Abstract
Measures of physical growth, such as weight and height have long been the predominant outcomes for monitoring child health and evaluating interventional outcomes in public health studies, including those that may impact neurodevelopment. While physical growth generally reflects overall health and nutritional status, it lacks sensitivity and specificity to brain growth and developing cognitive skills and abilities. Psychometric tools, e.g., the Bayley Scales of Infant and Toddler Development, may afford more direct assessment of cognitive development but they require language translation, cultural adaptation, and population norming. Further, they are not always reliable predictors of future outcomes when assessed within the first 12–18 months of a child’s life. Neuroimaging may provide more objective, sensitive, and predictive measures of neurodevelopment but tools such as magnetic resonance (MR) imaging are not readily available in many low and middle-income countries (LMICs). MRI systems that operate at lower magnetic fields (< 100mT) may offer increased accessibility, but their use for global health studies remains nascent. The UNITY project is envisaged as a global partnership to advance neuroimaging in global health studies. Here we describe the UNITY project, its goals, methods, operating procedures, and expected outcomes in characterizing neurodevelopment in sub-Saharan Africa and South Asia.
Keywords: Neurodevelopment, Child Health, Global Health, Healthy Development, Environmental Adversity, Low Field Magnetic Resonance Imaging
1. Introduction
Infancy and early childhood, from birth to 5 years of age, is a period of rapid and dynamic brain and cognitive development, which lays a foundation for future cognitive skills and abilities. On a macro anatomical level, a child’s brain expands in volume by more than 300 % across this period (Baribeau and Anagnostou, 2013), and is driven by the developing tissue microstructure and fiber architecture (Stiles and Jernigan, 2010). These microstructural changes include advancing white and gray matter myelination (Brody et al., 1987) and changing neuronal and synaptic density and organization (Houston et al., 2014). Collectively, these processes contribute to the development of mature and efficient functional brain networks (Miskovic et al., 2015; Faghiri et al., 2018) that support emerging cognitive functions, skills, and behavioral abilities (Luna et al., 2001; Peltzer-Karpf, 2012).
The rate and timing of developing neural systems are strongly shaped by genetic and environmental factors (and their interactions) beginning at the earliest stages of in-utero development and continuing across the lifespan (Bedi and Bhide, 1988, Nutrimenthe Research Group, 2013, Bick and Nelson, 2016, Vohr et al., 2017, Shankar et al., 2018, Fitzgerald et al., 2020, Charge Working Group neuro, 2020). During pregnancy, aspects of maternal health including malnutrition, infection(s), anemia, stress, depression, and fatigue can alter fetal neurodevelopment and pre- and post-natal brain connectivity via impaired neurogenesis, myelination, and other neurodevelopmental processes. Postnatally, functional brain systems depend on the carefully orchestrated delivery of growth factors and micro- and macro-nutrients (e.g., lipids, phospholipids, vitamins, and minerals) for optimal development. Deficient intake of iron, folic acid, vitamins A, D, K, B vitamins, choline, sphingolipids, and/or gangliosides can impair developing brain systems with lasting cognitive and behavioral consequences. In addition, environmental exposures to heavy metals, tobacco smoke, alcohol and other controlled substances, air pollution, and poor water quality (including human waste and feces) can also impact neurodevelopmental processes and brain development.
These exposures take place against a backdrop of demographic, socioeconomic, and other social determinants of health that can buffer or amplify their effects (Bedi and Bhide, 1988, Zhang et al., 2017, Kvaratskhelia et al., 2023, Resonance Consortium, 2023, Zugman et al., 2023). For example, access to quality healthcare and/or educational opportunities, clean water and sanitation, and warm, supportive, and attentive caregiving are all factors associated with beneficial neurodevelopmental outcomes. Maternal and family adversity, limited maternal autonomy, gender inequality, and other social and cultural norms can also differentially impact neurodevelopment depending on the child's sex and gender (Shroff et al., 2009).
Within the context of global health, measures of child growth (or, more specifically, growth faltering, e.g., stunting and wasting) are often the yardsticks by which interventions are measured and evaluated. Stunting, underweight status, and wasting are commonly defined as length or height-for-age Z-score (LAZ or HAZ), weight-for-age Z-score (WAZ), and weight-for-height Z-score (WHZ) less than −2 (i.e., more than 2 standard deviations below the mean), respectively. These and other measures of physical growth, such as head circumference (HC), and mid-upper-arm circumference (MUAC), are relatively quick and easy to reliably measure with sufficient training, and provide a general metric of child health and nutritional status (Tran et al., 2019). Moreover, they can be readily compared between children in different geographical regions and countries and monitored longitudinally to assess changes in population health (Vesel et al., 2019).
Based on measures of stunting and wasting, an estimated 170–250 million children worldwide under five years of age are failing to reach their developmental potential (Maternal, and Group Child Undernutrition Study, 2008, Maternal, and Group Child Nutrition Study, 2013, Committee Lancet Early Childhood Development Series Steering, 2017). This number is also commonly cited as the number of children who may be failing to reach their neurodevelopmental potential. However, given the limited association between physical growth and cognitive development and outcomes - relationships that can vary by environmental setting (Tran et al., 2019), the true burden of unattained neurodevelopment potential may be significantly higher (McCoy et al., 2016). While some of the same health and environmental factors that lead to stunting and/or wasting (e.g., malnutrition, nutritional deficiencies, environmental stress, and early disease and illness) also impact neurodevelopment (Nicolaou et al., 2020), physical growth accounts for only a small portion of the variance in cognitive performance (Tran et al., 2019). Further, interventions aimed at improving physical growth may have little impact on cognitive development, and, vice versa, interventions targeting neurodevelopment may not necessarily improve physical growth (Walker et al., 2006, Sokolovic et al., 2014). Thus, a more objective and direct measure of neurodevelopment may be warranted for the evaluation of interventions that are primarily directed toward neurodevelopmental outcomes.
Psychometric tools, including observational (e.g., the Bayley Scales of Infant and Toddler Development, BSID (Balasundaram and Avulakunta, 2023)) and parent-reported measures (e.g., the Ages and Stages Questionnaire, ASQ (Squires and Bricker, 2009) offer a more direct assessment of a child’s current cognitive and developmental status. Whilst many of these clinical tools are internationally recognized and have been validated across multiple contexts, they come with important caveats that may diminish their utility in some settings or as stand-alone measures. For use in many lower- and middle-income settings, these tools require language and cultural translation and population norming, particularly for use in multi-country comparisons of outcomes. Ongoing assessor training is also needed to ensure the validity and consistency of derived measures. More importantly, however, assessed performance within the first 12–18 months of a child’s life (corresponding to an important window of opportunity for intervention) is modestly associated with outcomes measured in later childhood (i.e., general intelligence or executive function skills at age 5 or 6 years) depending on the tool (Anderson and Burnett, 2017, Schonhaut et al., 2020, Mansson et al., 2021). Thus, while psychometric measures are an important tool for directly assessing neurodevelopment that can be used up to a population-level scale, they are not without challenges. In recognition of these challenges, neuroimaging tools have been examined as potential objective measures of underlying neurobiological mechanisms. These tools may better relate to neurodevelopmental outcomes and may complement traditional psychometric measures in assessing and evaluating intervention outcomes.
Neuroimaging methods, such as magnetic resonance imaging (MRI) and electrophysiology (electroencephalography, EEG) allow visualization and/or quantification of emerging brain structure and function, which may be an objective measure of brain maturation that is predictive of current and future cognitive abilities and performance. In addition to broad metrics of brain macrostructure and organization (e.g., total brain and regional tissue volumes), MRI allows the characterization of microstructural tissue organization, architecture, chemical composition, structural and functional connectivity, cellular metabolism, and brain physiology (e.g., blood flow). Many of these measures collected in infancy, and even in utero, are predictive of later childhood cognitive and academic skills (Thomason et al., 2018, Bugada et al., 2021, Yu et al., 2021), and are sensitive to the impact of nutritional deficiencies, adversity, stimulation, and other environmental factors (e.g., water cleanliness and air quality, sanitation and open defecation) (Sizonenko et al., 2013, , and Bank Australian Schizophrenia Research, 2018, E. R. A. Young Adult Follow-up team, 2020). However, while brain imaging does not require cultural or language translation and may be sensitive to neurodevelopmental differences associated with cultural context and the social environment, even the most portable and user-friendly neuroimaging methods are inherently limited in scalability.
Unfortunately, MRI systems that operate at high magnetic field strengths (e.g., 1.5 Tesla and above) require significant infrastructure and service support. This includes dedicated facilities, trained personnel, and ongoing service (e.g., helium refills, maintenance, and replacement of gradient and radio frequency hardware) that are expensive and difficult to procure in LMIC settings. An estimated 40 % of imaging equipment in low and middle income (LMIC) settings is unused due to the lack of service and parts availability. These factors have limited accessibility to MRI in LMIC geographies. For example, while the US has nearly one MRI scanner per 25,000 inhabitants, India and other countries in Southeast Asia and Sub-Saharan Africa may have fewer than one per 1.25 million (Ogbole et al., 2018). While alternative imaging methods such as EEG, and functional near-infrared spectroscopy (fNIRS) are more portable and less infrastructure-intensive, they still require significant personnel expertise for data acquisition and analysis.
As a consequence of this sparse availability, the collective knowledge of early anatomical neurodevelopment in children from LMIC settings is limited, and the potential utility of MRI as a tool in global health remains unclear. Only a handful of MRI neuroimaging studies have been performed outside of the ‘global north’ as highlighted by recent ‘growth curve’ analyses of life-course patterns of brain development (Initiative Alzheimer's Disease Neuroimaging, Investigators Alzheimer's Disease Repository Without Borders, Calm Team, C. A. N. Cam, Ccnp, Cobre, cVeda, Enigma Developmental Brain Age Working Group, Project Developing Human Connectome, FinnBrain, Study Harvard Aging Brain, Imagen, Kne, Aging Mayo Clinic Study of, Nspn, Pond, Prevent-Ad Research Group, Vetsa, 2022, Rutherford et al., 2022, and Enigma Lifespan Working Group, 2024). Less than 3 % of the contributed data came from a low or middle-income setting, and even less from the early life period beyond the neonatal period. Thus, while research from the North America, Europe, and Australia has shown the short and long-term effects of early childhood infection and disease, malnutrition, and other environmental adversities, the impact of these factors in children who live in LMIC settings, where these influences are far more common, is not yet well understood.
