Highlights
-
•
The native extracellular matrix, characterized by its diverse biochemical compositions, hierarchical microstructure, and dynamic mechanical properties, functions as an angiogenic biomaterial.
-
•
Physiological angiogenesis involves a coordinated interplay between growth factor signaling, integrin-mediated mechanosensing, and extracellular matrix dynamics.
-
•
Designer synthetic angiogenic biomaterials with tunable material properties offer an in vitro biosystem to investigate vascular biology.
-
•
The ECM's role in wound healing and tumor progression underscores its significance in clinical interventions, proposing new therapeutic strategies to improve therapeutic outcomes in angiogenesis-related diseases.
Keywords: Angiogenesis, Extracellular matrix, Growth factors, Cell adhesion, Biomaterials, Wound healing, Tumor vascularization
Abstract
While the extracellular matrix (ECM) has long been recognized for its structural contributions, anchoring cells for adhesion, providing mechanical support, and maintaining tissue integrity, recent efforts have elucidated its dynamic, reciprocal, and diverse properties on angiogenesis. The ECM modulates angiogenic signaling and mechanical transduction, influences the extent and degree of receptor activation, controls cellular behaviors, and serves as a reservoir for bioactive macromolecules. Collectively, these factors guide the formation, maturation, and stabilization of a functional vascular network. This review aims to shed light on the versatile roles of the ECM in angiogenesis, transcending its traditional functions as a mere structural material. We will explore its engagement and synergy in signaling modulation, interactions with various angiogenic factors, and highlight its importance in both health and disease. By capturing the essence of the ECM's diverse functionalities, we highlight the significance in the broader context of vascular biology, enabling the design of novel biomaterials to engineer vascularized tissues and their potential therapeutic implications.
Angiogenic microenvironment – The extracellular matrix composition, organization, and function
Angiogenesis, the formation of new blood vessels from pre-existing vasculature, is a fundamental process underpinning both physiological and pathological events [1]. From delivering oxygen and nutrients during embryonic development to facilitating wound healing, angiogenesis plays an important role in tissue homeostasis and regeneration [[2], [3], [4]]. Conversely, aberrations in angiogenesis contribute to many disorders such as cardiovascular diseases, chronic inflammation, diabetic ischemia, and tumorigenesis [[5], [6], [7]]. Historically, angiogenesis research has predominantly focused on investigating soluble angiogenic growth factors and cytokines, emphasizing their roles in controlling transcriptional regulation, processing, binding to cell-surface receptors and the activation of signaling pathways that control vessel growth [1,8]. However, the extracellular matrix (ECM), a complex network of proteins, glycoproteins, and proteoglycans, has emerged as a critical player that orchestrates growth factor (GF) binding, angiogenic signaling, and processes beyond ECM's basic structural features [4,9].
The native cell microenvironment, governed by the composition, organization, mechanical dynamics, and signaling regulators of the ECM, plays a pivotal role in angiogenesis [10]. Previously considered as a static structural component, the ECM has recently gained increased recognition for its influence on vascular network formation, maturation, and the maintenance of vasculature [[11], [12], [13], [14]]. Beyond functioning as a scaffolding material for mechanical support during multicellular morphogenesis, the ECM also serves as a coordinator of biochemical signals that actively modulate the behavior and function of endothelial cells (ECs) [15,16]. The key ECM components (Table. 1 and Fig. 1) contributing to angiogenesis can be broadly categorized to include the interstitial matrix and basement membrane. The interstitial matrix, which forms the primary structural framework of the ECM, is composed of fibrous proteins such as collagen and elastin. These compounds provide mechanical strength, elasticity, and integrity to tissues. Adhesive glycoproteins such as fibronectin are also present in the interstitial matrix, connecting to cell-surface integrins to support cell adhesion, migration, and cell-matrix interaction essential for vascular development. The vascular basement membrane, a dense mesh-like network underlying endothelial cell layers, is primarily composed of laminin and collagen type IV to provide cell adhesion and regulate blood-barrier functions. Pericytes, recruited by various angiogenic factors such as PDGF-BB and SDF1-α to wrap around the endothelium, play a critical role in maintaining vascular stability and homeostasis by promoting cell-cell interactions and depositing an array of ECM components, including fibronectin, laminin, collagen-IV, nidogen-1 and angiopoietin-1, during early vascular development, angiogenic sprouting, and vessel maturation and stabilization [17]. Both the interstitial ECM and vascular basement membrane are also abundant in heparan sulfate proteoglycans (HSPGs) and glycosaminoglycans (GAGs), for example heparin, perlecan and hyaluronic acid (HA). These glycans mediate GF sequestration, control vascular function, and are responsible for water retention, which resists compressive mechanical forces and provides hydration to the surrounding matrix.
Table 1.
Summary of common extracellular matrix proteins and their function in promoting angiogenesis [10,11,[22], [23], [24]].
| ECM | Biological Function | Context |
|---|---|---|
| Collagen I | Interstitial and granulation matrix, fibrous network, cell adhesion, contraction and migration, structural support, tensile strength | Bone formation, tissue repair, fibrosis |
| Collagen III | Interstitial and granulation matrix, colocalized with collagen I to provide structural integrity and support for soft tissues, cell attachment, proliferation and migration | Wound healing, blood vessel formation |
| Collagen IV | Basal lamina matrix, cell adhesion, migration, and differentiation, network forming, ECM organization and stabilization, regulate barrier permeability and function, growth factor sequestration | Blood-brain barrier, cell signaling |
| Laminin | Basal lamina matrix, cell survival, adhesion, proliferation, and migration, cell polarity and metabolism, interactions with other ECM proteins (e.g., Collagen IV, perlecan) to provide structural support for basement membrane, regulate barrier function, and promote tissue morphogenesis | Lung branching morphogenesis, vascular barrier function |
| Fibronectin | Interstitial, provisional and granulation matrix, cell adhesion, migration, proliferation, and differentiation, cell surface receptor binding and activation, GF sequestration, ECM organization, and angiogenesis | Mechanotransduction, angiogenesis, tissue repair, cancer dormancy |
| Proteoglycan | Regulation of growth factors and cytokines, structural organization of ECM to mediate cell signaling, tissue hydration and resilience, barrier function, modulation of enzymatic activity | Angiogenesis, embryonic development tissue repair, stem cell differentiation |
| Fibrinogen | Provisional matrix, converts to fibrin and form blood clots for structural support, cell adhesion and migration, modulates the activity of inflammatory cytokines, initial wound scaffold | Angiogenesis, wound healing, inflammation, tissue remodeling |
| Vitronectin | Cell adhesion and spreading, regulation of blood coagulation and fibrinolysis, matrix remodeling and immune response | Coagulation, wound healing, angiogenesis |
| Perlecan | Basement membrane heparan sulfate proteoglycan, cell adhesion and migration, cell signaling, growth factor regulation | Inflammation, cardiac development, cancer angiogenesis |
| Tenascin-C | Extracellular glycoprotein, structural support and organization of the ECM, multi-domain structure to interact with various other ECM proteins for cell adhesion, migration and tissue remodeling, upregulation in the stroma of tumors | Embryonic development, cancer progression and tumor angiogenesis |
| Elastin | Interstitial matrix, structural and mechanical support, tissue elasticity and resilience, regulation of cellular behavior, and tissue integrity and function | Tissue regeneration and function |
Fig. 1.
The extracellular matrix coordinates both biochemical cues and biophysical properties to regulate angiogenesis. A schematic representation of pro-angiogenic microenvironment with a selection of key extracellular matrix components involved in physiological angiogenesis. Highlighted here is the role of the extracellular matrix in modulating local growth factor sequestration and their binding affinity, and control of specific integrin activation, with an emphasis on cell-extracellular matrix signaling crosstalk between angiogenic pathway and integrin-mediated mechanosensing for angiogenesis. ECM: extracellular matrix; MMP: matrix metalloproteinases; FAC: focal adhesion complexes.
An angiogenic microenvironment exhibits complex chemical compositions and hierarchical microstructural organization, featuring primary ECM elements cohesively regulating the angiogenic signaling and processing in endothelial cells in a stiffness-dependent manner [[18], [19], [20]]. The native ECM, serving as a biomaterial, actively modulates cell behavior and remodels their microenvironment through its diverse molecular and mechanical properties, thereby effectively regulating angiogenic activities [15,21]. This biomolecular material system presents a platform to coordinate the spatiotemporal coordination of biochemical and biophysical cues, impacting various cellular behaviors such as cell adhesion, migration, proliferation, and differentiation involved in the process of angiogenic sprouting, invasion, and anastomosis of functional vasculature. Cells and cell-secreted factors interact dynamically with the highly specific ECMs and are regulated in turn by the microenvironmental cues that drive the outcomes of physiological and pathological angiogenesis.
