCLAB, and run on an ABI 3500 Genetic Analyzer (Thermo Fisher Scientific, Waltham, Massachusetts) at Georgia Southern University. Contiguous sequences were assembled using Sequencher version 5.4 software (GeneCodes Corp., Ann Arbor, Michigan). All newly generated sequences were deposited in GenBank under accessions PQ013240–PQ013269 and PP993596–PP993603 (Table 1).
Table 1.
Trematode parasites detected in recently metamorphosed leopard frogs (Rana pipiens) collected from SBN in 2022. For each parasite taxon, we present the species identification (based on morphology and genetics), the life stage found, infection prevalence (proportion of hosts with infection), the average number of parasites per host (including uninfected animals), the locations within the host where infection was observed, and an overview of the parasite's life cycle (typically from first intermediate to second intermediate to definitive host). Data based on a sample of 15 frogs.
| Species | Family | Stage | Prevalence | Load ±1 SE (range) | Location | Life cycle | GenBank Numbers | Citation |
|---|---|---|---|---|---|---|---|---|
| Brachylaima sp. | Brachylaimidae | adult | 6.6% | 14 | small intestine | Land snail, and small mammal | 28S (PQ013240) | 1 |
| Cephalogonimus americanus | Cephalogonimidae | metacercariae | 100% | 146.6 ± 36.3 | body cavity, mandible, mesentery, and skin | Aquatic snail, larval amphibian, and adult amphibian | ITS2+28S (PQ013241–PQ013245), 28S (PQ013252, PQ013253); cox1 (PP993596–PP993603) | 2 |
| Echinostoma spp. | Echinostomatidae | metacercariae | 86.7% | 45.7 ± 15.2 | kidneys | Aquatic snail, amphibian or fish, and bird or mammal | Not sequenced | 3 |
| Gorgoderina attenuata | Gorgoderidae | adult | 66.7% | 8.6 ± 1.2 | Urinary bladder, body cavity | Fingernail clams, larval amphibian or odonate, and adult amphibian | ITS region +28S (PQ013246–PQ013248), 28S (PQ013258) | 4 |
| Lechriorchis tygarti | Reniferidae | metacercariae | 100% | 24.9 ± 5.3 | mandible, mesentery, muscle, tongue, and skin | Aquatic snail, larval amphibian, and reptile | ITS region + 28S (PQ013249–PQ013251), 28S (PQ013259) | 5 |
| Ribeiroia ondatrae | Echinostomatidae | metacercariae | 100% | 75.2 ± 9.4 | body cavity, mandible, mesentery, muscle, tongue, and skin | Rams horn snail, amphibian or fish, and bird | 28S (PQ013260) | 6 |
2.5. Snail collection and dissections
Snails in the Planorbidae family are the only known first intermediate hosts of R. ondatrae (Johnson et al., 2004) and also host a number of other trematode taxa (Fernandez and Esch, 1991; Hannon et al., 2017). To test for the presence of R. ondatrae and other trematodes within the local snail population at SBN, we collected rams horn snails (Helisoma trivolvis; Fig. 4) every 5 m around the pond perimeter using a D-frame dipnet during each sampling visit. Because the prevalence of trematode infections in snails is often low (Richgels et al., 2013; Hobart et al., 2022), we sought to capture ∼100 or more individuals on each visit to increase detection probabilities. After measuring snail shell width, we gently crushed the shell using pliers and examined the tissues for evidence of infection under 10 to 60x magnification. Trematode parthenitae (clonally produced sporocysts and rediae), which are often accompanied by active cercariae (free-swimming infective stages), can occupy 20–40% of the host's tissue mass when mature and are often readily apparent upon dissection. Representative cercariae were put on slides and inspected at higher magnification (100–400x) to facilitate specific identification. Ribeiroia cercariae have a gymnocephalous morphotype (see Schell, 1985) with esophageal diverticula, although these structures can be harder to see on cercariae relative to metacercariae. They also lack the collar spines around the oral sucker than are evident in the morphologically similar and closely-related echinostome group, including the genera Echinostoma and Echinoparyphium (some of which also use rams horn snails). Infections without evident cercariae were classified as immature (prepatent). Voucher samples of parasite tissue from a subset of each morphotype were preserved for genetic analysis, as described above. A smaller number of Physa gyrina snails, which were also present but less abundant, were similarly examined and dissected.
3. Results
3.1. Malformation patterns
Across the three visits, 52 of 77 captured leopard frogs (late-stage larvae or recently metamorphosed frogs) exhibited one or more morphological abnormalities (67.5%). The frequency of abnormalities varied from 70% on 27 August (n = 23) to 72% on 2 September (n = 46) to 37.5% on the last visit (7 October), when only eight frogs were captured. Developmental stages examined ranged from Gosner 38 (late-stage limb development) to Gosner 46 (fully completed metamorphosis). Because malformed frogs often suffered >1 morphological abnormalities, we characterized 118 individual abnormalities. Thus, the average malformation severity was 2.31 ± 0.13 (range: 1 to 7), with 21 frogs exhibiting three or more individual abnormalities. All abnormalities involved the hind limbs, for which the most common abnormality was skin webbings (cutaneous fusion), comprising 51.7% of the total (Fig. 2A–D). In severe cases (e.g., Fig. 2D), the thigh and calf were fused for >50% of their length. Seventeen animals exhibited skin webbings on both hind limbs.