MRI systems that operate at lower magnetic fields (< 100mT) can overcome many of the obstacles that challenge access to conventional 1.5 and 3 Tesla (T) scanners (Sarracanie et al., 2015). In addition to the initial lower purchase cost (∼$200–400,000USD vs. $1.5–4 million), low and ultra-low field systems are more portable and require significantly less power (about the same as a common kitchen appliance). This makes them potentially more suitable for settings that lack reliable power grids or where power may only be possible through solar or wind generators. They also have a small magnetic fringe field (extending less than 6 feet in diameter), which makes them suitable for locations with limited infrastructure or that cannot afford to dedicate large rooms with magnetic and radio frequency shielding. Low and ultra-low field MRI (LF-MRI) systems also produce less acoustic noise than most high-field systems, allowing them to be integrated into clinical wards or research centers without additional noise derating or magnetic and radio frequency (RF)-shielding materials. Current commercially available systems, such as the Hyperfine Swoop, offer mobility, have small physical footprints, and do not require substantial operator expertise.
Given these attributes, LF-MRI may be an important and complementary tool for assessing neuroanatomy change and neurodevelopment in a global health context in LMIC settings. However, as many current low-field systems have been optimized for clinical applications in higher resource settings and hospitals, their utility for public health research, particularly in the early infant and childhood periods, is not clear but holds promising potential. While some of the first in vivo human MR images were acquired at field strengths much less than 100mT (e.g., Richard Damadian’s “Indomitable” operated at 50mT and the scanner of John Mallard and his group in Aberdeen, Scotland had a field strength of 40mT), systems less than 0.5 T were gradually replaced by higher field (1.5 T, 3 T, and above) systems in clinical and research settings through the 1980s and 1990s. However, continued exploration and development of low and ultra-low field strength MRI continued in research laboratories, with ongoing advancements in design, acquisition, and reconstruction methods. The commercialization of ultra low-field systems by Hyperfine in 2020 has rekindled interest in their use for in vivo human imaging, as seen in the steady increase in clinical use publications. A literature review for “low-Field”, “MRI”, “Human” and manually excluding field strengths of more than 100mT and strictly engineering papers yields 17 publications in 2023 and 2024 vs. less than 5 from 2018 to 2022). Despite this, low-field systems remain in the minority and lack the repertoire of imaging methods available on higher-field strength systems. To date, low field systems have only sporadically been used in neonatal and pediatric populations (Deoni et al., 2021, Cawley et al., 2022, Cawley et al., 2023, Sabir et al., 2023, Tu et al., 2023), or LMIC settings, and no large-scale studies in either HIC or LMIC settings have explored their use for clinical or neuroscience research.
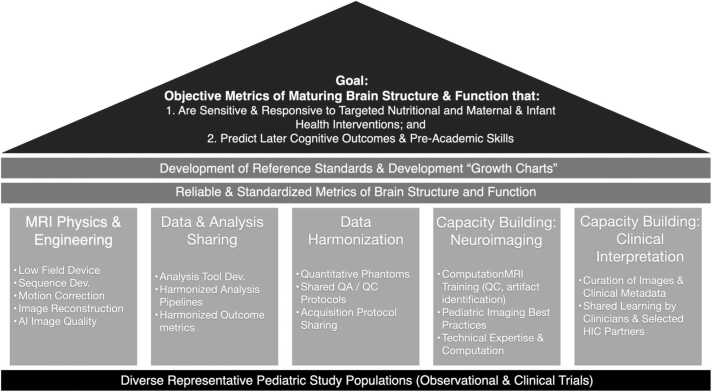
The UNITY Project (Ultra low-field Neuroimaging In The Young) is an ambitious multi-national and multi-institutional project aimed to accelerate development and deployment of ultra-low field MRI as an accessible neuroimaging modality, specifically for structural (volumetric, relaxometry, magnetization transfer, and diffusion imaging) and metabolic (lactate and perfusion) imaging of the brain. As a partnership between clinical, academic, and industrial researchers in high and lower-resource settings the primary goal of UNITY is to demonstrate the ability of LF-MRI to provide objective metrics of brain development that are predictive of current and future cognitive abilities; and sensitive and responsive to maternal and infant health interventions (Fig. 1).
Fig. 1.
The UNITY project aims to identify sensitive, responsive, and predictive measures of maturing brain structure and function by characterizing patterns of neurodevelopment across a large and diverse meta-cohort of children. To achieve this aim, the GlobalMap project includes 5 areas or ‘pillars’ of focus: 1. MRI physics and engineering to develop and optimize novel acquisition methods tailored for low-field MRI; 2. Data and analysis method sharing; 3. Data harmonization through shared protocols, phantoms, and rigorous QA/QC protocols; 4. Capacity building in low-field pediatric neuroimaging through site-by-site training of research and clinical personnel on patient handling, data acquisition, and data analysis; and 5. Capacity building in MRI physics and low-field MR image interpretation.
To achieve this ambitious goal, UNITY comprises five central research focus areas: 1. MRI physics development, including the development, optimization, and testing of anatomical and volumetric imaging, as well as methods sensitive to tissue microstructure and myelination in neonates, infants, and young children (but also applicable across the lifespan and for clinical decision making); 2. MR Image Analysis and Quality Improvement, including the development of artificial intelligence (AI) methods to improve image quality and the advancement of analytic methods (e.g., skull stripping, tissue segmentation, image normalization, and alignment) customized for pediatric populations and the unique contrast of LF-MRI images; 3. Data sharing and harmonization, including the use of quantitative phantoms and quality control and assurance protocols to help ensure inter-site and longitudinal intra-site data consistency; 4. Academic neuroimaging capacity building, including image analysis training, development of platforms for data sharing and shared analysis, and the formation and integration into local, regional, national, and international academic societies (e.g., national radiological societies, the International Society for Magnetic Resonance in Medicine, ISMRM); and 5. Clinical training and capacity building, including the development of training modules and the use of cloud-based platforms for radiological reading and interpretation (e.g., CollectiveMinds Radiology).
Research outcomes from these focal areas will optimize data collection in ongoing observational studies and clinical trials located across Sub-Saharan Africa (Ghana, Ethiopia, Kenya, Uganda, Malawi, Zambia, and South Africa) and South Asia (Pakistan, India, and Bangladesh) to help characterize patterns of brain development across these diverse populations and associated with differences in prenatal exposures, birth outcomes, nutritional status, social equality, and sociodemographic characteristics. These patterns will then be used to identify sensitive and predictive brain imaging metrics, and to help identify global and regional factors that influence developmental outcomes.
In this paper, we describe the organizational structure of UNITY, its goals, methods, and operating procedures, which we hope will provide a basis for shared large-scale epidemiological studies of neurodevelopment in HIC and LMIC settings.
2. METHODS
2.1. UNITY Project Overview
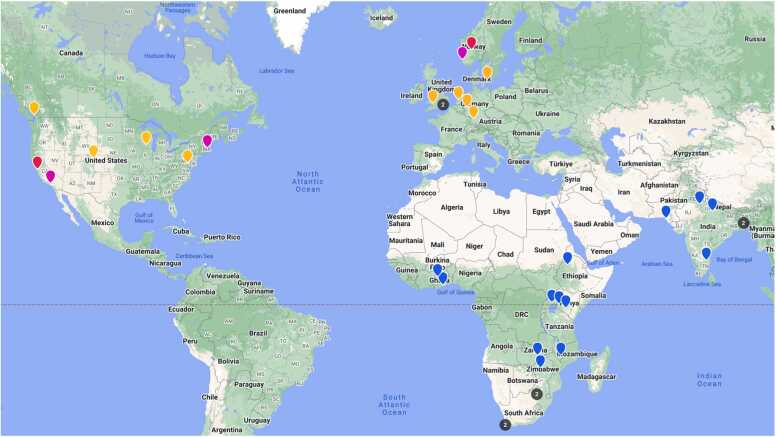
UNITY brings together academic researchers in MRI physics, engineering, computer science, and image analysis with clinical scientists, public health researchers, community action groups, and non-profit organizations located in high-resource countries (Australia, Canada, Germany, Sweden, the Netherlands, USA, and UK) and LMICs (Bangladesh, Ethiopia, Ghana, India, Kenya, Malawi, Pakistan, South Africa, Uganda, and Zambia). To date, 30 64mT Hyperfine Swoop MRI systems (Hyperfine.io, Guilford, CT) have been delivered and installed across a number of academic/research and clinical/hospital sites (Table 1, Fig. 2) in HIC and LMIC settings. A further 20 are planned by the end of 2024 to additional clinical sites in the current list of LMICs as well as Botswana, Guatemala, Zimbabwe, and others.
Table 1.