Growth factor signaling and integrin-mediated mechanotransduction crosstalk in angiogenesis
Physiological angiogenesis involves a coordinated interplay between GF sequestration and integrin-mediated mechanosensing to induce endothelial morphogenesis and assembly into new tubular structures [[25], [26], [27], [28]]. Angiogenesis is initiated in response to a hypoxic environment, causing a concentration gradient of pro-angiogenic growth factors to be released, activating quiescent blood vessels, and establishing sprouting [1,29]. Cell-secreted GFs diffuse through interstitial space and bind to sulfated molecules present in the ECM [30], where the distribution and organization of these highly negative constituents create different binding affinities to GFs, as well as control their local biodistribution and extent of cell-surface receptor activation. Although many GFs (e.g., basic fibroblast growth factor (bFGF), platelet-derived growth factor (PDGF), transforming growth factor-beta (TGF-β)), are involved in this process, vascular endothelial growth factor-A (VEGF-A) is the master modulator of angiogenesis [31,32]. It binds to the tyrosine kinase VEGFR-2 receptor on endothelial cells and enhances phosphorylation of Tyr1214, activating the angiogenic cascade and downstream signaling for new vessel growth [33]. Splicing variants in the VEGF-A gene generates different isoforms (e.g., VEGF-A121, VEGF-A165, and VEGF-A189 in humans) and alterations in the expression of these isoforms lead to changes in their binding affinity to the ECM, resulting in distinctive vascular patterns and morphologies [34]. For example, the non-ECM binding VEGF-A121 induces the formation of shorter, leaky blood vessels with larger vascular diameters and reduced branching, particularly notable in the tumor microenvironment, while VEGF-A189, with strong-ECM binding, causes aberrant branching and reduced capillary size [35,36]. However, VEGF-A165, with an intermediate ECM affinity, forms physiologically patterned vascular morphology, indicating the strength of GFs-ECM binding plays an important role in angiogenesis [[35], [36], [37]].
The specific ECM composition and their relative interactions with each other also control how GFs bind to the ECM and consequently impact the outcome of angiogenesis. Heparan sulfated proteoglycans, such as heparin and syndecan, have long been acknowledged as major ECM components capable of sequestering numerous GFs through electrostatically mediated interactions [38,39]. Less negatively charged, non-proteoglycan ECM proteins such as fibronectin, fibrinogen, vitronectin and tenascin C have now been revealed to exhibit GF-specific interactions and bioactivities [40]. Pioneering work from Hubbell and co-workers validated the highly promiscuous GF binding region of fibronectin, specifically the heparin-binding domain II within the 12th-14th type three repeats (FN III12–14), demonstrating a strong binding affinity to multiple GFs including PDGF, VEGF, IGF, TGF-β and bFGF families for enhanced cell migration [41,42]. Recent reports have elucidated how both proteoglycan molecules and non-proteoglycan proteins work cohesively to further potentiate GF immobilization and signaling. Studies have shown that heparin/heparan sulfate mediates conformational changes in fibronectin, exposing cryptic VEGF binding sites, thereby enhancing VEGF-fibronectin interactions and angiogenic signaling [43]. Kinetic models developed for this process suggest the ability of heparin to convert fibronectin from closed VEGF binding sites to an open conformation, proposing a catalytic activation mechanism of heparin in remodeling key ECM proteins to enhance GF binding affinity for angiogenesis [44].
The ECM and GFs do not regulate angiogenesis in isolation. They regulate and are regulated by endothelial cells through integrin receptors [45]. Integrins, a family of ubiquitous heterodimeric transmembrane cell adhesion proteins, serve as bidirectional molecular hubs connecting cells to the ECM. These molecules coordinate both growth factor signaling and adhesion mechanosensing to facilitate dynamic interactions between cells and their surrounding microenvironment, playing a critical role in angiogenesis [46,47]. Integrin heterodimers consist of specific αβ integrin pairs controlling the recognition and binding to cryptic domains in ECM proteins, initiating the formation of intracellular adhesion complexes for downstream signaling [45]. In endothelial cells, specific integrin pairs, for instance α3/α5β1 and αvβ3, have been recognized for their roles in vascular morphogenesis and vessel patterning [32]. However, activation of α3/α5β1 integrin promotes ECs to form organized vascular network while αvβ3 induces tortuous vessels with increased permeability, mostly upregulated in tumor ECs [46,48]. These findings highlight the dynamic role of specific integrin types as mechanical sensors in mediating the biochemical signaling that is essential for vascular morphogenesis.
The coexistence and proximity of binding site for both GF-receptors and integrins enables integrin-mediated direct activation of GF-receptors and vice versa, creating coactivation crosstalk between both signaling pathways. GF-receptor activation can directly initiate adhesion signaling, strengthening its binding affinity to the ECM in an integrin-specific manner. For instance, stimulating microvascular ECs with VEGF-A activates multiple integrins, including αvβ3, αvβ5, α5β1, and α2β1 [47], whereas stimulation of ECs with bFGF-2 decreases the expression of αvβ3 and α1β1 integrins [49]. Similarly, spatiotemporal regulation of specific integrins without GFs promotes the expression of GF receptors. Studies have shown that fibrinogen-mediated αvβ3 activation is required for upregulating bFGF-2 expression and enhancing EC proliferation [50], while inhibiting β3 integrin increases VEGF-A-mediated blood vessel permeability [51]. To synergize and amplify crosstalk between angiogenic signaling and adhesion, approaches tethering VEGF to the ECM have been applied to activate both integrins and GF-receptors simultaneously. This resulted in a sustained activation of VEGFR2 internalization and clustering with increased colocalization of β1 expression in human aortic ECs [52]. These findings suggest the formation of a complex cell-GF-integrin-matrix signaling loop where the presentation of GFs (soluble versus bound) and molecular associations between ligands and receptors jointly alter GF-receptor clustering and gene expression while concurrently engaging integrin-mediated mechanotransduction and cytoskeleton remodeling required for vascular morphogenesis (Fig. 2).
Fig. 2.
Extracellular matrix regulation of coordinated growth factor signaling and integrin-mediated mechanosensing for angiogenesis. Angiogenic growth factors secreted by cells are sequestered in the extracellular matrix and interact with various matrix components to partition different binding to their receptors and synergize with specific integrins at the cell surface to potentiate angiogenic signaling.
While the initiation of angiogenesis is primarily triggered by GF signaling, the directed invasion of endothelial sprouts into a hypoxic 3D matrix and subsequent processes of lumen formation, anastomosis, vessel stabilization, and maturation are highly influenced by the delicate balance of ECM degradation, matrix mechanical properties, and the regulation of GFs [1]. As ECs transition from quiescent to active, induced by VEGF and Notch signaling, they evolve into tip cells, adopting a more invasive phenotype characterized by numerous finger-like filopodia structures, followed by stalk cells [1]. These tip cells secret an array of matrix degrading enzymes, particularly matrix metalloproteases (MMPs) including 1, 2, 9 and MTI-MMP, to breakdown basement membrane proteins (e.g., laminin, collagen IV) and interstitial matrix proteins (e.g. collagen I and III) for sprout initiation, invasion, and extension [53]. An insufficient amount of MMPs fails to degrade the surrounding matrix, impeding EC sprouting and invasion. A reduction in MMP-2 activity is associated with the dysregulation of ECM degradation andcontributes to cardiac fibrosis [54]. Conversely, an excessive amount of MMPs significantly degrades the ECM and compromises matrix mechanical properties, yielding a disorganized and fragmented matrix, unable to support cell adhesion and multicellular migration required for functional angiogenesis [55]. This highlights the importance of balanced regulation in the case of MMP remodeling and matrix integrity (e.g., microstructure, organization, and stiffness).
Designer pro-angiogenic biomaterials
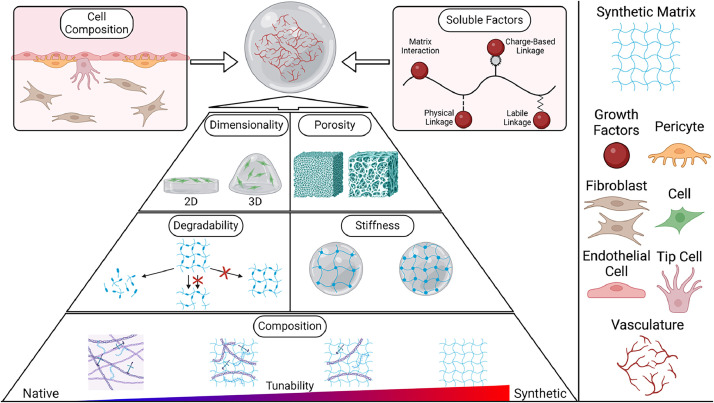
Biomaterials engineered with tissue-like properties have emerged as powerful tools for investigating how cells sense angiogenic stimuli and respond to mechanical properties, offering design principles to model angiogenesis [15,56]. The crosstalk between GF signaling pathways and ECM remodeling provides valuable information and guides engineers to develop angiogenic materials. Establishing in vitro biomaterial systems with tunable material properties (Fig. 3) enables the exploration of new vascular biology and translation of findings to enhance therapeutic angiogenesis outcomes. Native ECM-derived biopolymers, including collagen, fibrin and Matrigel, have been extensively used in angiogenesis studies owing to their ability to mimic the native angiogenic microenvironment by preserving biochemical and biophysical properties. However, these biological materials are limited by the difficulty of tuning and decoupling material properties. For example, to achieve a higher material stiffness typically requires increased crosslinking density and biochemical cues (e.g., adhesion ligands and GF binding domains) with uncontrolled protease-mediated degradation posing challenges for stability during long-term vessel culture [57]. To recapitulate critical aspects of native ECM while introducing necessary cues with tunability, synthetic biomimetic hydrogels offer an alternative approach to biological ECMs. Unlike their biological counterparts, synthetic biomaterials with selective crosslinking chemistries enable the control of many mechanical, structural and topological properties without altering biochemical composition (Fig. 3). These decoupled material properties facilitate the examination of how endothelial cells sense GF gradients, interact with the ECM, and integrate physical forces during angiogenesis [58].