Bony triangles (taumelia), in which one of the longbones in the limbs is folded into a triangular formation, was the next most common abnormality type (32.2%) (Fig. 2H–K). This generally occurs in the femur or tibiofibular longbones and leads to structural truncation of the limb, particularly in cases for which there are two or more such triangles in a single limb (e.g., Fig. 2H). Extra limbs and digits (polymelia and polydactyly) constituted an additional 11% of all abnormalities (Fig. 2E–G). Seven animals had a duplicated hind limb while there were six cases of an extra digit or foot. Extra limbs were always ventral to the primary limb, rather than dorsal as often seen in non-ranid frogs (Johnson et al., 2001b). An additional 4.2% of abnormalities involved shortened limbs or digits. Micro-CT scans revealed additional malformations of the skeletal structure often not evident (or difficult to characterize) during gross external examination (Fig. 3). For instance, some animals exhibited a thickening or duplication of the femur (Fig. 3A and C), increased joint spacing between tarsal bone (Fig. 3C), and additional bones near the ischium (Fig. 3B), all of which were not visible in gross examination.
3.2. Amphibian and snail dissections
All 15 dissected leopard frogs were infected with metacercariae of R. ondatrae (Fig. 4). The average number of cysts ±1 SE per frog was 75.2 ± 9.4 (range: 12 to 132), for which parasites were concentrated primarily around the base of the hind limbs (14%) and the tail resorption site (61.3%), with a secondary cluster by the distal end of the lower mandible (12.8%). Encysted metacercariae were often closely associated with observed malformations. In addition to R. ondatrae, we detected five other trematode parasites. Three of these were larval forms (metacercariae), including Lechriorchis tygarti, Cephalogonimus americanus, and Echinostoma spp. (see prevalence and mean loads in Table 1). Adult Brachylaima sp. and Gorgoderina sp. trematodes were also observed in the large intestine or body cavity of 1 and 10 frogs, respectively.
Of the 494 H. trivolvis dissected (Fig. 4), 93 (18.9%) were infected with one of five taxa of larval trematodes (range of 2.6–8.9% by taxa; Table 2). Larval stages of R. ondatrae were detected in 13 snails (2.6%), with other common infections including Zygocotyle sp., Echinostoma spp., C. americanus, and Australapatemon burti complex (Table 2). Two infections were classified as immature (prepatent) and could not be identified. Infected snails were generally larger than uninfected individuals, averaging 14.35 mm relative to 11.40 mm for uninfected snails. Among dissections of P. gyrina, 25% were infected with echinostomes (n = 20).
Table 2.
Trematodes parasites detected in freshwater snails collected from SBN in 2022. In total, 494 Helisoma trivolvis and 20 Physa gyrina snails were dissected and examined for larval trematodes. For each parasite taxon, we present the species identification (based on morphology and genetics), the morphotype of the cercaria (see Schell 1985), and the infection prevalence (proportion of hosts with infection). For evident infections without mature cercariae, “immature” is listed.
| Snail host | Species | Family | Morphotype | Prevalence |
|---|---|---|---|---|
| Helisoma trivolvis | Cephalogonimus americanus | Cephalogonimidae | Armatae | 8.9% |
| Helisoma trivolvis | Echinostome | Echinostomatidae | Echinostome | 0.8% |
| Helisoma trivolvis | Ribeiroia ondatrae | Echinostomatidae | Gymnocephalous | 2.6% |
| Helisoma trivolvis | Zygocotyle sp. | Zygocotylidae | Amphistome | 2.8% |
| Helisoma trivolvis | Immature | 0.4% | ||
| Helisoma trivolvis | Australapatemon burti complex | Strigeidae | Strigea | 3.0% |
| Physa gyrina | Echinostome | Echinostomatidae | Echinostome | 25% |
3.3. Parasite genetics
Most newly generated sequences, including several from larval stages, matched those in GenBank from morphologically identified adults (R. ondatrae, L. tygarti, and G. attenuata; see discussion on C. americanus below). In a few cases, our 28S sequences were not identical to previously published data in GenBank, but BLAST comparisons at least allow us to confidently identify our material to genus (i.e., Brachylaima sp.) or a likely family (i.e., eucotylid sp.). In the case of our two lineages of echinostomatid cercariae (PQ013266, PQ013267), both matched previously sequenced lineages of echinostomatids (e.g., MN815778 and KU896152). No intraspecific variation was detected among our 28S sequences of R. ondatrae (frog, n = 1; snail, n = 2; PQ013260–PQ013262), C. americanus (frog, n = 7; snail, n = 4; PQ013241–PQ013245, PQ013252–PQ013257), L. tygarti (frog, n = 4; PQ013249–PQ013251, PQ013259), G. attenuata (frog, n = 4; PQ013246–PQ013248, PQ013258), A. cf. burti complex (snail, n = 3; PQ013263–PQ013265), and a likely eucotylid sp. (snail, n = 2; PQ013268, PQ013269). Similarly, no differences were detected among ITS2 sequences of C. americanus (frog, n = 2; PQ013241, PQ013242) and ITS region sequences of L. tygarti (frog, n = 3; PQ013249–PQ013251) and G. attenuata (frog, n = 3; PQ013246–PQ013248). The cox1 sequences of C. americanus (frog, n = 7; PP993596–PP993603) lacked variation, but differ by 4.7% from C. americanus in GenBank (accession HM137633) collected in Mexico.