List of current contributing UNITY research and clinical sites.
| Hospital or Research Center | City, Country | Site PI | |
|---|---|---|---|
| Physics & Engineering Sites | University of British Columbia | Vancouver, BC, Canada | Shannon Kolind |
| University of Wisconsin, Madison | Madison, Wisconsin, USA | Douglas Dean III | |
| National Institutes of Health | Washington, DC, USA | Peter Basser | |
| Rhode Island Hospital | Providence, RI, USA | Viren D’Sa | |
| Centre for Neuroimaging Sciences, King’s College London | London, UK | Steven Wiliams | |
| St. Thomas Hospital, King’s College London | London, UK | Jo Hanjal | |
| CUBRIC Centre, Cardiff University | Cardiff, UK | Derek Jones | |
| Leiden University | Leiden, The Netherlands | Andrew Webb | |
| Lund University | Lund, Sweden | Emil Ljungberg | |
| University of Bonn | Bonn, Germany | Hemmen Sabir | |
| Max Plank Institute for Biological Cybernetics | Tubingen, Germany | Klaus Scheffler | |
| Data Analysis Sites (Scanners not Provided) | Children’s Hospital Los Angeles | Los Angeles, CA, USA | Natasha Lepore |
| Children's National Hospital | Washington, DC, USA | Marius Linguraru | |
| Murdoch Children's Research Institute | Melbourne, Australia | Marc Seal | |
| Clinical & Observational Study Sites | University of Cape Town | Cape Town, South Africa | Kirsten Donald |
| Kalafong Hospital | Pretoria, South Africa | Michael Pepper & Khomotso Masemola | |
| Chris Hani Baragwanath Academic Hospital | Johannesburg, South Africa | Michael Pepper, Sithembiso Velaphi & Firdose Nakwa | |
| Tygerberg Academic Hospital, Stellenbosch University | Cape Town, South Africa | Cilla Springer | |
| Makerere University & Kawempe Referral Hospital | Kampala, Uganda | Victoria Nankabirwa | |
| Women and Newborns Hospital, University Teaching Hospital | Lusaka, Zambia | Bridget Spelke | |
| Training & Research Unit of Excellence (TRUE) | Zomba, Malawi | Kamija Phiri | |
| Korle-Bu Teaching Hospital, | Accra, Ghana | Method Tuuli | |
| Kintampo Health Research Centre | Kintampoo, Ghana | Kwaku Poku Asante | |
| Felege Hiwot Regional Referral Hospital & Addis Continental Institute of Public Health | Bahir Dar, Ethiopia | Anne CC Lee/Yemane Berhane | |
| Jaramogi Oginga Odinga Teaching & Referral Hospital | Kisumu, Kenya | Dickens Onyango | |
| Cristian Medical College | Vellor, India | Beena Koshy | |
| Community Empowerment Lab | Lucknow, India | Aarti Kumar | |
| Aga Khan University Hospital | Karachi, Pakistan | Sidra Kaleem Jafri, Zahra Hoodbhoy | |
| Icddr,b | Dhaka, Bangladesh | Rashidul Haque |
Fig. 2.
Research sites that comprise the UNITY network include data collection and clinical partner sites (blue), physics and engineering development groups (orange), neuromodeling and analysis groups (pink), and education and clinical capacity groups (red). Black dots correspond to two sites in close proximity.
In addition to the data collection (clinical partners) and physics and engineering sites, the UNITY project also includes analytical hubs for data analysis and neuromodeling, clinical and research capacity-building centers, and industrial partners (Hyperfine, Flywheel, CollectiveMinds Radiology, and CaliberMRI) spread across high and lower income settings (Fig. 2).
2.1.1. Project Governance & Guiding Principles
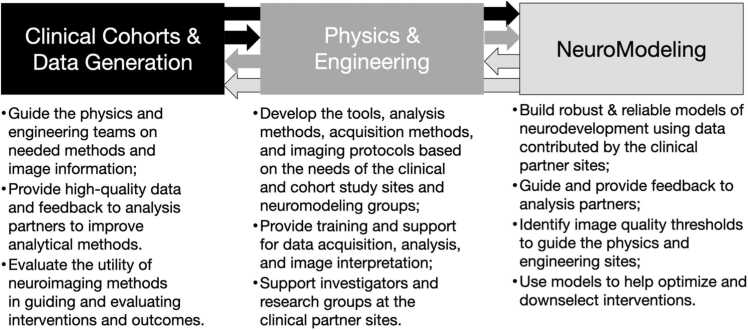
The structural organization of UNITY comprises an integrated network of partners comprising: 1. Clinical partners and data collection sites that have integrated LF-MRI alongside neurocognitive assessments into ongoing clinical trials and observational studies; 2. Physics and engineering sites focused on the development of LF-MRI pulse sequences and imaging methods driven by the needs of the clinical partners; 3. Image analysis and neuromodeling hubs to develop novel image analysis methods (e.g., segmentation, registration) and machine learning methods for improving image quality; and 4. International academic and clinical societies to foster local, regional, and international communities, knowledge transfer, and capacity building (Fig. 3). Each of these components is discussed further below.
Fig. 3.
General flow of knowledge and interaction between the main UNITY network components.
As a network structure, each clinical study site (CSS) of UNITY has its own unique site-specific structure, objectives, and outcomes. However, across the network of sites, care has been taken to align and harmonize specific data collection elements, including the neurocognitive assessments, neuroimaging protocol, and ideal neuro-related data collection timepoints. The physics and engineering (P&E) aspects of UNITY are coordinated from King’s College, London, and are driven by the research focus and needs of the CSSs. The P&E team also receives input from the image analysis and neuromodeling hubs (IAN) concerning image spatial resolution, signal- and contrast-to-noise, and other image quality characteristics that are needed for their modeling efforts. The IAN is also driven by the needs and desired outcomes of the CSSs. The individual CSSs are coordinated through individual grants from the Bill & Melinda Gates Foundation (BMGF), which acts as an independent monitor and assists with integration of harmonized neuroimaging protocols across sites. Whilst not directly involved in the research activities at each site, BMGF helps facilitate and maintain communication across them and with the P&E and IAN hubs.
2.2. Brief descriptions of clinical partner sites
UNITY builds upon and feeds into a unique foundation of LF-MRI neuroimaging data collected from large and diverse study populations across Sub-Saharan Africa and Southern Asia. Sites include a mixture of research, primary, and tertiary care centers, and span major clinical (e.g., Aga Khan University Hospital) and community health research centers (e.g., the Community Empowerment Lab). Whilst some of the larger clinical centers already have access to clinical neuroimaging systems, including 0.3–3 T MRI, neuroimaging research is nascent. In the smaller regional hospitals, as with the research facilities, radiological expertise is, at best, severely restricted (and often limited to X-ray and computed tomography, CT). This collection of sites encompasses a catchment area of more than 50 million families living in rural villages, urban slums, informal settlements, as well as upper-scale urban housing.
Felege Hiwot Comprehensive Specialized Hospital & Addis Continental Institute of Public Health, Bahir Dar (Ethiopia). The Bahir Dar Site is based at the Felege Hiwot Comprehensive Specialized Hospital and the study is organized through and led by Addis Continental Institute of Public Health (ACIPH). ACIPH is a center of excellence in Africa, focused on training and research in public health. ACIPH has worked with governmental and international organizations and academic institutions to firmly link the training of public health to practice and empirical research. The neurodevelopment study is a collaboration between ACIPH and the Brigham and Women’s Hospital/Harvard Medical School. The Felege Hiwot Hospital is a tertiary-level referral public hospital. Currently, more than 10 radiography technologists, 4 radiologists, and 3 biomedical engineers trained in the Hyperfine systems are operating the machine, interpreting results, and providing preventive maintenance. The radiology department within the 448-bed hospital also houses two X-Ray systems and one CT scanner. The Bahir Dar Site will conduct two studies. The first is a prospective accelerated longitudinal study to characterize typical healthy neurodevelopment in the first 5 years of life in a cohort of children from Bahir Dar city. In a second cohort, ACIPH will follow up infants from a pregnancy intervention study, the Enhancing Nutrition and Antenatal Infection Treatment study, and will examine the effects of prenatal interventions to optimize maternal nutrition and infection management in pregnancy on longer-term child neurodevelopment. The average salary of individuals served by the hospital is ∼4500 Birr ($80 USD)/month.
Aga Khan University Hospital (Pakistan) is the main teaching hospital for Aga Khan University (AKU) and is located in Karachi, Pakistan. AKUH is renowned for its comprehensive healthcare services, research, and educational programs, with a particular focus on maternal and child health. AKUH receives patients from primary healthcare clinics (PHCs) in the peri-urban coastal regions of the city. The PHCs are maintained by the Department of Pediatrics and Child Health at AKU and have quarterly household surveillance for key maternal and child health indicators along with antenatal care, immunization, and physician services for children under the age of 5 years. These sites are part of AKUH's broader commitment to addressing healthcare disparities and improving maternal and child health outcomes in underserved communities. They serve as essential hubs for healthcare delivery, research, and community engagement to promote the well-being of mothers and children in these areas. AKU is involved in a large-scale pregnancy risk and surveillance study (PRiSMA), which will follow children born to both healthy and anemic mothers. In addition to two Hyperfine Swoop systems (one located at the hospital and the second at a remote community PHC), the Department of Radiology at AKUH has 1.5 and 3 Tesla MRI scanners, a CT scanner, and 2 X-Ray systems, with 40 radiologists available on-site. Approximate average income for the serviced population is 100,000 PKR ($360 USD)/month.
Cape Town University, Cape Town (South Africa). The University of Cape Town (UCT) is a recognized centre of excellence with world-class research facilities including the Neuroscience Institute (NI). The UCT NI aims to build a global network addressing the brain-health priorities and challenges faced by the populations it serves through advancing research, training, and advocacy. The overarching research strategy is based on two conceptual domains, namely brain development across the lifespan and brain injuries. This site is home to an interdisciplinary team of researchers and clinicians, a network of collaborative projects, and state-of-the-art equipment and technology including a partnership with the Cape Universities Body Imaging Centre (CUBIC; http://www.cubic.uct.ac.za). CUBIC houses both a 3 T Siemens Skyra MRI as well as the Hyperfine Swoop scanner. The UCT team is championing cross-validation work across both MRI systems. This work is embedded within multi-modal pediatric research projects across several longitudinal birth cohort studies based in Gugulethu and other peri-urban regions of the Cape Town metropole. These high-risk communities are representative of many South African communities, with a high prevalence of maternal depression, childhood malnutrition, HIV exposure, exposure to violence, alcohol and drug use, and infectious diseases.
Christian Medical College (CMC), Vellore (India) is one of the premier teaching hospitals in India and has been in the forefront of primary to quaternary care services in the country for more than a century. The private non-profit hospital has ∼3700 inpatient beds across its seven campuses with approximately 10,500 outpatients per day. The hospital serves a broad community, including individuals from across India, Bangladesh, Srilanka, Maldives, Nepal, Bhutan, the Middle East, and central Africa. In addition to the Hyperfine Swoop, the hospital has access to 3 T MRI and high-resolution CT (with approx. 60,000 MRI and 70,000 CT scans performed per year). The radiology department consists of 100 radiologists and 150 radiographers, technicians, and other support staff. The child development research team is involved in the community with other institutional community teams in both urban and rural Vellore in a participatory and complementary model. CMC Vellore is involved in a large-scale pregnancy risk and surveillance study (PRiSMA), which will follow children born to healthy and anemic mothers. The average salary in the region is 31,000 INR ($372 USD)/month.