Fig. 3.
Designer approaches to engineer biomaterial properties for the generation of vascularized microtissues. Various designer parameters are employed to independently control specific properties of biomaterials, aiming to recapitulate the chemical, physical, and structural features of the native extracellular matrix to support vascularization. These parameters include dimensionality, porosity, degradability, stiffness, and tunable chemical composition.
To formulate effective synthetic, angiogenic biomaterials, certain considerations must be incorporated into the material design. These considerations include non-toxic, tunable, biomimetic characteristics, with cell-friendly gelation processes that support the regulation of GF sequestration and cell-matrix interactions in a way that contributes to clinical significance. Although synthetic materials typically lack biological functionality and cell-interactive capability, progress in macromolecular chemistry now enables conjugation of ECM-derived adhesive motifs (e.g., integrin-binding RGD sequence from fibronectin) and degradable sequences, derived from collagen I, into synthetic scaffolds to endow matrices with increased material complexity achieving controlled cellular function [59].
Inspired by how GFs induce angiogenic signaling, early efforts focused on incorporating key pro-angiogenic GFs in polymeric scaffolds and tuning network porosity (through changing crosslinking density) to attain a controlled release of those factors [60]. However, such “passive” regulation of GF diffusion exhibited a release kinetic profile that is highly dependent on the physiochemical properties of the materials used, where an optimized GF loading concentration from one system didn't achieve similar outcomes when applied to a different material, prompting further optimization and improved approaches. To modify the delivery strategy through a switch to “active” sequestration, GF-binding proteoglycans have been incorporated into various biomaterials and have become a popular approach that facilitates sustained release of multiple GFs and prolonged receptor activation to drive angiogenesis across diverse material categories [61]. However, native heparin/heparan sulfate isolated from animal tissues displays considerable heterogeneity in its chemical composition, molecular structure, and sulfate patterns, eliciting undesired responses such as hemorrhage during in vivo settings, posing challenges in controlling its various biological activities and raising concerns for clinical use [62]. Several alternative chemical routes have been implemented to gain synthetic control over sulfate patterns and spacing of charge density along the polymer backbone to better control biological functions including anticoagulation pharmacokinetics and GF binding affinity for angiogenesis. For example, Liu and Linhardt reported a chemoenzymatic synthesis leading to structurally homogeneous ultralow molecular weight (ULMW) heparin pentasaccharide with predictable in vivo pharmacological anticoagulation activities [63]. Exploiting similar approaches for angiogenesis application, bioengineers have since modified polymer backbones with sulfate moieties (e.g., styrene sulfonate units) and generated heparin-mimetic polymer conjugates that stabilize the bioactivity of bFGF upon delivery [64,65]. In addition to introducing sulfate residues, strategies involving heparin desulfation to create heparin derivatives with reduced sulfation patterns have allowed systematic investigation of how the GAG concentration and sulfation density cooperatively determine the retention of local VEGF165 and, consequently, the spatial organization of vascular patterns [66]. Beyond sulfated proteoglycans, ECM proteins (e.g., fibronectin and fibrinogen) containing heparin-binding motifs exhibit a promiscuous high-affinity capability for binding various GFs [42,67], where incorporating the heparin domain from fibrinogen into synthetic PEG hydrogels imparted GF-binding affinity and gained pro-angiogenic functions, effectively supporting chronic wound healing in diabetic mouse models [67].
Mechanical properties, particularly matrix stiffness, directly guide a broad range of cellular behavior [68,69], including adhesion, contraction, proliferation, collective migration, and differentiation of ECs during vascular network formation. Early work culturing ECs on 2D substrates revealed stiffness-dependent responses, where increased substrates stiffness (via increased crosslinking) led to enhanced EC spreading, extension, and proliferation [70]. Furthermore, substrate stiffness is also capable of stimulating the arterial and venous specification of endothelial progenitor cells (EPCs). Rigid substrates (∼130 kPa) promote arterial cell phenotypes and softer substates (∼5 kPa) induce venous lineages via the Ras/Mek mechanosensing pathway [71,72]. Given how EC morphology and behavior are influenced by substrate stiffness, researchers began to investigate the crosstalk between substrate stiffness and VEGF signaling. Pioneering studies by Ingber and colleagues reported a new mechanosensitive transcriptional mechanism for angiogenesis where ECM stiffness induced a VEGF-independent, biphasic response in VEGFR-2 expression level via balancing the activities between TFII-I and GATA-2, two antagonistic transcription factors [73]. This stiffness-mediated control of receptor expression was further supported by many other studies, confirming that increasing matrix stiffness also enhances VEGFR-2 internalization, ERK1/2 phosphorylation, cell proliferation and actin stress fibers formation via Rho-mediated signaling and actin contractility [20]. These findings highlight the essential role of matrix stiffness in cooperating GF binding, processing, and signaling to enhance angiogenic progression.
Seminal studies employing synthetic materials as a tool to modulate stiffness have uncovered central mechanosensing machineries and focal adhesion complexes transducing forces that drive vascular morphogenesis [9]. Substantial efforts have since been invested in developing cytocompatible crosslinking chemistry and 3D encapsulation protocols to model multicellular endothelial invasion and network assembly in vitro, transitioning from simplistic 2D platforms to complex, physiological relevant 3D systems [74]. While ECs cultured on 2D substrates exhibited a classic stiffness-dependent cellular response [75], the encapsulation and culturing ECs in 3D revealed a contrasting and complex cell behavior [76]. By integrating a tunable synthetic matrix with a microfluidic system to recapitulate the physiological 3D biomimicry of angiogenic sprouting, Chen and coworkers revealed a biphasic sprouting response mediated by both matrix stiffness and degradability, where both low and high degradability posed problems, promoting either single cell migration or restricting cell invasion. However, intermediate stiffness supported multicellular sprouting with strand-like invasions required for angiogenesis. These findings undoubtably underscore the interplay between various matrix properties and emphasize the importance of balancing matrix stiffness and degradability in regulating cellular behavior and angiogenesis in 3D context [77].
To further increase biophysical complexity and emulate the dynamic features of native ECM in synthetic biomaterials, recent advancements have shifted from static to dynamic hydrogel design [12], particularly focusing on capturing the non-linear and time-dependent viscoelastic mechanical properties in synthetic biomaterials. By tailoring the gelation mechanism, selecting between covalent versus non-covalent crosslinking, one can formulate substrates with minimal or significant stress relaxation. Cells cultured on these hydrogels developed a stress-relaxation dependent morphology wherein soft, stress relaxing matrices enhanced cell spreading and focal adhesion compared to their elastic counterparts with the same stiffness [78]. An alternative approach employed dynamic covalent crosslinking via imine and acylhydrazone coupling to generate adaptable hydrogels with an intrinsic dynamic equilibrium of bond association and disassociation, promoting vascular morphogenesis. In contrast, static bond formation from methacrylate-mediated UV polymerization created non-dynamic hydrogels that do not allow for network remodeling nor vascular network formation [79]. Collectively, these studies suggest that, in addition to stiffness, substrate non-linear mechanics and time-dependent stress relaxation are fundamental physical parameters that substantially impact cell behavior and function. While the mechanisms by which cells perceive such dynamic mechanical properties or how matrix time-dependent features regulate angiogenic signaling are not fully understood, continuing to introduce additional complexity in material design will open opportunities to fully capture cell-ECM interactions that mimic those found in the native ECM [79].
Our continuously evolving understanding of the ECM and its interactions with cells has propelled progress in developing innovative biomaterials for vascular tissue engineering [15,21,80]. Material-based in vitro systems allow for the isolation of cells from tissues and enable their culturing in 3D soft, tissue-like matrices with the ability to control the concentration and spatiotemporal regulation of dynamic materials properties, which has contributed significantly to identifying key regulators that govern angiogenic signaling. However, challenges remain as even the most complex biomaterials engineered thus far still cannot fully capture all the properties of the native ECM. Current biomaterials are typically constructed from a limited set of components, whereas in vivo, a diverse array of ECM components undergo constant remodeling orchestrated by multiple cells across nano-, micro-, and macro-scales [10,81,82]. Various cells function cohesively, exerting forces through a multitude of coordinated on-and-off binding interactions over a spectrum of receptors, from sensing mechanical gradient for durotaxis [83] and squeezing through dense matrix for sprouting invasion [84] to pulling fibers for ECM assembly and fostering enhanced focal adhesion and GF biodistribution [85]. Bridging this gap between in vitro biomaterials and the in vivo microenvironment remains a critical frontier in the pursuit of more effective and biomimetic biomaterials and biosystems for advancing angiogenesis research.
Clinical significance – ECM implications for new therapies and interventions
As a dynamic angiogenic scaffold, the ECM is integral in regulating both physiological and pathological outcomes. However, in the translation of angiogenic factors into clinical therapeutics, the significance of the ECM has long been overlooked, leading to considerable challenges arising from both efficacy and safety concerns. Here, we highlight two critical processes in which the ECM is significantly influential, for clinical applications in wound healing and tumor vascularization (Fig. 4).
Fig. 4.
Schematic representation and molecular components involved during different stages of wound healing versus tumor angiogenesis and progression. (a) Initial disruption of homeostasis, growth factor secretion during the inflammation stage, endothelial proliferation and granular tissue formation, and tissue remodeling leading to wound closure and scar tissue formation; (b) progression of tumor cluster formation, ECM remodeling, vascularization, and intravasation, allowing the primary tumor to disseminate to distant locations and form secondary tumors.