3.4. Water testing
Water testing resulted in normal (i.e., non-toxic according to the Colorado Department of Public Health and Environment, 2022) concentrations for all analytes tested (Table S1). The higher levels of Al, Ca, Fe, and Mg are likely the result of the geologic surroundings of the pond and are similar to what has been detected in other surface water samples in Boulder County, Colorado (M. Lawlor, personal communication; Keep it Clean Partnership, 2019).
4. Discussion
Multiple lines of evidence support the hypothesis that the outbreak of limb malformations observed in Colorado leopard frogs was caused by R. ondatrae infection. First, the types of limb malformations observed, including skin webbings, bony triangles, and duplicated feet or limbs, are strongly consistent with those induced experimentally following exposure of amphibian larvae to R. ondatrae cercariae. For example, Schotthoefer et al. (2003) found that the effects of R. ondatrae cercariae exposure on R. pipiens tadpoles in laboratory trials were both dose- and time-dependent. Exposure of early-stage tadpoles caused high mortality (72.5%), while exposure during limb growth led to the development of malformations in 16% of individuals. Similarly, in mesocosms, Strasburg and Boone (2021) reported that 86% of leopard frogs raised with R. ondatrae-infected snails developed one or more malformations, which included extra and missing limbs, bony triangles, and skin webbings. Experimental exposures of ∼15 other amphibian species to R. ondatrae cercariae, either in laboratory trials, mesocosms, or field cages, have similarly substantiated the link between infection and limb malformations (Johnson and Hartson, 2009; Johnson et al., 2012; Lunde et al., 2012; Roberts and Dickinson, 2012).
Second, the malformations observed in the current study mirror those detected at other parasite-linked hotspots involving northern leopard frogs, including several from Minnesota reported in the late 1990s and early 2000s (Duck Pond, Hibbing Pond, and the original Ney Pond that first drew attention to amphibian malformations) (see Souder, 2000; Lannoo et al., 2003; Vandenlangenberg et al., 2003; Hoppe, 2005; Sutherland, 2005; Johnson and Hartson, 2009). While the relative frequency of abnormality types can vary among amphibian species and the timing of exposure, these particular malformations appear to be consistently associated with R. ondatrae infection.
Finally, R. ondatrae was detected among all examined leopard frogs using morphological analyses and verified through 28S rDNA sequencing at infection loads (metacercariae per frog) high enough to account for observed malformations. For instance, the average number of metacercariae per frog was 75.2, whereas many laboratory experiments induce malformations with between 10 and 100 cercariae (of which <50% normally encyst as metacercariae) (Schotthoefer et al., 2007; Johnson et al., 2012). This pattern is consistent with the prevalence (2.6%) of snail hosts (H. trivolvis) infected with R. ondatrae at SBN. Typical prevalence values are often 5% or less (Peterson, 2007; Richgels et al., 2013; Hobart et al., 2022). Although several other trematodes were detected, none are known to induce malformations nor were closely associated with host limbs. Results of water chemistry analysis were also typical for the region, and the area is managed for recreation and habitat conservation (although there are many potential contaminants, including endocrine disruptors, for which no testing was done). Our results implicate R. ondatrae as the proximate cause of leopard frog limb malformations at SBN, but do not exclude the possibility of contributing co-factors.
An important question is what factors may have ultimately led to such high levels of R. ondatrae infection in 2022. Cases of mass malformations in amphibians are rare in Colorado, as are observations of R. ondatrae infection in the region generally. In the survey of US Fish and Wildlife Service National Wildlife Refuges, fewer than 1% of examined amphibians in Colorado were malformed, with only one collection supporting R. ondatrae infection. In unpublished surveys across the Front Range of Colorado, examination of 609 recently metamorphosed frogs of four species yielded <2% malformed, and no detections of R. ondatrae among the subset dissected to assess infection (Johnson, unpublished data). During annual monitoring of >100 wetland sites on OSMP properties from 2010 to present, biologists have also not detected the abnormal swimming or movements associated with malformed frogs at any other local wetlands.
We provide two hypotheses for the high numbers of R. ondatrae infection at SBN. First, shifts in the local distribution, survival or susceptibility of snail intermediate hosts could have contributed to higher infection pressure (cercariae exposure) for larval amphibians in the pond. For instance, Keller et al. (2021) suggested that an R. ondatrae-associated die-off of salamanders in California may have resulted from restoration efforts to deepen the pond, potentially leading to increased overwintering persistence of infected snails. Although vegetation was removed from SBN in 2013, likely altering habitat and the amount of open water, the pond is spring-fed with a stable water level in most years. Rams horn snails, which are locally widespread in Boulder County, are susceptible to R. ondatrae infection in experimental trials (unpublished data), suggesting the infection increase is unlikely to be associated with a recent change in snail susceptibility.