Community Empowerment Laboratory, Lucknow (India) is a community-entrenched global health research and innovation organization with more than two decades of experience in enacting and driving meaningful community-driven change in the health and survival of India’s children. The team is internationally recognized for its work in fostering and promoting kangaroo mothering care and other critical domains of maternal and newborn survival. The team has experience with onsite neurocognitive assessments and functional near-infrared spectroscopy (fNRIS) (Wijeakumar et al., 2019, Spencer et al., 2023), and has access to a high-field 3 T Philips Allegra scanner in Lucknow in addition to the LF-MRI Hyperfine Swoop that is located at the main research building in Shivgarh, a rural block in the Rae Bareli district, Uttar Pradesh, India. The site will conduct a large-scale longitudinal study focused on the impact of early mother ‘kangaroo’ care and breastfeeding. The mean salary in the region is 4200 INR ($50 USD)/month
Iccdr,b Dhaka (Bangladesh) The International Centre for Diarrheal Disease Research, Bangladesh (icddr,b) is a large multi-disciplinary international and national scientific research site that includes a main hospital campus as well as affiliated satellite sites, e.g., an urban site (Mirpur). Two Hyperfine Swoop systems have been delivered to the main campus and one satellite site with Dhaka, complement an existing 1.5 T MRI system and 128-channel research-dedicated EEG system. The sites will engage in pediatric research related to early child care and advancing women’s economic empowerment and labour force involvement. Average salary of participants enrolled in ongoing studies is approx. 26,000 BDT ($235 USD)/month.
Jaramogi Oginga Odinga Teaching & Referral Hospital, Kisumu (Kenya). The Kenya site of the Pregnancy Risk Stratification Innovation and Measurement Alliance (PRiSMA) MNH study is led by the Kenya Medical Research Institute (KEMRI)-Centre for Global Health Research (KEMRI-CGHR) in collaboration with the United States Centre for Disease Control (CDC), Kisumu County Department of Health, and Siaya County Department of Health, in western Kenya. Participants are drawn from two Health and Demographic Surveillance System (HDSS) areas, located in western Kenya: (1) a rural district, Siaya County; (2) An urban area of Kisumu County. The scanner is hosted at Jaramogi Oginga Odinga Teaching & Referral Hospital (JOOTRH). JOOTRH is located in Kisumu City and is the main referral hospital for over 7 million residents of 10 counties in western Kenya. The site is involved in a large-scale pregnancy risk and surveillance study (PRiSMA), which will follow children born to healthy and anemic mothers. Average Salary in the area is approximately 35,000 Kenyan Shilling ($265 USD)/month.
Kintampo Health Research Centre (KHRC), Kintampo (Ghana) KHRC area includes communities of nine adjoining districts in the Bono East Region of Ghana. The total resident population of the research area is about 600,000 and approximately 30,000 pregnancies are identified and recorded by KHRC each year. This is one of the largest populations involved in continual research surveillance in sub-Saharan Africa. KHRC works collaboratively with the Kintampo Municipal Hospital, therefore has access to MRI (1.5 T) and EEG equipment in addition to the on-site Hyperfine Swoop. KHRC is involved in a large-scale pregnancy risk and surveillance study (PRiSMA), which will follow children born to healthy and anemic mothers. Average salary in the region is approx. 1500 Ghanian Cedis ($113 USD)/month.
Korle-Bu Teaching Hospital, Accra (Ghana). The Korle-Bu Teaching hospital is the largest academic medical center and the largest public hospital in Ghana, with 2000 beds. In addition to the Hyperfine Swoop scanner, the clinical radiology department operates a 1.5 T Toshiba Vantage MRI scanner, two CT scanners and four X-Ray (including a mobile unit) systems. There are currently eight Specialist and five Senior Specialist Radiologists at the hospital. The Hyperfine system will be used as part of a longitudinal follow-up study assessing structural brain development and function in children from birth to 12 months, including the potential impact of maternal and infant anemia. Average salary within the area is approx. 2000 Ghanian Cedi ($150 USD)/month.
Makerere University & Kawempe Referral Hospital (KRH), Kampala (Uganda) The school of public health at Makerere university (MakSPH) is part of the Makerere university college of health sciences. The university is one of the oldest in Africa (established in 1922) and is recognized as a leading academic and research institution in Sub-Saharan Africa. The research site is located at the Kawempe national referral hospital (KNRH), a public tertiary hospital in urban Kampala, Uganda. Kawempe National Referral Hospital has one radiologist and nine radiographers. The hospital has an X-ray machine and a CT scan machine but no access to MRI. The hospital has a capacity of 200 beds and manages a considerable workload, delivering an average of 65 babies per day. A second study site, at the Ndejje Health Center IV, operates at a lower level within the Ugandan healthcare system and has no resident radiologist. In addition, the health center has no X-ray, CT, or MRI. Two Hyperfine Swoop systems have been delivered to each study site. The systems will be used to investigate longitudinal brain development in healthy children from 0 to 5 years of age, as well as in low-birth weight infants and the impact of early nutritional supplementation (Flaherman et al., 2023). Both institutions primarily serve low-income communities, with average salaries of approx. 250,000 UGX ($65 USD)/month.
University of Stellenbosch, Stellenbosch (South Africa). The research site is located at Tygerberg Hospital, Parow, Cape Town. Tygerberg is the largest hospital in the Western Cape and acts as a teaching hospital in conjunction with the University of Stellenbosch’s Health Science Faculty. The research unit includes two assessment rooms, a neurophysiology laboratory (EEG and eye-tracking) and a neuroimaging room where the Hyperfine Swoop is situated. The current, Biomarkers of Neurodevelopmental Outcomes Study (BONO) aims to assess the cognitive, socioemotional, neurodevelopmental and general health outcomes in up to 2000 children (aged 6–17 years) from surrounding communities. Our participants are from two surrounding lower socioeconomic residential areas and their mean monthly income in 2019 (feasibility study) was ZAR 5883 ($370 USD)/month.
University of Pretoria (South Africa). The LF-MRI units for the Neonatal Encephalopathy with Suspected Hypoxic Ischaemic Encephalopathy (NESHIE) study have been placed at Kalafong (University of Pretoria) and Chris Hani Baragwanath Academic Hospital (CHBAH; University of the Witwatersrand). Both sites are based in the Gauteng province of South Africa and are tertiary level state hospitals with limited access to 1.5 T MRI scanners (namely Phillips and GE MRI scanners at Kalafong and CHBAH, respectively) as well as CT, PET, ultrasound, and X-Ray facilities. Together, the ∼1100 and ∼3200-bed public hospitals have 64 and 185-bed neonatal unites, respectively. The radiology department at Kalafong has four radiology consultants and four registrars, whilst CHBAH has thirteen radiology consultants and twenty-three rotating registrars. The Hyperfine Swoops systems at the two hospitals will be used as part of a study of neonatal encephalopathy with suspected hypoxic ischaemic encephalopathy (NESHIE), specifically as a means through which point-of-care proximal imaging biomarkers can be determined in moderate-to-severe term (GA ≥ 36 weeks) NESHIE neonates. The LF-MRI component of the NESHIE study has been actively enrolling patients since November 2021 and involves comparing same-day LF-MRI and 1.5 T MR images from babies with moderate-severe NESHIE. Average salary of individuals served by both Kalafong and CHBAH is less than 5000 Zar ($265 USD)/month.
University Teaching Hospital, Lusaka (Zambia). The University Teaching Hospital (UTH) is the principal medical training institution for the University of Zambia. Post-graduate physician training is available in the Departments of Anesthesia, Internal Medicine, Obstetrics and Gynecology, Pediatrics, Surgery, and Pathology. UTH is a public hospital with approximately 2000 beds, 21 operating rooms, and 10 ventilator-accessible intensive care unit beds. It provides a full range of primary, secondary, and tertiary health and medical services on both an inpatient and outpatient basis. In addition, it serves as the country's specialist centre receiving referrals from all over Zambia. Specialty services at UTH include internal medicine subspecialties (i.e., infectious disease, cardiology, gastroenterology), neonatology, orthopedics, urology, transplant medicine, pediatric surgery, radiology (CT capabilities), physical therapy, and prosthesis fitting and production. In addition to the Hyperfine Swoop, Imaging services include: CT, plain radiography, DEXA scan, ultrasound/echo, and fluoroscopy. There are currently 2 radiologists (physicians) with16 radiographers and 28 radiography technicians. UTH is involved in a large-scale pregnancy risk and surveillance study (PRiSMA), which will follow children born to healthy and anemic mothers. Average income for the population in Lusaka is $231 USD/month.
Zomba Central Hospital (ZCH), Zomba (Malawi) The Training and Research Unit of Excellence (TRUE) in Zomba, Malawi, is a research institution with over 14-year track record of local and international collaborative research. Focused on infectious diseases, malaria, and nutrition, it operates within the Zomba Central Hospital complex, which serves a population of approximately 4.5 million in the southeastern region, 70 km from Blantyre. TRUE's research has yielded innovative health solutions, including those addressing early-life risks like anemia and malaria, incorporated into WHO guidelines. The site focuses on maternal and child health, maintaining a birth cohort of about 5000 children under 5. The Hyperfine Swoop system is located at ZCH and is integrated into a number of ongoing maternal and child health studies. Average salary of participants in the area is less than 10,000 Malawian Kwacha ($6 USD)/month.
Fig. 4.
A snapshot of images of the Hyperfine Swoop systems installed at many of the identified UNITY sites, including members of the study teams.
Cohorts across the included study sites (Table 2) encompass both healthy full-term infants, as well as those born with adverse birth outcomes (including preterm delivery, small-for-gestational-age, and hypoxic-ischemic encephalopathy), maternal and child diet and nutrition status (specifically maternal prenatal anemia), socioeconomic and environmental adversities, and differing gender and social norms and equality. The expected meta-cohort aims for more than 10,000 mother-child dyads with neuroimaging performed at multiple time points throughout the first year of life (typically at 3, 6, and 12 months of age), and then less frequently (biannually or annually) from 1 to 5 years of age and older.
Table 2.