Wound healing
Wound healing is a highly coordinated process that involves substantial morphogenetic changes in the cellular structures, and their interactions with the surrounding matrix and soluble factors [86]. The wound healing cascade is typically described as 4 tightly regulated steps: (i) homeostasis, which lasts from a few minutes to hours and involves the formation of fibrin clots to stop injured tissues from bleeding; (ii) the inflammatory phase, characterized by the recruitment of immune cells (e.g., neutrophils, macrophages) to the injury site to clean the wound bed, typically within the first few days (1∼3 days); (iii) the proliferation stage, which requires the formation of a vascularized, matrix-rich granulation tissues, followed by epidermal migration and cell division for reepithelialization and restoration of barrier integrity, lasting approximately days to weeks, and (iv) maturation/remodeling, the final stage of wound healing which can last from weeks, months to years is driven by myofibroblasts and the continuous turnover remodeling of the newly deposited provisional matrix for wound closure (Fig. 4a) [86,87]. Angiogenesis is a critical step during normal wound healing, where the formation of granulation tissue, a process that occurs in the late inflammation stage and early proliferation stage, serves as a foundation for rapid wound remodeling and healing [88]. Granulation tissue is a highly vascularized provisional matrix composed of multiple cell types including endothelial cells and fibroblasts, and cell-secreted ECM proteins [89]. Conventional 2D scratch wound assay doesn't recapitulate cell migration in 3D nor capture the complexity of cell-matrix interactions required for vascularization and granulation tissue formation during the healing of a multilayered and structured tissue [90,91]. To capture the coordinated interactions of endothelial cells and fibroblasts in a 3D matrix in the context of physiological wound closure, Tefft et al. developed a humanized in vitro system of vascularized wound healing and granulation tissue formation. This system reveals the dynamics of cellular migration and contraction-mediated closure of three-dimensional wounds with cell-deposited extracellular matrix, representative of early granulation tissue formation [92]. Failure to form a vascularized granulation tissue can lead to the development of chronic wounds typically associated with cardiovascular ischemic conditions, diabetic ulcers, and cancer [93]. Chronic wounds often exhibit biochemical abnormalities in the ECM shown by in vivo studies where cells in chronic wounds fail to secrete sufficient angiogenic factors to induce endothelial migration and vascular invasion [94]. Thus, delivering exogenous GFs to the location of desired angiogenesis has emerged as a promising and broadly applicable approach in the clinical setting.
While preclinical trials of delivering several GFs (e.g., GM-CSF, bFGF and VEGF) to the wound sites have shown initial promise in promoting and accelerating the healing and tissue regeneration, those small randomized clinical trials have yet to yield significant outcomes [95]. Becaplermin, a recombinant human platelet-derived growth factor-BB (PDGF-BB) commercially known as Regranex, is the only marketed angiogenic drug approved by the FDA in 1997 used for the topical treatment of diabetic foot ulcers [96,97]. However, challenges persist, necessitating the administration of supraphysiological doses and repeated injections to counteract enzymatic degradation and rapid clearance of becaplermin in the wound bed, raising considerable safety concerns particularly increased risks of systemic cancer.
Limited success in clinical outcomes can be partially attributed to the fact that wound angiogenesis is not solely regulated by soluble angiogenic GFs but also by interactions with ECMs in their surrounding microenvironment. Soluble angiogenic factors alone don't effectively attract vessel invasion or sustain functional vasculature in vivo without the appropriate structural and mechanical cues. The vasculature resulting from uncontrolled soluble GF stimulation is highly disorganized, and the tortuous hyper-branched capillary networks ultimately fail to support stable vessels and sufficient perfusion [10,98,99]. In chronic wounds, excessive protease activities degrade newly synthesized matrix proteins and decrease mechanical properties significantly, creating a disorganized microstructure lacking key ECM proteins needed to regulate GF signaling and support appropriate multicellular migration and sprouting invasion [100]. Early endeavors delivering GFs to scarred or atrophied vocal fold tissues [101] and stem cell injections for chronic wounds treatment exhibited limited effectiveness, suggesting the pivotal role of a vascularized ECM structure in fostering the healing of chronic wounds. A recent randomized, open-label and controlled clinical trial evaluated safety and efficacy of Oasis® wound matrix, a porcine small-intestine submucosa derived ECM graft composed of collagen, glycosaminoglycans, proteoglycans, fibronectin, and growth factors (including bFGF and TGF-β), for the treatment of 130 patients with Stage III and IV full-thickness pressure ulcers. With the combination of key ECM components and GFs, this extracellular wound biocomposite demonstrated an improved outcome, promoting a 90 % reduction in the ulcer area for 55 % of the patient group who were treated with Oasis wound matrix versus 38 % of the control patient group, treated with standard care [102]. These clinical findings reinforce the idea that the implementation of ECM in combination with GFs has proven effective in clinical chronic wound treatment. The coordinated interactions within a 3D microenvironment are crucial for angiogenic signaling, matrix mechanosensing, cellular migration, tissue vascularization and the response to injury and subsequent repair.
When comparing wound angiogenesis versus tumor vascularization, both similarities and differences emerge. A tightly regulated GF gradient is necessary to initiate vessel sprouting during the early stages of wound healing, whereas dysregulated and sustained GFs facilitated intravasation during cancer metastasis. Maintaining a balance between matrix degradation and ECM production is critical for promoting physiological wound healing. In contrast, in the tumor microenvironment, overexpression of MMPs significantly decreases the mechanical integrity of the surrounding cellular matrix [53], promoting cooperative dissemination of cancer cells to secondary locations via migrating along tortuous and leaky vessels for invasion [103,104].
Tumor vascularization and progression
One of the hallmarks for cancer metastasis and tumor progression involves vessel co-operation mediated cell migration through an ECM-rich stroma where cancer cells can disseminate along the vasculature via intravasation and extravasation [105]. The timeline for tumor metastasis can be complex and varies widely depending on the type of cancer, the tumor microenvironment and location, and individual patient factors. A general outline of the stages includes primary tumor formation (months to years), followed by intravasation, where tumor cells invade and circulate in the bloodstream, and extravasation, where circulating tumor cells adhere to the endothelium and extravasate to invade the surrounding tissue (weeks to months) [106]. After initial colonization, micrometastasis occurs, with small, often clinically undetectable tumor clusters beginning to vascularize and grow into larger, clinically detectable secondary tumors at a different location, a process typically taking months to years [104,106].
While genetic mutations in tumor cells undoubtedly initiate malignancy, the metastatic progression is typically associated with an angiogenic switch and significantly influenced by biochemical gradients, ECM components and the organization of microstructures in the tumor microenvironment [104]. In the 1970s, Judah Folkman was the first to observe that tumor tissues were often highly vascularized with fragile and leaky blood vessels. He later proposed that tumor angiogenesis is a key contributor to tumor survival and growth, with early in vivo experiments demonstrating that tumor implants didn't grow and remained dormant in the absence of neovascularization [107]. These findings embarked a new concept where blocking angiogenesis could lead to tumor dormancy, and therefore the development of anti-angiogenesis drugs has emerged as a major approach for anticancer treatment [108].
Chemokines and GFs are among key tumor cell activators driving the transition of tumor cells from a quiescent state to an invasive phenotype to promote metastasis and tumor angiogenesis. Contrasting normal blood vessel, tumor vasculature typically exhibits many structural and functional abnormalities, including tortuous architecture, irregular blood flow, low oxygen levels, leakiness, and phenotypic heterogeneity in vessel size, shape, and diameter) [109]. The hypoxia-induced expression of hypoxia-inducible factor (HIF) has been shown to upregulate downstream VEGF signaling [110]. Together with disorganized ECM microstructure and dysregulated VEGF gradient, HIF contributes to tumor angiogenesis [103]. Given the essential role of VEGF in promoting vascular hyperpermeability and tumorigenesis [111], four FDA-approved anti-angiogenic drugs have primarily targeted for VEGF pathway and are currently in clinical testing. Although these anti-angiogenic drugs initially demonstrated modest efficacy in slowing metastatic cancer progression in some patients, recent clinical trials have started to show drug resistance, failing to achieve overall survival improvements. For instance, a phase III clinical trial of bevacizumab, the first FDA-approved monoclonal antibody targeting VEGF, for patients with early-stage colorectal carcinoma, resulted in no benefit to disease-free survival, raising questions regarding the efficacy of antiangiogenic agents in blocking different stages of tumor progression [112].