Second, increases in local or regional activity by avian definitive hosts could have enhanced amphibian infections through the concentrated addition of parasite eggs into the pond, fueling higher snail prevalence with R. ondatrae. Birds represent an important vehicle for the dispersal and colonization of many complex life cycle trematodes (Hechinger and Lafferty, 2005; Gutiérrez et al., 2019). Ribeiroia infections have been recorded in approximately 50 species of birds, including many migratory water birds that frequent freshwater ponds and lakes (e.g. Johnson et al., 2004; Johnson and McKenzie, 2009). Johnson and Haas (2021) hypothesized that the relative rarity of R. ondatrae infection and amphibian malformations in the Intermountain West may stem from a relatively low abundance of migratory birds (see also Hartson et al., 2011). The high prevalence of R. ondatrae in snails from SBN indicates that the site experienced a high rate of infected bird activity in 2021–2022. Such an event could be stochastic, particularly for a small pond environment, where even a small number of infected birds could have a large effect on the patterns of snail infection prevalence. It could also be associated with unusual drought conditions experienced in most western states in 2021–2022 (USDA, 2022), potentially driving some migratory birds eastward in search of more stable water sources. Interestingly, another pathogenic trematode (Clinostomum marginatum) found in amphibians and transmitted by birds also exhibited an unusual epizootic in a nearby Boulder pond in 2022 (Calhoun and Johnson, unpublished data).
Equally important to understand are the potential implications of parasite-induced malformations for the viability of the leopard frog population in and around SBN and the Boulder Creek watershed. Leopard frogs have declined substantially in the Front Range over the past half century (e.g. Corn and Fogleman, 1984; Livo, 1997; Lambert, 2006; Johnson et al., 2011) and breeding sites that produce large numbers of metamorphic frogs have become increasingly scarce. OSMP properties contains the greatest number of leopard frog breeding locations on public land in Boulder County, and likely along the Front Range, so understanding impacts of parasites affecting these populations is paramount to maintaining stable populations for land managers tasked with conserving wildlife resources. Infection by R. ondatrae can pose a threat to individual frogs or the population for several reasons. Malformed frogs typically do not survive to sexual maturity, starving or being eaten because of impaired movement abilities (Goodman and Johnson, 2011). Perhaps more importantly, high levels of R. ondatrae infection can substantially increase mortality of larval amphibians, suggesting that malformed metamorphic individuals represent only the ‘tip of the iceberg’ with respect to the proportion of the population affected. In laboratory studies, exposure of larval amphibians to even modest numbers of cercariae can cause high mortality because of the parasites' active penetration and destruction of host tissue (Johnson et al., 1999; Johnson et al., 2012; but note that Strasburg and Boone 2021 reported no significant effects of infection on leopard frog survival, despite high exposures). In natural aquatic environments, infection has also been linked to increased mortality in amphibians. Keller et al. (2021) reported a die-off in two species of endangered salamanders associated with high R. ondatrae infection in California, where dead individuals exhibited large numbers of R. ondatrae metacercariae in proximity to gross lesions and necrotic tissue. Wilber et al. (2020) combined information on the statistical distribution of parasites among hosts and laboratory experiments in a modeling framework to show that R. ondatrae infection likely kills 13–40% of tadpoles in a pond where it occurs annually, with a range of up to 90% in some California populations with high loads and involving susceptible species.
We emphasize, however, that early life stages of amphibians often experience high mortality (e.g. Martof, 1956; Calef, 1973), and whether R. ondatrae infections have lasting impacts on the population depends on (1) whether parasite-induced mortality is additive or compensatory and, perhaps more importantly, (2) whether high infection exposure occurs over multiple years (and also occurs at neighboring breeding sites). If malformation outbreaks are short-lived, their effects may be limited to specific annual cohorts, allowing opportunities for recovery by amphibian populations with long-lived adults. Thus far, there have been no long-term studies to investigate the consequences of parasite-induced malformations for amphibian population viability. Continued surveillance of infection and malformations patterns of this and surrounding ponds in conjunction with efforts to evaluate activity by potential definitive hosts (e.g., camera trapping of waterbirds) will be valuable steps toward understanding the potential implications of this phenomenon for local leopard frog populations and determining whether cases of mass malformations in amphibians will become more frequent in the future. Such surveys may also provide additional insights into the environmental factors contributing to elevated levels of R. ondatrae infection, with potential relevance to both historical and contemporary observations of amphibian malformations.
CRediT authorship contribution statement
Pieter T.J. Johnson: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Dana M. Calhoun: Writing – review & editing, Validation, Project administration, Methodology, Investigation, Data curation, Conceptualization. Tyler J. Achatz: Writing – review & editing, Resources, Methodology, Investigation, Formal analysis. Stephen E. Greiman: Writing – review & editing, Resources, Methodology, Investigation, Formal analysis. Adrian Gestos: Software, Resources, Methodology, Investigation. William H. Keeley: Writing – review & editing, Resources, Methodology, Investigation, Conceptualization.
Declaration of competing interest
The authors declare no conflict of interest. All authors have approved the manuscript, and we hereby attest the manuscript is not under consideration for publication elsewhere.
Acknowledgements
We thank City of Boulder staff Michael Lawlor for facilitating the analysis of water samples and Christina Fairbanks for reporting original malformations. For assistance with sample collection and processing, we thank Sophie Elliot, Katelyn Johnson, and Kyle Johnson, Jasmine Groves, and Carmela Bonato. Elizabeth Falendyze provided helpful input on micro-CT results. Analyses were performed at MIMIC, CU Boulder (RRID:SCR_019307). This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijppaw.2024.100965.
Contributor Information
Pieter T.J. Johnson, Email: pieter.johnson@colorado.edu.
Dana M. Calhoun, Email: dana.calhoun@colorado.edu.
Tyler J. Achatz, Email: tyler.achatz@mga.edu.
Stephen E. Greiman, Email: sgreiman@georgiasouthern.edu.
Adrian Gestos, Email: adrian.gestos@colorado.edu.