Overview of observational and clinical trials that are included in the UNITY network.
| Study Location | Cohort Name | Study Type | Cohort Age Range | Cohort Type | Cohort Size | Imaging Timepoints |
|---|---|---|---|---|---|---|
| CEL, Lucknow, India | India Longitudinal Cohort | Longitudinal Observation Study | 3 Months - 5 Years | Healthy Community Sample | 180 | 3, 9, 15, 21, 30, 60 Months |
| Makerere University & Kawempe Referral Hospital, Uganda | PRIMES | Longitudinal Study following Nutritional Supplementation | 3–12 Months | RCT of low-birth infants with and without early nutritional supplementation for 30 days at 1 Month | 80 | 3, 6, 12 Months |
| Makerere University & Kawempe Referral Hospital, Uganda | Uganda Longitudinal Cohort | Longitudinal Observation Study | Birth - 5 Years | Healthy Community Sample | 180 | 3, 6, 12, 18, 24, 30, 36, 42, 48, 54, and 60 Months |
| University of Cape Town, South Africa | DoLPHIN 2 Plus+ | Randomized Clinical Drug Trial | 2–4 Years of Age | Observational follow up of HIV-exposed uninfected children nested within the drug trial and HIV-unexposed uninfected children from the same high-risk community. | 120 | 24–60 Months |
| University of Cape Town, South Africa | Khula | Longitudinal Observation Study | Birth - 2 Years | Healthy Community Sample | 300 | 3, 6, 12, 18, 24 Months |
| Kalafong Hospital (Pretoria) and Chris Hani Baragwanath Academic Hospital (Johannesburg), South Africa | NESHIE | Longitudinal Observation Study | 0–6 Months | Patients with moderate-severe NESHIE | 200 | 0–6 H of life*, 3–6 Days OR 7–14 Days, and 3–6 Months |
| Blantyre, Malawi | Khula | Longitudinal Observation Study | Birth - 2 Years | Healthy Community Sample | 300 | 3, 6, 12, 18, 24 Months |
| Stellenbosch University, Cape Town South Africa | BONO | Longitudinal Observation Study | Birth - 7 Years | Community Sample | 2000 | 1, 2, 3, 4, 5, 6, 7 Years |
| Korle-Bu Teaching Hospital, Ghana | Accra Neuroimaging Study | Longitudinal Observation Study | 0–12 Months | Community Sample | 120 | 3, 6, 12 Months |
| CMC Vellore, India | PRiSMA | Longitudinal Observation Study | 0–12 Months | Drawn from larger study of mothers with and without antenatal anemia | 300 | 3, 6, 12 Months |
| Kintampo Health Research Center, Ghana | PRiSMA | Longitudinal Observation Study | 0–12 Months | Drawn from larger study of mothers with and without antenatal anemia | 300 | 3, 6, 12 Months |
| Kisumu, Kenya | PRiSMA | Longitudinal Observation Study | 0–12 Months | Drawn from larger study of mothers with and without antenatal anemia | 300 | 3, 6, 12 Months |
| Lusaka, Zambia | PRiSMA / ZAPPS | Longitudinal Observation Study | 0–12 Months | Drawn from larger study of mothers with and without antenatal anemia | 300 | 3, 6, 12 Months |
| AKU Hospital, Pakistan | PRiSMA | Longitudinal Observation Study | 0–12 Months | Drawn from larger study of mothers with and without antenatal anemia | 300 | 3, 6, 12 Months |
| AKU Hospital, Pakistan | MINE | Longitudinal Observation Study | 1–36 Months | General hospital & community sample | 250 | 12, 24, 36 Months |
| Iccdr,b Bangladesh | BEAN | Cross-Sectional Observation Study | Birth - 12 Years | A community cohort longitudinal EEG, NIRS, and MRI study of neurodevelopment | 210 | 3, 12, 24, 48 and 96 Months |
| Zomba Central Hospital, Zomba | Birth Cohort | Mixed Cross-Sectional and Longitudinal Cohort | Birth - 5 Years | Community sample. | 5000 | 3, 12, 24, 36, 48, 60 Months |
| Zomba Central Hospital, Zomba | REVAMP | Longitudinal RCT | Birth - 2 Years | RCT of maternal IV-Iron infusion for the treatment of antenatal anemia | 200 | 6, 12, 24 Months |
| Felege Hiwot Comprehensive Specialized Hospital, Bahir Dar, Ethiopia; Addis Continental Institute of Public Health | BCD (Bahirdar Child Development) | Prospective accelerated longitudinal cohort | 6 Months-5 Years | Healthy community sample | 210 | 6, 12, 18, 24, 30, 36, 42, 48, 60 Months |
| Felege Hiwot Comprehensive Specialized Hospital, Bahir Dar, Ethiopia; Addis Continental Institute of Public Health | ENAT Infant Follow up | Longitudinal Infant follow up of Offspring of ENAT study | 12–24 Months | Randomized pragmatic effectiveness study of maternal nutrition interventions (Balanced Energy protein supplement, iodized salt, IFA) and enhanced infection management (genitourinary tract infection treatment and deworming) | 60 | 12, 24 Months |
Individually, analysis of cohort-specific data will provide important insight into the use of low-field MRI in understanding the neurological impact of specific conditions, nutritional deficiencies, and environmental adversities and evaluating potential therapeutic interventions. Collectively, data integrated from all cohorts will also provide new insight into ‘neurotypical’ development from birth through age 5 years across these geographies, as well as allow examination of how regional and geographical differences in health and environmental factors prevalent in lower resource “global south” settings affect neurodevelopment.
2.3. Site setup and staff training
2.3.1. Import and shipping of the hyperfine swoop into LMIC settings
The Hyperfine Swoop LF-MRI system (operating at 64mT) is FDA 510k cleared for brain imaging for all ages. The Swoop was chosen for this project due to its portability, ease of installation and use, limited infrastructure requirements (e.g. no cryogens), and immediate commercial availability at scale. Whilst many of the procedures are novel and undergoing continuous improvement, the protocols developed as part of the UNITY project will inform a broad range of future ultra-low-field neuroimaging studies.
Importing the systems into each country presented unique regulatory challenges from various governmental agencies (Health, Energy, and Economic Development). For study sites located in LMICs, PATH, a global health non-profit, worked closely with Flexport (flexport.com), the logistics provider, to navigate local regulatory and import requirements to facilitate delivery of the MRI systems. In the absence of a uniform regulatory regime, each country’s regulatory procedures were unique and required a novel approach to facilitate delivery of the device to the respective study sites. Common challenges included attaining import tax waivers, managing conflicting or inconsistent application of regulations, and optimizing delivery strategies.
Given the cost of the Hyperfine Swoop device, some value added tax (VAT) and importation taxes in certain geographies had the potential to place an undue financial burden on the project. Most countries, however, would provide waivers and/or tax exemptions predicated on the nature of the device, its intended use, and/or ownership status. Letters of ‘donation’ (Botswana, Ghana) or ‘transfer of ownership’ (Ethiopia, Malawi, Zambia), for example, were often sufficient, or providing evidence that the device was intended for scientific purposes, helped reduce or eliminate the tax exposure.
Conflicting regulation, or the engagement of other local agencies, was also experienced. The local regulatory agency of South Africa, SAHPRA, asked for completed forms from the Medical Device Unit and the Radiation Control Unit, in addition to licensing requirements for import, import of an electromagnetic device, and use of an electromagnetic device. The former required EC certificate and EC declaration of conformity, while the latter did not, as the two were operating under the requirements of two national laws that were not harmonized. An exemption letter had to be obtained from the Ministry of Health in order to move forward. In Ghana, the team had to engage with the Nuclear Regulatory Authority for an import permit (including completing and submitting a “Notification of radioactive material transport” and an application to “Authorize the registration to use radiological materials) despite the device – like all MRI systems – not containing radiological materials.
The cost to deliver the MRI systems by air freight was significantly higher than by sea. Though the transit time was longer and more likely subject to delays, cargo ships facilitated in-country delivery for most study sites. Study sites in landlocked countries, such as Ethiopia, Malawi, and Zambia, made use of air freight, whereas Botswana received their device via land from South Africa. In each instance, local logistics partners and hospital coordinators were instrumental in the successful allocation of MRI systems to the respective study sites. Final mile delivery logistics relied on close attention to details including securing the availability of pallet jacks/forklifts during delivery, identifying a location where the crate will be unloaded, and walking the delivery pathway to avoid narrow door widths, inclines, uneven areas, and gradients greater than 5 degrees.
2.3.2. Ongoing service and support for the swoop scanner
An important aspect of UNITY is the sustainability of the imaging systems, specifically with respect to ongoing service maintenance, repairs, and software updates. This has traditionally been a challenge in many LMIC settings, where the lack of parts and service are not always readily available despite service contracts with manufacturers that can represent several thousands of dollars. This has resulted in “equipment graveyards” with non-functional imaging systems and other medical equipment taking up precious space in often over-crowded hospitals. In lieu of a traditional service contract with Hyperfine, UNITY provides direct support for dedicated personnel to oversee the operation of the UNITY scanners and provide direct phone or video-based support for connectivity issues, software updates, and operation issues. For service calls that cannot be diagnosed remotely or that require in-person support (e.g., more in-depth diagnostics, parts replacement), UNITY has a dedicated service engineer who will travel between sites. In addition, working with Hyperfine, in-depth training on scanner service has also been provided, allowing sites to perform a higher degree of maintenance than would be commonly possible.
2.3.3. IRB Considerations
As part of the importation process of research-dedicated scanners, local ethics approval was required for each system. As with high-field systems, this required reporting of known risks associated with ultra-low-field MRI, including possible discomfort and claustrophobia, scanner noise, energy deposition, and potential for peripheral nerve stimulation (PNS). The open concept and head-only nature of the Hyperfine Swoop helps to minimize participant discomfort associated with claustrophobia (in particular in smaller children and infants). Foam padding and inflatable pads (e.g., PearlTec www.pearl-technology.ch), and immobilizers (e.g., Med-Vac) are used to help position the infant and child’s head in the system and minimize motion. Although the Hyperfine system is relatively quiet compared to high-field scanners, headphones, and ear plugs/protectors are used to reduce acoustic noise levels to less than 40 dB, well below established FDA guidelines of 99 dB. Similarly, whilst PNS and SAR (specific absorption rate, a measure of energy deposition) limits are often encountered on higher field systems, they do not pose a significant risk on the Swoop system because it lacks the hardware to switch gradients quick enough to induce PNS, operates at a radio frequency that carries little energy, and has hardware duty-cycle limits that further limit SAR.