As highlighted, the ECM is a critical regulator that potentiates the activity of angiogenic factors to promote angiogenesis, which could be one possible contributing factor to the unsuccessful clinical outcome of anti-angiogenic therapy. For example, antiangiogenic therapy targeting VEGF signaling is commonly administrated to metastatic colorectal cancer patients, unfortunately, the survival rate is limited due to acquired drug resistance. A recent study by Fukumura and colleagues discovered that VEGF inhibition remodels the ECM in the tumor microenvironment and significantly increases the expression of HA and sGAGs in both mouse models and in patient samples [113]. The alteration in ECM composition and mechanics is correlated with increased tumor stiffness of colorectal cancer liver metastases, making them more difficult for treatment [113]. Similarly, McDonald and coworkers investigated the temporal effects of VEGF inhibitors on tumor revascularization using spontaneous RIP-Tag2 tumors in Lewis lung carcinomas mouse model. Although the inhibitors of VEGF-receptor signaling were effective in blocking angiogenesis and reducing tumor vascularity (60 %), rapid vascular regrowth was observed upon VEGF inhibitor withdrawal. This process was facilitated by the presence of “left-behind” laminin- and collagen-IV- rich vascular basement membrane proteins, which function as scaffolding material guiding rapid restoration of the tumor vasculature accompanied by surviving pericytes for vessel stabilization [114]. Recent efforts have also suggested that matrix proteins play a critical role in maintaining tumor dormancy or switching to a metastatic state [115,116]. Employing an in vitro cell culture system where structural ECM proteins can be added individually or in combination, Barney et al. revealed that dormant breast cancer cells deposited and assembled a fibronectin-rich matrix characterized by α5β1 integrin-mediated adhesion and ROCH-associated tension, while switching from dormancy to proliferative stage requires MMP-2-mediated fibronectin degradation [117]. In contrast, Weaver and coworkers revealed that inflammatory stromal cells upregulated lysyl hydroxylase 2 (LH2) to induce collagen crosslinking and stromal stiffening induces, which significantly correlates with tumor progression and disease specific mortality [118]. Together, these data emphasize that tumor microenvironmental properties including the ECM composition and microstructure play a critical role in tumor regression and revascularization, suggesting that local tumor microenvironment could be a co-targeting entity for improved anti-angiogenic therapeutic interventions.
Challenges and future horizons
As we reflect on the extensive literature surrounding angiogenesis, it is evident that the role of ECM extends far beyond just cell adhesion and structural functions. The intricate interplay between the ECM and angiogenic processes elucidates its dynamic contributions, spanning molecular signaling to multicellular responses, highlighting the ECM not just a passive framework but as an interactive modulator during physiological and pathological angiogenesis. Such insights have initiated the concept of angiogenic ECM mimicry which has emerged as a central theme in the design of the next generation of synthetic ECM biomaterials. These analogs are designed to endow the cooperative angiogenic signaling and cell mechanosensing, aiming to leverage the inherent complexity and multifunctionality of native ECM to influence angiogenesis. Emerging biotechnologies, such as microfluidics, organ-on-chips, and 3D bioprinting, have enabled biosystems to recapitulate tissue- and organ-level physiology and functionality that are not possible with conventional 2D or 3D culture systems [119]. Integrating biomaterials with microfluidics systems for tissue culture, studies have revealed previously unappreciated fluid mechanics that play a critical role in regulating angiogenic sprouting and maintaining hemostasis. For example, with user-defined flow profiles, steady perfusion at flow rates resembling physiological shear stress promoted the establishment of a functional vascular barrier [120], whereas increasing the shear stress flow rate to a threshold approximately 10 dyn/cm−2 triggers angiogenic sprouting [121]. In parallel, 3D bioprinting employs top-down approaches to precisely organize biological elements for scaling-up those miniaturized tissue constructs [122,123], presenting new opportunities to fabricate functional tissue substitutes for organ transplantation [124,125]. As the landscape of angiogenesis research continues to evolve, it becomes increasingly clear that our future endeavors will hinge on our ability to synergize biomaterial innovation and technological advances across multidiscipline studies, combining insights from vascular biology, materials science, bioengineering, device microfabrication, tissue manufacturing, and clinical research. Together, these collaborative efforts will drive the course for innovative, effective, and patient-specific angiogenic interventions for regenerative medicine.
CRediT authorship contribution statement
Jaxson R. Libby: Writing – original draft, Conceptualization. Haley Royce: Writing – original draft, Conceptualization. Sarah R. Walker: Writing – review & editing. Linqing Li: Writing – review & editing, Writing – original draft, Supervision, Project administration, Investigation, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to acknowledge the financial support from the New Hampshire BioMade provided by the National Science Foundation through EPSCoR Research Infrastructure Improvement Award IIA 1757371 (LL), the National Institute of General Medical Sciences of the National Institute of Health, P20GM113131 (SRW, LL) and R35GM155450 (LL). The schematics presented in all Figures were generated using BioRender.com.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.bbiosy.2024.100097.
Appendix. Supplementary materials
Data availability
No data was used for the research described in the article.
References
- 1.Potente M., Gerhardt H., Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146:873–887. doi: 10.1016/j.cell.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 2.Carmeliet P., Jain R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao Y., Xie L. Unique bone marrow blood vessels couple angiogenesis and osteogenesis in bone homeostasis and diseases. Ann N Y Acad Sci. 2020;1474:5–14. doi: 10.1111/nyas.14348. [DOI] [PubMed] [Google Scholar]
- 4.Humphrey J.D., Schwartz M.A. Vascular mechanobiology: homeostasis, adaptation, and disease. Annu Rev Biomed Eng. 2021;23:1–27. doi: 10.1146/annurev-bioeng-092419-060810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okonkwo U.A., Chen L., Ma D., Haywood V.A., Barakat M., Urao N., DiPietro L.A. Compromised angiogenesis and vascular Integrity in impaired diabetic wound healing. PLoS ONE. 2020;15 doi: 10.1371/journal.pone.0231962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nuevo-Tapioles C., Santacatterina F., Stamatakis K., Núñez de Arenas C., Gómez de Cedrón M., formentini L., Cuezva J.M. Coordinate β-adrenergic inhibition of mitochondrial activity and angiogenesis arrest tumor growth. Nat Commun. 2020;11:3606. doi: 10.1038/s41467-020-17384-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Annex B.H., Cooke J.P. New directions in therapeutic angiogenesis and Arteriogenesis in peripheral arterial disease. Circ Res. 2021;128:1944–1957. doi: 10.1161/CIRCRESAHA.121.318266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 9.Humphrey J.D., Dufresne E.R., Schwartz M.A. Mechanotransduction and extracellular matrix homeostasis. Nat Rev Mol Cell Biol. 2014;15:802–812. doi: 10.1038/nrm3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marchand M., Monnot C., Muller L., Germain S. Extracellular matrix scaffolding in angiogenesis and capillary homeostasis. Semin Cell Dev Biol. 2019;89:147–156. doi: 10.1016/j.semcdb.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 11.Hynes R.O. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216–1219. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burdick J.A., Murphy W.L. Moving from static to dynamic complexity in hydrogel design. Nat Commun. 2012;3:1269. doi: 10.1038/ncomms2271. [DOI] [PubMed] [Google Scholar]
- 13.Uto K., Tsui J.H., DeForest C.A., Kim D.-H. Dynamically tunable cell culture platforms for tissue engineering and mechanobiology. Prog Polym Sci. 2017;65:53–82. doi: 10.1016/j.progpolymsci.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei Z., Lei M., Wang Y., Xie Y., Xie X., Lan D., Jia Y., Liu J., Ma Y., Cheng B., Gerecht S., Xu F. Hydrogels with tunable mechanical plasticity regulate endothelial cell outgrowth in vasculogenesis and angiogenesis, Nat. Commun. 2023;14:8307. doi: 10.1038/s41467-023-43768-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Briquez P.S., Clegg L.E., Martino M.M., Mac Gabhann F., Hubbell J.A. Design principles for therapeutic angiogenic materials. Nat Rev Mater. 2016;1:15006. [Google Scholar]