William H. Keeley, Email: keeleyw@bouldercolorado.gov.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Ankley G.T., Degitz S., Diamond S., Tietge J. Assessment of environmental stressors potentially responsible for malformations in North American anuran amphibians. Ecotoxicol. Environ. Saf. 2004;58:7–16. doi: 10.1016/j.ecoenv.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Beaver P.C. The morphology and life history of Psilostomum ondatrae Price, 1931 (Trematoda: Psilostomidae) J. Parasitol. 1939;25:383–393. [Google Scholar]
- Bishop D.W. Polydactyly in the tiger salamander. J. Hered. 1947;38:290–293. [PubMed] [Google Scholar]
- Bolek M.G., Snyder S.D., Janovy Jr J. Alternative life cycle strategies and colonization of young anurans by Gorgoderina attenuata in Nebraska. J. Parasitol. 2009;95:604–616. doi: 10.1645/GE-1813.1. [DOI] [PubMed] [Google Scholar]
- Bowerman J., Johnson P.T.J., Bowerman T. Sublethal predators and their injured prey: linking aquatic predators and severe limb abnormalities in amphibians. Ecology. 2010;91:242–251. doi: 10.1890/08-1687.1. [DOI] [PubMed] [Google Scholar]
- Bowles J., Blair D., McManus D.P. Genetic variants within the genus Echinococcus identified by mitochondrial DNA sequencing. Mol. Biochem. Parasitol. 1992;54:165–173. doi: 10.1016/0166-6851(92)90109-w. [DOI] [PubMed] [Google Scholar]
- Calef G.W. Natural mortality of tadpoles in a population of Rana aurora. Ecology. 1973;54:741–758. [Google Scholar]
- Calhoun D., Leslie K., Riepe T., Achatz T., McDevitt-Galles T., Tkach V., Johnson P.T.J. Patterns of Clinostomum marginatum infection in fishes and amphibians: integration of field, genetic, and experimental approaches. J. Helminthol. 2020;94:1–10. doi: 10.1017/S0022149X18001244. [DOI] [PubMed] [Google Scholar]
- Calhoun D.M., Groves J., Schaffer P.A., Achatz T.A., Greiman S.E., Johnson P.T.J. Epizootic of Clinostomum marginatum (Trematoda: Clinostomidae) in Ambystoma tigrinum from Colorado: investigation through genomics, histopathology, and noninvasive imagery. J. Wildl. Dis. 2024 submitted for publication. [Google Scholar]
- Colorado Department of Public Health and the Environment Integrated water quality monitoring and assessment report. 2022. https://cdphe.colorado.gov/impaired-waters
- Colorado Parks and Wildlife State wildlife action plan. 2015. https://cpw.state.co.us/aboutus/Pages/StateWildlifeActionPlan.aspx
- Converse K.A., Mattsson J., Eaton-Poole L. Field surveys of Midwestern and Northeastern fish and wildlife service lands for the presence of abnormal frogs and toads. J. Iowa Acad. Sci. 2000;107:160–167. [Google Scholar]
- Corn P.S., Fogleman J.C. Extinction of montane populations of the northern leopard frog (Rana pipiens) in Colorado. J. Herpetol. 1984;18:147–152. [Google Scholar]
- Derycke S., Remerie T., Vierstraete A., Backeljau T., Vanfleteren J., Vincx M., Moens T. Mitochondrial DNA variation and cryptic speciation within the free-living marine nematode Pellioditis marina. Mar. Ecol. Prog. Ser. 2005;300:91–103. [Google Scholar]
- Eaton-Poole L., Pinkney A.E., Green D.E., Sutherland D.R., Babbitt K.J. In: Multiple Stressor Effects in Relation to Declining Amphibian Populations. Linder G., Krest L.E., Sparling S., editors. ASTM International; U.K: 2003. Investigation of frog abnormalities on national wildlife refuges in the Northeast US; pp. 34–49. [Google Scholar]
- Esteban J.G., Muñoz-Antoli C. In: The Biology of Echinostomes: from the Molecule to the Community. Toledo R., Fried B., editors. Springer; New York, New York: 2009. Echinostomes: systematics and life cycles; pp. 1–14. [Google Scholar]
- Fernandez J., Esch G.W. Guild structure of larval trematodes in the snail Helisoma anceps: patterns and processes at the individual host level. J. Parasitol. 1991;77:528–539. [PubMed] [Google Scholar]
- Gentz E.J. Medicine and surgery of amphibians. ILAR J. 2007;48:255–259. doi: 10.1093/ilar.48.3.255. [DOI] [PubMed] [Google Scholar]
- Gibson D.I., Jones A., Bray R.A. CABI; London, U.K: 2002. Keys to the Trematoda. [Google Scholar]
- Goodman B.A., Johnson P.T.J. Disease and the extended phenotype: parasites control host performance and survival through induced changes in body plan. PLoS One. 2011;6 doi: 10.1371/journal.pone.0020193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosner K.L. A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica. 1960;16:183–190. [Google Scholar]
- Gutiérrez J.S., Piersma T., Thieltges D.W. Micro‐and macroparasite species richness in birds: the role of host life history and ecology. J. Anim. Ecol. 2019;88:1226–1239. doi: 10.1111/1365-2656.12998. [DOI] [PubMed] [Google Scholar]