Although the Swoop system has a low magnetic field strength and a small 5 Gauss line “footprint”, there remains potential for harm to individuals with metal prosthetics or implants (including extensive dental work i.e., braces, aneurysm clips, cardiac pacemakers, or deep brain stimulators, or insulin pumps). These individuals are not allowed near the scanner (within the area circumscribed by the 5 Gauss line).
2.3.4. Site Preparation
Preceding and following the arrival of the Swoop imaging system (Fig. 5), clinical partners were engaged with members of the import team, Hyperfine, and experts in pediatric imaging to ensure an appropriate site for the device, identify and address challenges with local connectivity, and arrange online and in-person training for the study staff.
Fig. 5.
Pictures of Swoop arriving at a clinical cohort site. Following unloading of the scanner and accessory crate (a and b), the scanner crate is opened and protectors removed (c). The scanner can then be driven out of the grant and into the facility (d, e, and f) to its desired location. Here final set up and unpacking is performed (g) before being ready to image. From arrival to set up can take between 30 and 60 minutes depending on access and distance from delivery point to final scan room.
Preferred siting conditions for the Swoop system include an unobstructed and relatively flat route from the delivery location to the scanner storage area free of ramps greater than 5 degrees and doorways narrower than 92 cm; a restricted access area to limit unintentional exposure to the system’s magnetic field; and an electrical outlet with the appropriate amperage and voltage.
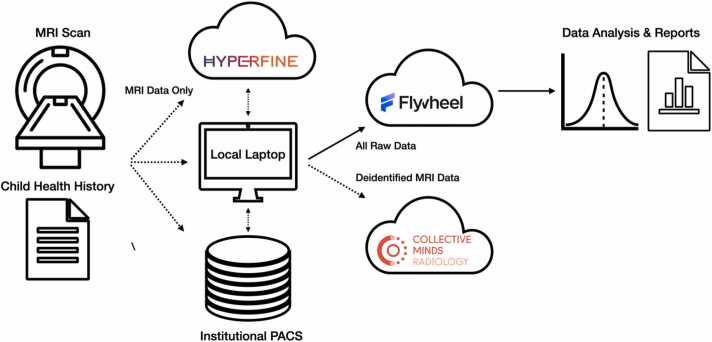
Depending on local requirements, the imaging system was connected either directly to the hospital Picture Achieving and Communication System (PACS) or the internet for image transfer. Non-PACS-connected systems utilized the Hyperfine cloud-based repository if internet connectivity was sufficient, or was hard-line (ethernet) connected to a provided Apple MacBook Pro laptop, with data curation performed using the open-source Horos image viewer (https://horosproject.org/). Whilst high-speed WiFi hubs and 5 G LTE cellular networks are commonplace in HIC hospitals, universities, and clinical care settings, this is not the case in many LMIC settings where cellular modems providing internet via 3 G and 4 G networks are more typical and require consideration for data transfer.
2.3.5. Site Training
In-person safety training and imaging protocol development (Fig. 6) was provided initially by a team of pediatric imaging researchers from either the Advanced Baby Imaging Lab at Hasbro Children’s Hospital, Providence RI, or the University of Cape Town, Cape Town, South Africa. Following the delivery of the scanner, the team would arrive to assist with unboxing, set-up, and usage over a 3–5 day period. Training encompassed general safety protocols, moving and positioning the scanner via its built-in motor and drive wheel, and approaches to non-sedated infant and pediatric neuroimaging, which include many of the same techniques developed for pediatric imaging at 3 T (Dean et al., 2014, Wedderburn et al., 2020). Particular focus was placed on how to prepare infants and children (and their families) for scanning, ideal positioning and alignment in the scanner, and quality checking the scans in real-time. Training also covered the use of accessories, including pediatric immobilizers (such as those from Pearltec and MedVac) and foam cushions to restrict infant motion during the scan and to reduce scanner noise. The training audience and imaging teams varied by LMIC site and included, but not limited to, medical and clinical staff, radiology staff, research staff, and IT and biomedical technical staff, where they were available.
Fig. 6.
Pictures of In-person training beginning with a general safety orientation and introduction to the scanner (a), demonstrations and training on scanner driving and positioning (b), initial protocol setup and scanner interface (c), practice scanning on each other (d) and, finally, positioning and scanning of infants and toddlers (e and f).
Safety concerns at some of the participating sites preclude participant travel from their homes to the image centers at night, precluding the option of nighttime imaging. Specific strategies for daytime scanning were, thus, developed and troubleshot at each site that was adapted to their unique setting and context. As part of the training, the imaging teams at each site participated in practical demonstrations before scanning each other and, if research ethics were in place, infants. Following each scanning session, a debriefing was held to identify and propose changes to address challenges. A usability study involving user interviews with trainees was conducted to gain a deeper understanding of MRI users' needs, desires, and experiences; perceptions around training and operation of the scanner in the LMIC sites; common issues across research sites and recommendations for improvements; and best practices for implementing MRI in LMIC settings.
The training was structured in such a way as to give ample time for each member of the imaging team(s) to practice operating the scanner. This helped to ensure that once training was concluded the team was ready to scan independently and to train other members of their team in the future. Training videos describing the essential steps in unpacking the Hyperfine scanner, driving it, setting it up for scanning of research subjects, cleaning, and quality control scanning were recorded and shared between partners within the project. Additional training materials, including pictorial instruction sheet providing step-by-step instructions visually demonstrating the relevant procedures for cleaning, moving, and scanning participants of different ages have also been made and distributed across sites. This has helped facilitate rapid training of new staff in the project and adherence to the protocols.
In addition to the in-person training performed at site initiation, on-going training is also facilitated through KCL, Brown University, and the University of Cape Town via regular check-ins and image-review sessions on Collective Minds Radiology (discussed further later in this overview). Site-visits are also organized yearly, and include members from BMGF, KCL, Hyperfine, ISMRM, Collective Minds, FlyWheel, and other organizations within the CSSs network.
2.4. MRI Physics, Sequence Development, Imaging Protocols, and Data Acquisition Methods
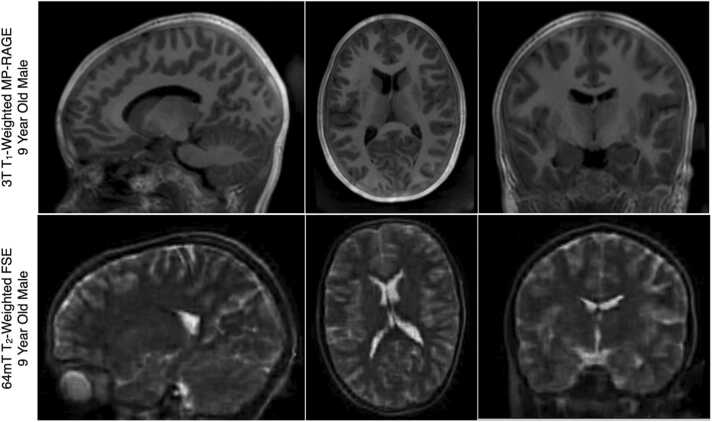
Image quality and tissue contrast (i.e., signal-to-noise and contrast-to-noise ratios, SNR and CNR) in MRI is inherently related to: 1. Magnetic field strength; 2. Tissue relaxation characteristics; 3. Imaging sequence and acquisition parameters (i.e., echo time, repetition time, etc.); 4. Image voxel size; and 5. Acquisition time. The reduction in field strength from 1.5 T or 3 T to 0.064 T results in a significant loss of signal and SNR decrease. Further, the T1 relaxation time is fundamentally related to field strength, and is also significantly reduced at 64mT. The factors yield visible quality and contrast differences between T1 and T2-weighted images acquired at ultra low and high magnetic field, e.g., Fig. 7.
Fig. 7.
Example (top) 3 T T1-weighted and (bottom) 64mT T2-weighted anatomical images of a 9-year old male child. Owing to differences in T1 and T2 relaxation parameters, T2-weighted imaging is preferred at low-field and is the primary image contrast of the UNITY common protocol.
In addition to these considerations, the Hyperfine Swoop system was originally developed for adult imaging in acute clinical care settings with T1 and T2-weighted Fast Spin Echo (FSE), Fluid Attenuated Inversion Recovery (FLAIR), and single-direction diffusion-weighted imaging (with diffusion encoding along the anterior-posterior axis of the head) sequences based on fast spin echo (FSE) acquisitions and optimized for adult tissue characteristics and contrast. Using these, a basic standardized “core” protocol has been developed that consists of volumetric T1 and T2 weighted imaging and quantitative T2 imaging (Table 3, Table 4), but with parameters optimized for the neonatal, infant, and pediatric brain. This protocol is designed to provide basic anatomical and volumetric measures to achieve the desired aims of the UNITY project and address the research questions at the individual studies. Including child positioning and localizer scans, the protocol requires approximately 20–25 minutes.
Table 3.
The main imaging protocol used across the CSSs for neonates and infants less than 1 month of age.
|
FOV (X x Y x Z) cm3 |
Resolution (X x Y x Z) mm3 |
TE / TR / TI (ms) | Time (min:sec) | |
|---|---|---|---|---|
| T2FSE (axial) | 21 ×18×18 | 2.0 ×2.0 ×2.0 | 371 / 2000 / NA | 14:38 |
| T1IR-FSE (coronal) | 22 ×18×18 | 2.0 ×2.0 ×2.0 | 7.6 / 1250 / 400 | 14:53 |
| T1IR-FSE (coronal) | 22 ×18×18 | 2.0 ×2.0 ×2.0 | 7.6 / 1250 / 500 | 14:53 |
| T2Mapping (optional) | 20 ×18×22 | 1.7 ×1.7 ×5 | 41, 81, 122, 163, 204, 244, 285, 326, 366, 407 / 2000 / NA | 9:22 |
Table 4.