- 16.Wang W.Y., Jarman E.H., Lin D., Baker B.M. Dynamic endothelial stalk cell–matrix interactions regulate angiogenic sprout diameter. Front Bioeng Biotechnol. 2021;9:620128. doi: 10.3389/fbioe.2021.620128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Payne L.B., Zhao H., James C.C., Darden J., McGuire D., Taylor S., Smyth J.W., Chappell J.C. The pericyte microenvironment during vascular development. Microcirculation. 2019;26:e12554. doi: 10.1111/micc.12554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santos L., Fuhrmann G., Juenet M., Amdursky N., Horejs C.-M., Campagnolo P., Stevens M.M. Extracellular stiffness modulates the expression of functional proteins and growth factors in endothelial cells. Adv Healthc Mater. 2015;4:2056–2063. doi: 10.1002/adhm.201500338. [DOI] [PubMed] [Google Scholar]
- 19.Sack K.D., Teran M., Nugent M.A. Extracellular matrix stiffness controls VEGF signaling and processing in endothelial cells. J Cell Physiol. 2016;231:2026–2039. doi: 10.1002/jcp.25312. [DOI] [PubMed] [Google Scholar]
- 20.LaValley D.J., Zanotelli M.R., Bordeleau F., Wang W., Schwager S.C., Reinhart-King C.A. Matrix stiffness enhances VEGFR-2 internalization, signaling, and proliferation in endothelial cells. Converg Sci Phys Oncol. 2017;3:44001. doi: 10.1088/2057-1739/aa9263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee J.-H., Parthiban P., Jin G.-Z., Knowles J.C., Kim H.-W. Materials roles for promoting angiogenesis in tissue regeneration. Prog Mater Sci. 2021;117 [Google Scholar]
- 22.Sottile J. Regulation of angiogenesis by extracellular matrix. Biochim Biophys Acta Rev Cancer. 2004;1654:13–22. doi: 10.1016/j.bbcan.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 23.Heissig B., Hattori K., Friedrich M., Rafii S., Werb Z. Angiogenesis: vascular remodeling of the extracellular matrix involves metalloproteinases. Curr Opin Hematol. 2003;10:136–141. doi: 10.1097/00062752-200303000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Rouwkema J., Khademhosseini A. Vascularization and angiogenesis in tissue engineering: beyond creating static networks. Trends Biotechnol. 2016;34:733–745. doi: 10.1016/j.tibtech.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 25.Kretschmer M., Rüdiger D., Zahler S. Mechanical aspects of angiogenesis. Cancers. 2021;13:4987. doi: 10.3390/cancers13194987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flournoy J., Ashkanani S., Chen Y. Mechanical regulation of signal transduction in angiogenesis. Front Cell Dev Biol. 2022;10:933474. doi: 10.3389/fcell.2022.933474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rauff A., Manning J.C., Hoying J.B., LaBelle S.A., Strobel H.A., Stoddard G.J., Weiss J.A. Dynamic biophysical cues near the tip cell microenvironment provide distinct guidance signals to angiogenic neovessels. Ann Biomed Eng. 2023;51:1835–1846. doi: 10.1007/s10439-023-03202-4. [DOI] [PubMed] [Google Scholar]
- 28.Bakhshandeh B., Ranjbar N., Abbasi A., Amiri E., Abedi A., Mehrabi M.-R., Dehghani Z., Pennisi C.P. Recent progress in the manipulation of biochemical and biophysical cues for engineering functional tissues. Bioeng Transl Med. 2023;8:e10383. doi: 10.1002/btm2.10383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 30.da Costa D., Reis R.L., Pashkuleva I. Sulfation of glycosaminoglycans and its implications in human health and disorders. Annu Rev Biomed Eng. 2017;19:1–26. doi: 10.1146/annurev-bioeng-071516-044610. [DOI] [PubMed] [Google Scholar]
- 31.Olsson A.-K., Dimberg A., Kreuger J., Claesson-Welsh L. VEGF receptor signalling ? in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 32.Simons M., Gordon E., Claesson-Welsh L. Mechanisms and regulation of endothelial VEGF receptor signalling. Nat Rev Mol Cell Biol. 2016;17:611–625. doi: 10.1038/nrm.2016.87. [DOI] [PubMed] [Google Scholar]
- 33.Shibuya M. Vascular endothelial growth factor (VEGF) and its receptor (VEGFR) signaling in angiogenesis: a crucial target for anti- and pro-angiogenic therapies. Genes Cancer. 2011;2:1097–1105. doi: 10.1177/1947601911423031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ruhrberg C., Gerhardt H., Golding M., Watson R., Ioannidou S., Fujisawa H., Betsholtz C., Shima D.T. Spatially restricted patterning cues provided by heparin-binding VEGF-A control blood vessel branching morphogenesis. Genes Dev. 2002;16:2684–2698. doi: 10.1101/gad.242002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park J.E., Keller G.-A., Ferrara N. The vascular endothelial growth factor (VEGF) isoforms: differential deposition into the subepithelial extracellular matrix and bioactivity of extracellular matrix-bound VEGF. Mol Biol Cell. 1993;4:1317–1326. doi: 10.1091/mbc.4.12.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vempati P., Popel A.S., Gabhann F.Mac. Extracellular regulation of VEGF: isoforms, proteolysis, and vascular patterning. Cytokine Growth Factor Rev. 2014;25:1–19. doi: 10.1016/j.cytogfr.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jeremy Grunstein R.H.F.G., Masbad Joseph J., Johnson R.S. Isoforms of vascular endothelial growth factor act in a coordinate fashion to recruit and expand tumor vasculature. Mol. Cell. Biol. 2000;20:7282–7291. doi: 10.1128/mcb.20.19.7282-7291.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Capila I., Linhardt R.J. Heparin–protein interactions. Angew Chemie Int Ed. 2002;41:390–412. doi: 10.1002/1521-3773(20020201)41:3<390::aid-anie390>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 39.Nugent M.A., Zaia J., Spencer J.L. Heparan sulfate-protein binding specificity. Biochem (Basel) 2013;78:726–735. doi: 10.1134/S0006297913070055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martino M.M., Briquez P.S., Güç E., Tortelli F., Kilarski W.W., Metzger S., Rice J.J., Kuhn G.A., Müller R., Swartz M.A., Hubbell J.A. Growth factors engineered for super-affinity to the extracellular matrix enhance tissue healing. Science. 2014;343:885–888. doi: 10.1126/science.1247663. [DOI] [PubMed] [Google Scholar]
- 41.Wijelath E.S., Rahman S., Namekata M., Murray J., Nishimura T., Mostafavi-Pour Z., Patel Y., Suda Y., Humphries M.J., Sobel M. Heparin-II domain of fibronectin is a vascular endothelial growth factor-binding domain. Circ Res. 2006;99:853–860. doi: 10.1161/01.RES.0000246849.17887.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martino M.M., Hubbell J.A. The 12th–14th type III repeats of fibronectin function as a highly promiscuous growth factor-binding domain. FASEB J. 2010;24:4711–4721. doi: 10.1096/fj.09-151282. [DOI] [PubMed] [Google Scholar]
- 43.Mitsi M., Hong Z., Costello C.E., Nugent M.A. Heparin-mediated conformational changes in fibronectin expose vascular endothelial growth factor binding sites. Biochemistry. 2006;45:10319–10328. doi: 10.1021/bi060974p. [DOI] [PubMed] [Google Scholar]
- 44.Mitsi M., Forsten-Williams K., Gopalakrishnan M., Nugent M.A. A catalytic role of heparin within the extracellular matrix. J Biol Chem. 2008;283:34796–34807. doi: 10.1074/jbc.M806692200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kechagia J.Z., Ivaska J., Roca-Cusachs P. Integrins as biomechanical sensors of the microenvironment. Nat Rev Mol Cell Biol. 2019;20:457–473. doi: 10.1038/s41580-019-0134-2. [DOI] [PubMed] [Google Scholar]
- 46.Friedlander M., Brooks P.C., Shaffer R.W., Kincaid C.M., Varner J.A., Cheresh D.A. Definition of two angiogenic pathways by distinct αv integrins. Science (1979) 1995;270:1500–1502. doi: 10.1126/science.270.5241.1500. [DOI] [PubMed] [Google Scholar]
- 47.Byzova T.V., Goldman C.K., Pampori N., Thomas K.A., Bett A., Shattil S.J., Plow E.F. A mechanism for modulation of cellular responses to VEGF: activation of the integrins. Mol. Cell. 2000;6:851–860. [PubMed] [Google Scholar]
- 48.Li S., Nih L.R., Bachman H., Fei P., Li Y., Nam E., Dimatteo R., Carmichael S.T., Barker T.H., Segura T. Hydrogels with precisely controlled integrin activation dictate vascular patterning and permeability. Nat Mater. 2017;16:953–961. doi: 10.1038/nmat4954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Klein S., Giancotti F.G., Presta M., Albelda S.M., Buck C.A., Rifkin D.B. Basic fibroblast growth factor modulates integrin expression in microvascular endothelial cells. Mol Biol Cell. 1993;4:973–982. doi: 10.1091/mbc.4.10.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sahni A., Francis C.W. Stimulation of endothelial cell proliferation by FGF-2 in the presence of fibrinogen requires αvβ3. Blood. 2004;104:3635–3641. doi: 10.1182/blood-2004-04-1358. [DOI] [PubMed] [Google Scholar]
- 51.Robinson S.D., Reynolds L.E., Wyder L., Hicklin D.J., Hodivala-Dilke K.M. β3-integrin regulates vascular endothelial growth factor-a–dependent permeability. Arterioscler Thromb Vasc Biol. 2004;24:2108–2114. doi: 10.1161/01.ATV.0000143857.27408.de. [DOI] [PubMed] [Google Scholar]
- 52.Chen T.T., Luque A., Lee S., Anderson S.M., Segura T., Iruela-Arispe M.L. Anchorage of VEGF to the extracellular matrix conveys differential signaling responses to endothelial cells. J Cell Biol. 2010;188:595–609. doi: 10.1083/jcb.200906044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rundhaug J.E. Matrix metalloproteinases and angiogenesis. J Cell Mol Med. 2005;9:267–285. doi: 10.1111/j.1582-4934.2005.tb00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Van Linthout S., Seeland U., Riad A., Eckhardt O., Hohl M., Dhayat N., Richter U., Fischer J.W., Böhm M., Pauschinger M., Schultheiss H.-P., Tschöpe C. Reduced MMP-2 activity contributes to cardiac fibrosis in experimental diabetic cardiomyopathy. Basic Res Cardiol. 2008;103:319–327. doi: 10.1007/s00395-008-0715-2. [DOI] [PubMed] [Google Scholar]