- Haas S.E., Reeves M.K., Pinkney A.E., Johnson P.T.J. Continental‐extent patterns in amphibian malformations linked to parasites, chemical contaminants, and their interactions. Global Change Biol. 2018;24:e275–e288. doi: 10.1111/gcb.13908. [DOI] [PubMed] [Google Scholar]
- Hamilton R.S. University of Colorado; 1949. Natural History of the Rocky Mountains Tiger Salamanders (Ambystoma tigrinum) and the Occurrence of Polydactylism in a Local Population. [Google Scholar]
- Hannon E.R., Calhoun D.M., Chadalawada S., Johnson P.T.J. Circadian rhythms of trematode parasites: applying mixed models to test underlying patterns. Parasitology. 2017;145:783–7921. doi: 10.1017/S0031182017001706. [DOI] [PubMed] [Google Scholar]
- Hartson R.B., Orlofske S.A., Melin V.E., Dillon R.T., Johnson P.T.J. Land use and wetland spatial position jointly determine amphibian parasite communities. EcoHealth. 2011;8:485–500. doi: 10.1007/s10393-011-0715-9. [DOI] [PubMed] [Google Scholar]
- Hechinger R.F., Lafferty K.D. Host diversity begets parasite diversity: bird final hosts and trematodes in snail intermediate hosts. Proc. Biol. Sci. 2005;272:1059–1066. doi: 10.1098/rspb.2005.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helgen J., McKinnell R.G., Gernes M.C. In: Status and Conservation of Midwestern Amphibians. Lannoo M.J., editor. University of Iowa Press; Iowa City: 1998. Investigation of malformed Northern leopard frogs in Minnesota; pp. 288–297. [Google Scholar]
- Hobart B.K., Moss W.E., McDevitt‐Galles T., Stewart Merrill T.E., Johnson P.T.J. It's a worm‐eat‐worm world: consumption of parasite free‐living stages protects hosts and benefits predators. J. Anim. Ecol. 2022;91:35–45. doi: 10.1111/1365-2656.13591. [DOI] [PubMed] [Google Scholar]
- Hoppe D.M. History of Minnesota frog abnormalities: do recent findings represent a new phenomenon? J. Iowa Acad. Sci. 2000;107:86–89. [Google Scholar]
- Hoppe D.M. In: Amphibian Declines: the Conservation Status of United States Species. Lannoo M.J., editor. University of California Press; Berkeley, CA: 2005. Malformed frogs in Minnesota: history and interspecific differences; pp. 103–108. [Google Scholar]
- Johnson P.T.J., Calhoun D.M., Stokes A.N., Susbilla C.B., McDevitt‐Galles T., Briggs C.J., Hoverman J.T., Tkach V.V., de Roode J.C. Of poisons and parasites—the defensive role of tetrodotoxin against infections in newts. J. Anim. Ecol. 2018;87:1192–1204. doi: 10.1111/1365-2656.12816. [DOI] [PubMed] [Google Scholar]
- Johnson P.T.J., Haas S.E. Why do parasites exhibit reverse latitudinal diversity gradients? Testing the roles of host diversity, habitat and climate. Global Ecol. Biogeogr. 2021;30:1810–1821. doi: 10.1111/geb.13347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P.T.J., Hartson R.B. All hosts are not equal: explaining differential patterns of malformations in an amphibian community. J. Anim. Ecol. 2009;78:191–201. doi: 10.1111/j.1365-2656.2008.01455.x. [DOI] [PubMed] [Google Scholar]
- Johnson P.T.J., Lunde K.B., Haight R.W., Bowerman J., Blaustein A.R. Ribeiroia ondatrae (Trematoda: Digenea) infection induces severe limb malformations in western toads (Bufo boreas) Can. J. Zool. 2001;79:370–379. [Google Scholar]
- Johnson P.T.J., Lunde K.B., Ritchie E.G., Launer A.E. The effect of trematode infection on amphibian limb development and survivorship. Science. 1999;284:802–804. doi: 10.1126/science.284.5415.802. [DOI] [PubMed] [Google Scholar]
- Johnson P.T.J., Lunde K.B., Ritchie E.G., Reaser J.K., Launer A.K. Morphological abnormality patterns in a California amphibian community. Herpetologica. 2001;57:336–352. [Google Scholar]
- Johnson P.T.J., Lunde K.B., Thurman E.M., Ritchie E.G., Wray S.N., Sutherland D.R., Kapfer J.M., Frest T.J., Bowerman J., Blaustein A.R. Parasite (Ribeiroia ondatrae) infection linked to amphibian malformations in the western United States. Ecol. Monogr. 2002;72:151–168. [Google Scholar]
- Johnson P.T.J., Lunde K.B., Zelmer D.A., Werner J.K. Limb deformities as an emerging parasitic disease in amphibians: evidence from museum specimens and resurvey data. Conserv. Biol. 2003;17:1724–1737. [Google Scholar]
- Johnson P.T.J., McKenzie V.J. In: The Biology of Echinostomes: from the Molecule to the Community. Toledo R., Fried B., editors. Springer; New York, New York: 2009. Effects of environmental change on helminth infections in amphibians: exploring the emergence of Ribeiroia and Echinostoma infections in North America; pp. 249–280. [Google Scholar]
- Johnson P.T.J., McKenzie V.J., Peterson A.C., Kerby J.L., Brown J., Blaustein A.R., Jackson T. Regional decline of an iconic amphibian associated with elevation, land‐use change, and invasive species. Conserv. Biol. 2011;25:556–566. doi: 10.1111/j.1523-1739.2010.01645.x. [DOI] [PubMed] [Google Scholar]