The main imaging protocol used across the CSSs for children older than 1 month of age.
|
Image Matrix (X x Y x Z) |
Resolution (X x Y x Z) mm3 |
TE / TR / TI (ms) | Time (min:sec) | |
|---|---|---|---|---|
| T2FSE (axial) | 112 ×136×40 | 1.5 ×1.5 ×5 | 180 / 2000 / NA | 2:15 |
| T2FSE (coronal) | 112 ×44×124 | 1.5 ×5×1.5 | 220 / 2000 / NA | 2:22 |
| T2FSE (sagittal) | 36 ×136×124 | 5 ×1.5 ×1.5 | 225 / 2000 / NA | 2:12 |
| T1IR-FSE (axial) | 112 ×138×40 | 1.5 ×1.5 ×5 | 6.6 / 880 / 354 | 6:11 |
| T2Mapping (optional) | 20 ×18×22 | 1.7 ×1.7 ×5 | 41, 81, 122, 163, 204, 244, 285, 326, 366, 407 / 2000 / NA | 9:22 |
Complementing these core acquisitions, a central component of the UNITY project is the development of dedicated neonatal and pediatric-optimized acquisition protocols, as well as more sensitive methods to interrogate tissue microstructure. When or where possible we aim to enhance the core protocol with MTR, MWI, diffusion, and other acquisitions as they are developed. To this end, recent advancements have been made concerning motion-tolerant and contrast-optimized neonatal protocols (Cawley et al., 2023), quantitative T1 and T2 imaging methods (qT1 and qT2, respectively), magnetization transfer ratio (MTR) imaging (UBC), multicomponent relaxometry and myelin water imaging (MWI)(Dvorak et al., 2023) (UBC), and diffusion sensor MRI (CUBRIC) Fig. 8.
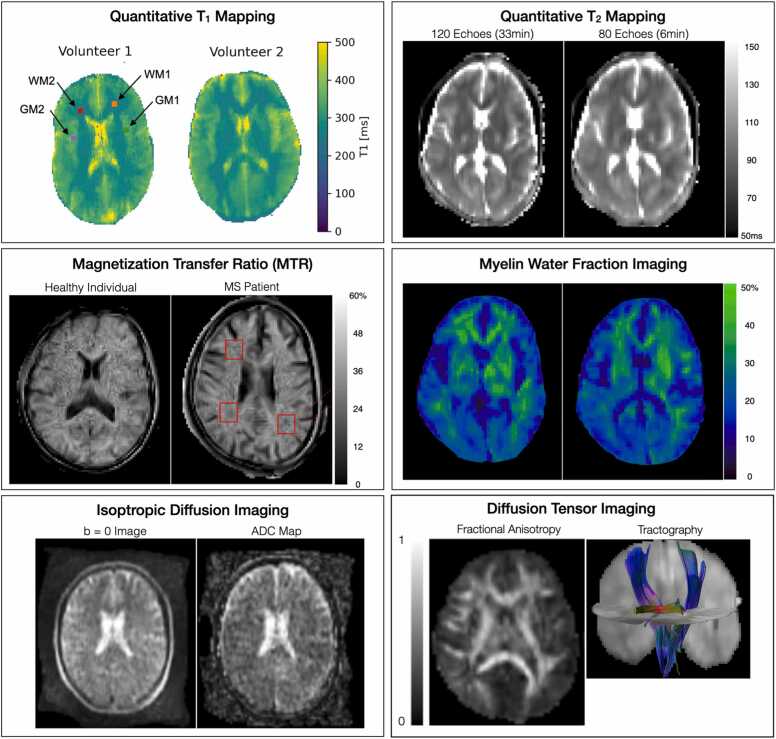
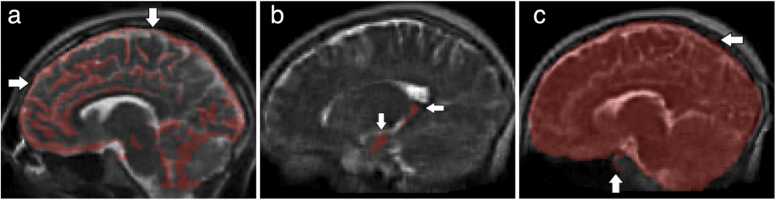
Fig. 8.
Representative quantitative maps and images from the advanced acquisition methods, including single and multiple component relaxometry for quantitative T1, T2, and myelin water fraction imaging, magnetization transfer imaging, isotropic diffusion-weighted imaging, and diffusion tensor imaging and tractograhy of the cortico-spinal tracts and splenium of the corpus callosum. These methods are intended to provide increased sensitivity to microstructure change associated with early neurodevelopment and the impact of nutritional and other interventions.
Enabling these developments requires strong academic-industrial collaboration and partnership between the P&E teams and the Hyperfine hardware engineering and sequence development teams. This collaboration has facilitated the development of an open sequence development interface for altering sequence parameters, and access to the raw k-space imaging data for offline processing using state-of-the-art image reconstruction tools (e.g., BART, Riesling, ISMRMRD). Given the unique nature of the Hyperfine operating system, initial sequence development for the project has been carried out via dedicated ‘sprints’ held at the Hyperfine Development center in Guilford, CT, with work then performed back at individual home institutions. These development sprints bring together a small group of academic MR physicists and the Hyperfine sequence development team around a central focused theme (e.g., MTR, diffusion tensor MR) for 1–2 weeks of in-depth work and development, including extension and refinement of the sequence development interface. Sprints occur approximately quarterly and are preceded by in-depth discussions between the teams to identify the need, propose two or three potential “best bet” avenues to address the need, and lay the necessary groundwork such that the in-person time is maximized. Needs are driven by the clinical partner sites and study focuses. For example, the strong emphasis on understanding the impact of anemia and iron deficiency on early neurodevelopment has guided the development of qT2, MTR, and MWI methods due to the potential impact of anemia on neurodevelopment and myelination (Georgieff, 2008, Mercer et al., 2022).
2.4.1. Core neonatal imaging protocol (< 1 Month)
2.4.2. Core infant and child imaging protocol (>1 Month)
Due to the inherent reduced signal (and, consequently, lower signal-to-noise ratio (SNR)) at low magnetic field, LF-MRI images are often acquired with anisotropic spatial resolution, increasing signal through large through-plane resolutions (e.g., 1.5 mm×1.5 mm x 5 mm) whilst maintaining reasonable acquisition times (e.g., 5 minutes). Isotropically-resolved acquisitions are possible, but with increased acquisition times that can also confer motion sensitivity, particularly in older pediatric populations who find lying still for extended periods (10–15 minutes) challenging. To achieve high-resolution structural imaging in children 3 months of age and older, we have adopted a super-resolution reconstruction approach that produces isotropically-resolved images from three anisotropic images acquired along three orthogonal orientations (Deoni, O'Muircheartaigh et al., 2022), e.g., Fig. 7. AI-based approaches, such as SynthSR will also be used to recreate “synthetic” high-resolution anatomical T1-weighted images from lower-resolution T1 and/or T2 images (Initiative Alzheimer's Disease Neuroimaging, 2021, Iglesias et al., 2023). While SynthSR has not yet been trained on data from infants and young children, such work is ongoing. It is worth noting however, that positive results have been obtained using SynthSR in pediatric populations, lending confidence in its utility even without addition infant-focused training (Cooper et al., 2024). Beyond approaches trained on low-resolution data which was synthetically generated from high-field tissue segmentation labels, we are also training custom neural networks on paired empirical ultra-low-field and high-field scans.
Unlike protocols used at higher field strengths, which focus on T1 weighted images as the main anatomical and structural measure (with T2 weighted images occasionally included for children less than 1 year), the structural imaging component of our LF-MRI protocol focuses on T2 weighted images across the age span (Fig. 7). We have found the T2-FSE images provide consistently better tissue contrast across the age span than the current T1-weighted inversion-prepared FSE images (Deoni et al., 2021, Deoni et al., 2022). However, work is ongoing to improve T1-weighted contrast through the use of more rapid steady-state sequences (e.g., spoiled gradient or inversion-prepared balanced free precession), which may also offer improved signal-to-noise per unit scan time.
Adding additional information on tissue microstructure, quantitative T2 mapping via a multi-echo FSE acquisition is also an integral component of the core protocol. Tissue differences in T2 may be related to changes in iron, lipid, and myelin content - providing insight into potential changes in brain iron and myelination hypothesized to be impacted by maternal antenatal anemia, malnutrition, and environmental adversities. To provide improved sensitivity to myelination changes, steady-state free-precession magnetization transfer (SSFP-MT) may also be collected, though this approach is not part of the core protocol.
2.4.3. Infant and pediatric data acquisition methods
Building on methods for non-sedated pediatric imaging developed at Brown University and other child imaging centers (Dean et al., 2014, Wedderburn et al., 2020), non-clinical imaging for UNITY is performed during natural sleep without the aid of sedation. Given the diversity of research sites, a single one-size-fits-all approach to pediatric imaging is unlikely to succeed. Instead, each site has adapted its approaches after the initial training sessions to maximize success given local constraints and challenges. For example, whilst evening or night-time scanning is generally preferred (particularly for infants 6–9 months of age through to age 4–5 years), participant safety when returning home at night has forced some sites to emphasize daytime scanning during naps, though this often requires the family to return two or more times to complete the imaging session successfully. Alternatively, some sites in clinical settings may admit the mother, providing the family with a room so that scanning can be performed at night without the need to immediately return home after dark. Other differences between the sites, is the use of individual ‘private’ rooms in which the child can fall asleep before being moved into the scanner (allowing multiple families and children to be scanned in a single night) vs. having the child fall asleep in the scanner (limiting the number of children per day or night).
The Malawi and Uganda sites, for example, have found strong success in scanning children up to 2 years of age during the day by bringing in multiple families, allowing them to fall asleep in different areas of the center or hospital and then moving them to the scanner. If the child wakes, the mother is encouraged to feed the child and stay until they fall asleep. In contrast, all scanning at Aga Khan University is done during the day with a single mother-child at a time, and often the child is scanned awake.
Depending on a child’s age, a custom-built “baby tray” or vacuum immobilizer (Fig. 9) can be used to help restrain the infant and position them within the scanner. Memory foam and/or inflatable cushions are further used to help secure the infant and reduce head motion in the scanner. For children older than 2 years, imaging is challenged by their reduced nap time. This can be countered by allowing the child to watch a favorite movie. Unlike larger 1.5 T or 3 T scanners, which have space within the bore and head coil for a mirror system to watch a projected movie, space within the Swoop scanner is tightly constrained. To address this, mini projectors (e.g., FATORK Mini Projector, or more expensive CineBeam Ultra Short Throw LED Home Theater) mounted to the base of the scanner can be used to project a video onto the inner surface of the scanner bore, allowing it to be viewed by the child. VR headsets, like the Oculus or Apple Vision may also be viable alternatives but have not yet been tested. Sound is played out loud (rather than through headphones) due to space constraints within the head coil itself as well as the reduced acoustic noise of the scanner.