- 55.Caley M.P., Martins V.L.C., O'Toole E.A. Metalloproteinases and wound healing. Adv Wound Care. 2015;4:225–234. doi: 10.1089/wound.2014.0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Song H.-H.G., Rumma R.T., Ozaki C.K., Edelman E.R., Chen C.S. Vascular tissue engineering: progress, challenges, and clinical promise. Cell Stem Cell. 2018;22:340–354. doi: 10.1016/j.stem.2018.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Caliari S.R., Burdick J.A. A practical guide to hydrogels for cell culture. Nat Methods. 2016;13:405–414. doi: 10.1038/nmeth.3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tibbitt M.W., Anseth K.S. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol Bioeng. 2009;103:655–663. doi: 10.1002/bit.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liang Y., Li L., Scott R.A., Kiick K.L. 50th anniversary perspective: polymeric biomaterials: diverse functions enabled by advances in macromolecular chemistry. Macromolecules. 2017;50:483–502. doi: 10.1021/acs.macromol.6b02389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tayalia P., Mooney D.J. Controlled growth factor delivery for tissue engineering. Adv Mater. 2009;21:3269–3285. doi: 10.1002/adma.200900241. [DOI] [PubMed] [Google Scholar]
- 61.Sakiyama-Elbert S.E. Incorporation of heparin into biomaterials. Acta Biomater. 2014;10:1581–1587. doi: 10.1016/j.actbio.2013.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hirsh J., Heparin N. Engl. J. Med. 1991;324:1565–1574. doi: 10.1056/NEJM199105303242206. [DOI] [PubMed] [Google Scholar]
- 63.Xu Y., Masuko S., Takieddin M., Xu H., Liu R., Jing J., Mousa S.A., Linhardt R.J., Liu J. Chemoenzymatic synthesis of homogeneous ultralow molecular weight heparins. Science. 2011;334:498–501. doi: 10.1126/science.1207478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nguyen T.H., Kim S.-H., Decker C.G., Wong D.Y., Loo J.A., Maynard H.D. A heparin-mimicking polymer conjugate stabilizes basic fibroblast growth factor. Nat Chem. 2013;5:221–227. doi: 10.1038/nchem.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Paluck S.J., Nguyen T.H., Maynard H.D. Heparin-Mimicking Polymers: synthesis and Biological Applications. Biomacromolecules. 2016;17:3417–3440. doi: 10.1021/acs.biomac.6b01147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Limasale Y.D.P., Atallah P., Werner C., Freudenberg U., Zimmermann R. Tuning the local availability of VEGF within glycosaminoglycan-based hydrogels to modulate vascular endothelial cell morphogenesis. Adv Funct Mater. 2020;30 [Google Scholar]
- 67.Martino M.M., Briquez P.S., Ranga A., Lutolf M.P., Hubbell J.A. Heparin-binding domain of fibrin(ogen) binds growth factors and promotes tissue repair when incorporated within a synthetic matrix. Proc Natl Acad Sci. 2013;110:4563–4568. doi: 10.1073/pnas.1221602110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Engler A.J., Sen S., Sweeney H.L., Discher D.E. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 69.Li L., Eyckmans J., Chen C.S. Designer biomaterials for mechanobiology. Nat Mater. 2017;16:1164–1168. doi: 10.1038/nmat5049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yeung T., Georges P.C., Flanagan L.A., Marg B., Ortiz M., Funaki M., Zahir N., Ming W., Weaver V., Janmey P.A. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil Cytoskeleton. 2005;60:24–34. doi: 10.1002/cm.20041. [DOI] [PubMed] [Google Scholar]
- 71.Xue C., Zhang T., Xie X., Zhang Q., Zhang S., Zhu B., Lin Y., Cai X. Substrate stiffness regulates arterial-venous differentiation of endothelial progenitor cells via the Ras/Mek pathway. Biochim. Biophys. Acta Mol Cell Res. 2017;1864:1799–1808. doi: 10.1016/j.bbamcr.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 72.Wong L., Kumar A., Gabela-Zuniga B., Chua J., Singh G., Happe C.L., Engler A.J., Fan Y., McCloskey K.E. Substrate stiffness directs diverging vascular fates. Acta Biomater. 2019;96:321–329. doi: 10.1016/j.actbio.2019.07.030. [DOI] [PubMed] [Google Scholar]
- 73.Mammoto A., Connor K.M., Mammoto T., Yung C.W., Huh D., Aderman C.M., Mostoslavsky G., Smith L.E.H., Ingber D.E. A mechanosensitive transcriptional mechanism that controls angiogenesis. Nature. 2009;457:1103–1108. doi: 10.1038/nature07765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Baker B.M., Chen C.S. Deconstructing the third dimension – how 3D culture microenvironments alter cellular cues. J Cell Sci. 2012;125:3015–3024. doi: 10.1242/jcs.079509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li L., Griebel M.E., Uroz M., Bubli S.Y., Gagnon K.A., Trappmann B., Baker B.M., Eyckmans J., Chen C.S. A protein-adsorbent hydrogel with tunable stiffness for tissue culture demonstrates matrix-dependent stiffness responses. Adv Funct Mater. 2024;34 doi: 10.1002/adfm.202309567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Crosby C.O., Zoldan J. Mimicking the physical cues of the ECM in angiogenic biomaterials. Regen Biomater. 2019;6:61–73. doi: 10.1093/rb/rbz003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Trappmann B., Baker B.M., Polacheck W.J., Choi C.K., Burdick J.A., Chen C.S. Matrix degradability controls multicellularity of 3D cell migration. Nat Commun. 2017;8:371. doi: 10.1038/s41467-017-00418-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chaudhuri O., Gu L., Darnell M., Klumpers D., Bencherif S.A., Weaver J.C., Huebsch N., Mooney D.J. Substrate stress relaxation regulates cell spreading. Nat Commun. 2015;6:6365. doi: 10.1038/ncomms7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wei Z., Schnellmann R., Pruitt H.C., Gerecht S. Hydrogel network dynamics regulate vascular morphogenesis. Cell Stem Cell. 2020;27:798–812.e6. doi: 10.1016/j.stem.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ngo M.T., Harley B.A.C. Angiogenic biomaterials to promote therapeutic regeneration and investigate disease progression. Biomaterials. 2020;255:120207. doi: 10.1016/j.biomaterials.2020.120207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gilbert P.M., Weaver V.M. Cellular adaptation to biomechanical stress across length scales in tissue homeostasis and disease. Semin Cell Dev Biol. 2017;67:141–152. doi: 10.1016/j.semcdb.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bubli S.Y., Smolag M., Blackwell E., Lin Y.-C., Tsavalas J.G., Li L. Inducing an LCST in hydrophilic polysaccharides via engineered macromolecular hydrophobicity. Sci Rep. 2023;13:14896. doi: 10.1038/s41598-023-41947-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hartman C.D., Isenberg B.C., Chua S.G., Wong J.Y. Vascular smooth muscle cell durotaxis depends on extracellular matrix composition. Proc Natl Acad Sci. 2016;113:11190–11195. doi: 10.1073/pnas.1611324113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nguyen D.-H.T., Stapleton S.C., Yang M.T., Cha S.S., Choi C.K., Galie P.A., Chen C.S. Biomimetic model to reconstitute angiogenic sprouting morphogenesis in vitro. Proc Natl Acad Sci. 2013;110:6712–6717. doi: 10.1073/pnas.1221526110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Baker B.M., Trappmann B., Wang W.Y., Sakar M.S., Kim I.L., Shenoy V.B., Burdick J.A., Chen C.S. Cell-mediated fibre recruitment drives extracellular matrix mechanosensing in engineered fibrillar microenvironments. Nat Mater. 2015;14:1262–1268. doi: 10.1038/nmat4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Talbott H.E., Mascharak S., Griffin M., Wan D.C., Longaker M.T. Wound healing, fibroblast heterogeneity, and fibrosis. Cell Stem Cell. 2022;29:1161–1180. doi: 10.1016/j.stem.2022.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Peña O.A., Martin P. Cellular and molecular mechanisms of skin wound healing. Nat Rev Mol Cell Biol. 2024 doi: 10.1038/s41580-024-00715-1. [DOI] [PubMed] [Google Scholar]
- 88.Bao P., Kodra A., Tomic-Canic M., Golinko M.S., Ehrlich H.P., Brem H. The role of vascular endothelial growth factor in wound healing. J Surg Res. 2009;153:347–358. doi: 10.1016/j.jss.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Arif S., Attiogbe E., Moulin V.J. Granulation tissue myofibroblasts during normal and pathological skin healing: the interaction between their secretome and the microenvironment. Wound Repair Regen. 2021;29:563–572. doi: 10.1111/wrr.12919. [DOI] [PubMed] [Google Scholar]
- 90.Veith A.P., Henderson K., Spencer A., Sligar A.D., Baker A.B. Therapeutic strategies for enhancing angiogenesis in wound healing. Adv Drug Deliv Rev. 2019;146:97–125. doi: 10.1016/j.addr.2018.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Martinotti S., Ranzato E. In: Scratch wound healing assay bt – Epidermal Cells: methods and protocols. Turksen K., editor. Springer US; New York, NY: 2020. pp. 225–229. [DOI] [PubMed] [Google Scholar]