- Johnson P.T.J., Preston D.L., Hoverman J.T., Richgels K.L. Biodiversity decreases disease through predictable changes in host community competence. Nature. 2013;494:230–233. doi: 10.1038/nature11883. [DOI] [PubMed] [Google Scholar]
- Johnson P.T.J., Preu E.R., Sutherland D.R., Romansic J.M., Han B., Blaustein A.R. Adding infection to injury: synergistic effects of predation and parasitism on amphibian malformations. Ecology. 2006;87:2227–2235. doi: 10.1890/0012-9658(2006)87[2227:aitise]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Johnson P.T.J., Reeves M.K., Krest S.K., Pinkney A.E. In: Ecotoxicology of Amphibians and Reptiles. Sparling. Linder D.W., Bishop G., Krest C.A., editors. CRC Press; California: 2010. A decade of deformities; pp. 511–536. [Google Scholar]
- Johnson P.T.J., Rohr J.R., Hoverman J.T., Kellermanns E., Bowerman J., Lunde K.B. Living fast and dying of infection: host life history drives interspecific variation in infection and disease risk. Ecol. Lett. 2012;15:235–242. doi: 10.1111/j.1461-0248.2011.01730.x. [DOI] [PubMed] [Google Scholar]
- Johnson P.T.J., Sutherland D.R., Kinsella J., Lunde K.B. Review of the trematode genus Ribeiroia (Psilostomidae): ecology, life history, and pathogenesis with special emphasis on the amphibian malformation problem. Adv. Parasitol. 2004;57:191–253. doi: 10.1016/S0065-308X(04)57003-3. [DOI] [PubMed] [Google Scholar]
- Keller S., Roderick C.L., Caris C., Grear D.A., Cole R.A. Acute mortality in California tiger salamander (Ambystoma californiense) and Santa Cruz long-toed salamander (Ambystoma macrodactylum croceum) caused by Ribeiroia ondatrae (Class: Trematoda) Int. J. Parasitol.: Parasites and Wildlife. 2021;16:255–261. doi: 10.1016/j.ijppaw.2021.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keep it Clean Partnership Water quality report. 2019. https://www.keepitcleanpartnership.org/our-watershed/monitoring/
- Kiesecker J.M. vol. 99. 2002. pp. 9900–9904. (Synergism between Trematode Infection and Pesticide Exposure: a Link to Amphibian Limb Deformities in Nature? Proceedings of the National Academy of Sciences). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudlai O., Kostadinova A., Pulis E.E., Tkach V.V. A new species of Drepanocephalus Dietz, 1909 (Digenea: Echinostomatidae) from the double-crested cormorant Phalacrocorax auritus (Lesson) (Aves: Phalacrocoracidae) in North America. Syst. Parasitol. 2015;90:221–230. doi: 10.1007/s11230-015-9550-7. [DOI] [PubMed] [Google Scholar]
- Lambert B. Northern Leopard Frog (Rana pipiens) statewide status assessment and targeted inventory in Grand County. Final report. Colorado Division of Wildlife, Colorado Springs, Colorado. 2006 [Google Scholar]
- Lang B.Z. The life cycle of Cephalogonimus americanus Stafford, 1902 (Trematoda: Cephalogonimidae) J. Parasitol. 1968;10:945–949. [Google Scholar]
- Lannoo M., Sutherland D., Jones P., Rosenberry D., Klaver R., Hoppe D., Johnson P., Lunde K., Facemire C., Kapfer J. In: Multiple Stressor Effects in Relation to Declining Aphibian Populations. Linder G.L.E., Krest S., Sparling D., editors. ASTM International; U.K: 2003. Multiple causes for the malformed frog phenomenon; pp. 25–34. [Google Scholar]
- Livo L.J. 1997. City of Boulder 1996 Amphibian and Reptile Survey, City of Boulder Open Space Department, Boulder, CO. [Google Scholar]
- Lunde K.B., Johnson P.T.J. A practical guide for the study of malformed amphibians and their causes. J. Herpetol. 2012;46:429–441. [Google Scholar]
- Lunde K.B., Resh V.T., Johnson P.T.J. Using a whole-ecosystem manipulation to understand host-parasite interactions and how they vary with study venue. Ecosphere. 2012;3:84–91. [Google Scholar]
- Martof B. Growth and development of the green frog, Rana clamitans, under natural conditions. Am. Midl. Nat. 1956;55:101–117. [Google Scholar]
- Mas-Coma S., Montoliu I. The life cycle of Brachylaima ruminae n. sp. (Trematoda: Brachylaimidae), a parasite of rodents. Z. für Parasitenkd. 1986;72:739–753. doi: 10.1007/BF00925095. [DOI] [PubMed] [Google Scholar]
- Meteyer C.U., Loeffler I.K., Fallon J.F., Converse K.A., Green E., Helgen J.C., Kersten S., Levey R., Eaton‐Poole L., Burkhart J.G. Hind limb malformations in free‐living northern leopard frogs (Rana pipiens) from Maine, Minnesota, and Vermont suggest multiple etiologies. Teratology. 2000;62:151–171. doi: 10.1002/1096-9926(200009)62:3<151::AID-TERA3>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Ouellet M. Society of Environmental Toxicology and Chemistry. SETAC) Press; Pensacola, Florida: 2000. Amphibian deformities: current state of knowledge, Ecotoxicology of Amphibians and Reptiles; pp. 617–661. [Google Scholar]