- 92.Tefft J.B., Chen C.S., Eyckmans J. Reconstituting the dynamics of endothelial cells and fibroblasts in wound closure. APL Bioeng. 2021;5:16102. doi: 10.1063/5.0028651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McGrath M.H., Emery J.M.I.I.I. The effect of inhibition of angiogenesis in granulation tissue on wound healing and the fibroblast. Ann Plast Surg. 1985;15:105–122. doi: 10.1097/00000637-198508000-00004. [DOI] [PubMed] [Google Scholar]
- 94.Okonkwo U.A., DiPietro L.A. Diabetes and wound angiogenesis. Int J Mol Sci. 2017;18:1419. doi: 10.3390/ijms18071419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Niezgoda J.A., Van Gils C.C., Frykberg R.G., Hodde J.P., Group O.D.U.S. Randomized clinical trial comparing OASIS wound matrix to Regranex gel for diabetic ulcers. Adv. Skin Wound Care. 2005;18:258–266. doi: 10.1097/00129334-200506000-00012. [DOI] [PubMed] [Google Scholar]
- 96.Chan R.K., Liu P.H., Pietramaggiori G., Ibrahim S.I., Hechtman H.B., Orgill D.P. Effect of recombinant platelet-derived growth factor (Regranex®) on wound closure in genetically diabetic mice. J Burn Care Res. 2006;27:202–205. doi: 10.1097/01.BCR.0000202898.11277.58. [DOI] [PubMed] [Google Scholar]
- 97.Rennert R.C., Rodrigues M., Wong V.W., Duscher D., Hu M., Maan Z., Sorkin M., Gurtner G.C., Longaker M.T. Biological therapies for the treatment of cutaneous wounds: phase III and launched therapies. Expert Opin. Biol. Ther. 2013;13:1523–1541. doi: 10.1517/14712598.2013.842972. [DOI] [PubMed] [Google Scholar]
- 98.Chaudhuri O., Cooper-White J., Janmey P.A., Mooney D.J., Shenoy V.B. Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature. 2020;584:535–546. doi: 10.1038/s41586-020-2612-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ruehle M.A., Eastburn E.A., LaBelle S.A., Krishnan L., Weiss J.A., Boerckel J.D., Wood L.B., Guldberg R.E., Willett N.J. Extracellular matrix compression temporally regulates microvascular angiogenesis. Sci Adv. 2020;6:eabb6351. doi: 10.1126/sciadv.abb6351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yager D.R., Nwomeh B.C. The proteolytic environment of chronic wounds. Wound Repair Regen. 1999;7:433–441. doi: 10.1046/j.1524-475x.1999.00433.x. [DOI] [PubMed] [Google Scholar]
- 101.Li L., Stiadle J.M., Lau H.K., Zerdoum A.B., Jia X., Thibeault S.L., Kiick K.L. Tissue engineering-based therapeutic strategies for vocal fold repair and regeneration. Biomaterials. 2016;108:91–110. doi: 10.1016/j.biomaterials.2016.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Brown-Etris M., Milne C.T., Hodde J.P. An extracellular matrix graft (Oasis® wound matrix) for treating full-thickness pressure ulcers: a randomized clinical trial. J Tissue Viability. 2019;28:21–26. doi: 10.1016/j.jtv.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 103.Anderson N.M., Simon M.C. The tumor microenvironment. Curr Biol. 2020;30:R921–R925. doi: 10.1016/j.cub.2020.06.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Valastyan S., Weinberg R.A. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147:275–292. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.De Palma M., Biziato D., Petrova T.V. Microenvironmental regulation of tumour angiogenesis. Nat Rev Cancer. 2017;17:457–474. doi: 10.1038/nrc.2017.51. [DOI] [PubMed] [Google Scholar]
- 106.Borriello L., Karagiannis G.S., Duran C.L., Coste A., Oktay M.H., Entenberg D., Condeelis J.S. The role of the tumor microenvironment in tumor cell intravasation and dissemination. Eur J Cell Biol. 2020;99 doi: 10.1016/j.ejcb.2020.151098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 108.Abdollahi A., Folkman J. Evading tumor evasion: current concepts and perspectives of anti-angiogenic cancer therapy. Drug Resist Updat. 2010;13:16–28. doi: 10.1016/j.drup.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 109.Bernabeu M.O., Köry J., Grogan J.A., Markelc B., Beardo A., d'Avezac M., Enjalbert R., Kaeppler J., Daly N., Hetherington J., Krüger T., Maini P.K., Pitt-Francis J.M., Muschel R.J., Alarcón T., Byrne H.M. Abnormal morphology biases hematocrit distribution in tumor vasculature and contributes to heterogeneity in tissue oxygenation. Proc Natl Acad Sci. 2020;117:27811–27819. doi: 10.1073/pnas.2007770117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Moon E.J., Mello S.S., Li C.G., Chi J.-T., Thakkar K., Kirkland J.G., Lagory E.L., Lee I.J., Diep A.N., Miao Y., Rafat M., Vilalta M., Castellini L., Krieg A.J., Graves E.E., Attardi L.D., Giaccia A.J. The HIF target MAFF promotes tumor invasion and metastasis through IL11 and STAT3 signaling. Nat Commun. 2021;12:4308. doi: 10.1038/s41467-021-24631-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Senger D.R., Galli S.J., Dvorak A.M., Perruzzi C.A., Harvey V.S., Dvorak H.F. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- 112.Allegra C.J., Yothers G., O'Connell M.J., Sharif S., Petrelli N.J., Colangelo L.H., Atkins J.N., Seay T.E., Fehrenbacher L., Goldberg R.M., O'Reilly S., Chu L., Azar C.A., Lopa S., Wolmark N. Phase III trial assessing Bevacizumab in stages II and III Carcinoma of the Colon: results of NSABP Protocol C-08. J Clin Oncol. 2010;29:11–16. doi: 10.1200/JCO.2010.30.0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rahbari N.N., Kedrin D., Incio J., Liu H., Ho W.W., Nia H.T., Edrich C.M., Jung K., Daubriac J., Chen I., Heishi T., Martin J.D., Huang Y., Maimon N., Reissfelder C., Weitz J., Boucher Y., Clark J.W., Grodzinsky A.J., Duda D.G., Jain R.K., Fukumura D. Anti-VEGF therapy induces ECM remodeling and mechanical barriers to therapy in colorectal cancer liver metastases. Sci Transl Med. 2016;8:360ra135. doi: 10.1126/scitranslmed.aaf5219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mancuso M.R., Davis R., Norberg S.M., O'Brien S., Sennino B., Nakahara T., Yao V.J., Inai T., Brooks P., Freimark B., Shalinsky D.R., Hu-Lowe D.D., McDonald D.M. Rapid vascular regrowth in tumors after reversal of VEGF inhibition. J Clin Invest. 2006;116:2610–2621. doi: 10.1172/JCI24612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lu P., Weaver V.M., Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol. 2012;196:395–406. doi: 10.1083/jcb.201102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Winkler J., Abisoye-Ogunniyan A., Metcalf K.J., Werb Z. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat Commun. 2020;11:5120. doi: 10.1038/s41467-020-18794-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Barney L.E., Hall C.L., Schwartz A.D., Parks A.N., Sparages C., Galarza S., Platt M.O., Mercurio A.M., Peyton S.R. Tumor cell–organized fibronectin maintenance of a dormant breast cancer population. Sci Adv. 2020;6:eaaz4157. doi: 10.1126/sciadv.aaz4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Maller O., Drain A.P., Barrett A.S., Borgquist S., Ruffell B., Zakharevich I., Pham T.T., Gruosso T., Kuasne H., Lakins J.N., Acerbi I., Barnes J.M., Nemkov T., Chauhan A., Gruenberg J., Nasir A., Bjarnadottir O., Werb Z., Kabos P., Chen Y.-Y., Hwang E.S., Park M., Coussens L.M., Nelson A.C., Hansen K.C., Weaver V.M. Tumour-associated macrophages drive stromal cell-dependent collagen crosslinking and stiffening to promote breast cancer aggression. Nat Mater. 2021;20:548–559. doi: 10.1038/s41563-020-00849-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bhatia S.N., Ingber D.E. Microfluidic organs-on-chips. Nat Biotechnol. 2014;32:760–772. doi: 10.1038/nbt.2989. [DOI] [PubMed] [Google Scholar]
- 120.Polacheck W.J., Kutys M.L., Yang J., Eyckmans J., Wu Y., Vasavada H., Hirschi K.K., Chen C.S. A non-canonical Notch complex regulates adherens junctions and vascular barrier function. Nature. 2017;552:258–262. doi: 10.1038/nature24998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Galie P.A., Nguyen D.-H.T., Choi C.K., Cohen D.M., Janmey P.A., Chen C.S. Fluid shear stress threshold regulates angiogenic sprouting. Proc Natl Acad Sci. 2014;111:7968–7973. doi: 10.1073/pnas.1310842111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Skylar-Scott M.A., Uzel S.G.M., Nam L.L., Ahrens J.H., Truby R.L., Damaraju S., Lewis J.A. Biomanufacturing of organ-specific tissues with high cellular density and embedded vascular channels. Sci Adv. 2019;5:eaaw2459. doi: 10.1126/sciadv.aaw2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Miller J.S., Stevens K.R., Yang M.T., Baker B.M., Nguyen D.-H.T., Cohen D.M., Toro E., Chen A.A., Galie P.A., Yu X., Chaturvedi R., Bhatia S.N., Chen C.S. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat Mater. 2012;11:768–774. doi: 10.1038/nmat3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Grigoryan B., Paulsen S.J., Corbett D.C., Sazer D.W., Fortin C.L., Zaita A.J., Greenfield P.T., Calafat N.J., Gounley J.P., Ta A.H., Johansson F., Randles A., Rosenkrantz J.E., Louis-Rosenberg J.D., Galie P.A., Stevens K.R., Miller J.S. Multivascular networks and functional intravascular topologies within biocompatible hydrogels. Science. 2019;364:458–464. doi: 10.1126/science.aav9750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lee A., Hudson A.R., Shiwarski D.J., Tashman J.W., Hinton T.J., Yerneni S., Bliley J.M., Campbell P.G., Feinberg A.W. 3D bioprinting of collagen to rebuild components of the human heart. Science. 2019;365:482–487. doi: 10.1126/science.aav9051. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.