- Peterson N.A. Seasonal prevalence of Ribeiroia ondatrae in one population of Planorbella trivolvis (= Helisoma trivolvis), including notes on the larval trematode component community. Comp. Parasitol. 2007;74:312–318. [Google Scholar]
- Reeves M.K., Medley K.A., Pinkney A.E., Holyoak M., Johnson P.T.J., Lannoo M.J. Localized hotspots drive continental geography of abnormal amphibians on US wildlife refuges. PLoS One. 2013;8 doi: 10.1371/journal.pone.0077467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richgels K.L., Hoverman J.T., Johnson P.T.J. Evaluating the role of regional and local processes in structuring a larval trematode metacommunity of Helisoma trivolvis. Ecography. 2013;36:854–863. [Google Scholar]
- Riepe T.B., Calhoun D.M., Johnson P.T.J. Comparison of direct and indirect techniques for evaluating endoparasite infections in wild-caught newts (Taricha torosa and T. granulosa) Dis. Aquat. Org. 2019;134:137–146. doi: 10.3354/dao03365. [DOI] [PubMed] [Google Scholar]
- Roberts C., Dickinson T. Ribeiroia ondatrae causes limb abnormalities in a Canadian amphibian community. Can. J. Zool. 2012;90:808–814. [Google Scholar]
- Rosine W.N. Polydactylism in a second species of amphibia in Muskee Lake, Colorado. Copeia. 1955;1955:136. 136. [Google Scholar]
- Schell S.C. University Press of Idaho; Moscow, ID: 1985. Handbook of Trematodes of North America North of Mexico. [Google Scholar]
- Schotthoefer A.M. University of Illinois at Urbana-Champaign; 2003. Environmental, Landscape, and Host-Related Factors Associated with Parasitism and its Effects on Larvae and Metamorphic Frogs of Rana pipiens. [Google Scholar]
- Schotthoefer A.M., Labak K.M., Beasley V.R. Ribeiroia ondatrae cercariae are consumed by aquatic invertebrate predators. J. Parasitol. 2007;93:1240–1243. doi: 10.1645/GE1129R.1. [DOI] [PubMed] [Google Scholar]
- Sessions S.K., Ruth S.B. Explanation for naturally occurring supernumerary limbs in amphibians. J. Exp. Zool. 1990;254:38–47. doi: 10.1002/jez.1402540107. [DOI] [PubMed] [Google Scholar]
- Snyder S.D., Tkach V.V. Neosychnocotyle maggiae, n. gen., n. sp. (Platyhelminthes: Aspidogastrea) from freshwater turtles in northern Australia. J. Parasitol. 2007;93:399–403. doi: 10.1645/GE-1001R.1. [DOI] [PubMed] [Google Scholar]
- Souder W. A plague of frogs: the horrifying true story. Hyperion Books. 2000 [Google Scholar]
- Strasburg M., Boone M.D. Effects of trematode parasites on snails and Northern Leopard Frogs (Lithobates pipiens) in pesticide-exposed mesocosm communities. J. Herpetol. 2021;55:229–236. [Google Scholar]
- Stuart S.N., Chanson J.S., Cox N.A., Young B.E., Rodrigues A.S., Fischman D.L., Waller R.W. Status and trends of amphibian declines and extinctions worldwide. Science. 2004;306:1783–1786. doi: 10.1126/science.1103538. [DOI] [PubMed] [Google Scholar]
- Sutherland D. In: Amphibian Declines: the Conservation Status of United States Species. Lannoo M.J., editor. University of California Press; Calfornia: 2005. Parasites of North American frogs; pp. 109–123. [Google Scholar]
- Talbot S.B. A description of four new trematodes of the subfamily Reniferinae with a discussion of the systematics of the subfamily. Trans. Am. Microsc. Soc. 1934;53:40–56. [Google Scholar]
- Tkach V., Pawlowski J.W. A new method of DNA extraction from the ethanol-fixed parasitic worms. Acta Parasitol. 1999;44:147–148. [Google Scholar]
- Tkach V.V., Littlewood D.T.J., Olson P.D., Kinsella J.M., Swiderski Z. Molecular phylogenetic analysis of the Microphalloidea ward, 1901 (Trematoda: Digenea) Syst. Parasitol. 2003;56:1–15. doi: 10.1023/a:1025546001611. [DOI] [PubMed] [Google Scholar]
- United States Department of Agriculture . 2022. Drought conditions in Western States in summers of 2021, 2022 were the most intense in 20 years.https://www.ers.usda.gov/data-products/chart-gallery/gallery/chart-detail/?chartId=104849 [Google Scholar]
- Vandenlangenberg S.M., Canfield J.T., Magner J.A. A regional survey of malformed frogs in Minnesota (USA) Environ. Monit. Assess. 2003;82:45–61. doi: 10.1023/a:1021684723301. [DOI] [PubMed] [Google Scholar]
- Wake D.B., Vredenburg V.T. Are we in the midst of the sixth mass extinction? A view from the world of amphibians. Proc. Natl. Acad. Sci. USA. 2008;105:11466–11473. doi: 10.1073/pnas.0801921105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilber M.Q., Briggs C.J., Johnson P.T.J. Disease's hidden death toll: using parasite aggregation patterns to quantify landscape‐level host mortality in a wildlife system. J. Anim. Ecol. 2020;89:2876–2887. doi: 10.1111/1365-2656.13343. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.