Fig. 9.
Images of child participants in Hyperfine Swoop ranging in age from 1 to 3 months (a, b) using a baby tray positioning insert and foam head pads, 1 year (c) using a blue Med-Vac Immobilizer and head pads, and 5 years (d) using just ear and head pads to minimize motion.
2.4.4. Data Harmonization and the UNITY Phantom
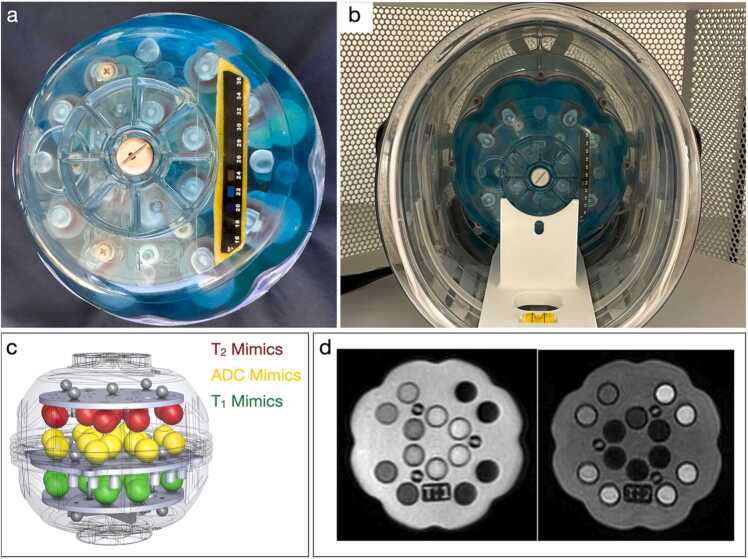
To address known longitudinal intra and inter-site challenges to robust data integration, a custom UNITY phantom was designed by CaliberMRI (Boulder, CO) for use across the project sites. To ensure reliable and consistent positioning of the phantom in the scanner across sites, a physical cradle was also developed that allows unambiguous positioning and leveling of the phantom in the scanner head coil. The UNITY Phantom (CaliberMRI Model 137) (Fig. 10) was inspired by the “NIST/ISMRM” phantom (Stupic et al., 2021) and measures 170 mm in diameter to fit the unique head coil of the Hyperfine Swoop scanner.
Fig. 10.
The CaliberMRI UNITY phantom (a) outside and (b) inside the scanner. The phantom includes 3 sets of T1, T2, and ADC mimics arranged in parallel trays within the phantom (c) that provide differing contrast on T1 and T2-weighted and quantitative images (d). In addition, the phantom includes a spatial resolution grid, and internal and external temperature strips (a). A positioning cradle allows the phantom to be unambiguously positioned in the scanner (b).
The phantom includes 14 T1, 14 T2, and 14x diffusion calibration solution mimics that span the range of healthy to diseased human tissues in the respective parameter space. Approximate ranges of relaxation values at 3 T are ∼20–1900 ms for T1, ∼10–550 ms for T2, and apparent diffusion coefficient (ADC) of ∼400–2000 mm2/s. While “ground truth” measurements of the relaxation times at 64mT are not yet available, the mimics have known concentrations, have high stability, and are SI-traceable to NIST at 3 T. For this work, absolute quantitative accuracy is not of paramount importance. Rather the initial intention is to use these mimics to measure potential drifts in longitudinal and cross-site measurements. To assess geometric distortion and allow calibration of volume measures, the phantom includes a 3-dimensional array of 15 fiducial markers, a slice profile wedge, and a resolution insert.
Given the sensitivity of relaxometry measures to temperature (Bottomley et al., 1984, Bottomley et al., 1987), the UNITY phantom includes an MR-readable thermometer, with a readable range of 15–24°C (Keenan et al., 2020). However, given that many of the sites in UNTY have seasonal temperatures that exceed 24°C and lack air conditioning or climate control, a liquid crystal strip thermometer with a temperature range of 16–36°C was also incorporated into the design.
Quality Assurance Protocol. The QA protocol consists of a set of five scans, a total duration of 15 min. A dedicated sequence is used to assess the phantom temperature. Two T2w axial scans are acquired to assess signal-to-noise ratio (SNR), contrast-to-noise ratio (CNR), and geometric distortions. Additional T2w scans in coronal and sagittal orientation are acquired to complement the assessment of geometric distortions. See Table 5 for a summary of scan parameters.
Table 5.
Short QA protocol used to assess SNR and geometric distortions.
| Parameter | Temperature scan | T2w axial | T2w sag | T2w cor |
|---|---|---|---|---|
| Base sequence | FISP | FSE | FSE | FSE |
| Resolution (mm3) | 3×3x5 mm | 1.6×1.6×5 | 1.6×1.6×5 | 1.6×1.6×5 |
| TE/TR (ms) | 4.11/11.53 | 194.8/2000 | 238.4/2000 | 231.6/2000 |
| Duration (s) | 61.3 | 156.6 | 130.7 | 138.6 |
| ETL | N/A | 80 | 80 | 80 |
| ESP | N/A | 4.9 | 6.0 | 5.8 |
| TI | N/A | N/A | N/A | N/A |
Abbreviations: ESP - Echo spacing, ETL – Echo train length, TE – Echo time, TR – Repetition time, TI – Inversion time, N/A – Not applicable. *For the T2-mapping sequence, ESP and TE is given as the same value
The QA protocol may be performed daily, though the frequency can be adjusted (more or less frequently) depending on factors such as environmental conditions, unique patient concerns, and scanner operations (including software and hardware updates, and moving locations). The QA scans are primarily evaluated via the cloud-based qCal-MR software suite, a product of CaliberMRI, for detailed, repeatable configuration, operation, and quantitative statistical confirmation. A detailed description of the quantitative QA assessment is outside the scope of this paper and will be described in a separate publication.
2.5. Data governance, sharing, and analysis
2.5.1. Data governance and flow
As a guiding principle, UNITY follows a model of global open access consistent with FAIR (Findable, Accessible, Interoperable, Reusable) practices for sharing data, analytic tools, processing pipelines, and publications. This implies that (a) knowledge, information, and data gained from the project be promptly and broadly disseminated and (b) the methods and tools be made available and accessible to all. Fig. 11 visually illustrates the data flow, including low-field MRI data and relevant contextual family and child health data (e.g., child age, birth date, biological sex - detailed below), from each site to central repositories for storage, harmonized analyses, sharing, and community engagement.
Fig. 11.
Data Flow. MRI and health history information collected at each site may be spread across different devices, including Hyperfine Cloud for Hyperfine MRI data (if institutionally allowed), PACS (if available) or on a local laptop. From here, raw MRI and contextual data are uploaded to Flywheel for analysis and sharing. MRI data of questionable quality or with potential artifacts or incidental findings can also be shared with Collective Minds for community feedback. Cohort-specific and UNITY-wide analyses performed on Flywheel then form the basis of scientific reports and publications.
Given differences in institution and country policies with respect to data security and sharing, the specific flow of MRI data may take one of three different routes: 1. Uploaded from scanner to Hyperfine Cloud - an Internet cloud-based PACs server provided by Hyperfine, with data then downloaded to a local laptop; 2. Directly from the scanner to a local laptop (where internet connectivity is not available or institutional policies don’t allow the use of Hyperfine Cloud; or 3. From the scanner to an institutional PACS and then to a local laptop. From the laptop, data is then uploaded to Flywheel and (optionally) Collective Minds radiology for analysis and community feedback. Each site is responsible for uploading all data to Flywheel and performing an initial QC evaluation of the data.
An MRI data core, based at King’s College, London, provides support to the individual sites and acts as a liaison between the sites and Flywheel. This group also assists with sharing permissions, oversees data access and contributor agreements, and provides a secondary QC check of data. Finally, this group also assists with the development, testing, and deployment of image processing algorithms.
2.5.2. Data storage, curation, and sharing
Flywheel provides a common and secure data storage, curation, and analysis platform. Here, harmonized analysis pipelines can be developed, tested, and run across all data, with appropriate tracking of data provenance, without the need to install additional local hardware infrastructure and software. Most imaging centers and labs traditionally adopt common analysis packages (e.g., SPM (Ashburner and Friston, 2000), FreeSurfer (Fischl, 2012), FSL (Jenkinson et al., 2012), AFNI (Cox, 1996), or imageJ (Schneider, Rasband, and Eliceiri, 2012), amongst others) that include both analysis methods for tissue segmentation, labeling, registration, and regression as well as image viewers, or combine different open source analysis tools and image viewers, such as from the Neuroimaging tools & Resources Collaboratory (NITRC). However, these tools often carry a steep learning curve for new users and require additional data management and computation resources to store data and ensure the same version of tools and processing parameters are used across studies. Flywheel offers a streamlined and all-in-one approach to data storage, viewing, and analysis via harmonized containerized algorithms (aka ‘gears’), removing much of the data management overhead and allowing novice sites to engage in data analysis rapidly. Researchers can access the data generated from the Hyperfine scanners and process algorithms on the platform securely with role-based access controls.
Instances of FlyWheel have been deployed using public cloud services in the US and India to address the data-sharing restrictions in India. For sites that were restricted by their IRB from open data sharing across the network, FlyWheel allows distributed processing (i.e., federated learning) via a multi-tenant SaaS cloud platform, allowing machine learning models to be trained across all datasets without providing individual access to raw data.
Complementing Flywheel, Collective Minds Radiology is a cloud-based platform for crowd-sourced radiology. Investigators with questions regarding image quality, artifacts, and possible incidental findings or presence of pathology can upload de-identified data to the UNITY group on Collective Minds and solicit feedback and opinions from other members of the group. This resource also provides a platform for ongoing training on data collection and harmonization, artifact identification, and other acquisition-related aspects via “image review sessions” during which sites can review recently acquired data in an effort to ensure data consistency and quality is met even with changing personnel or protocols.
Data sharing is an expectation for all BMGF funded projects and partners, and UNITY is no exception. However, data sharing across the UNITY sites must also comply with local and national regulations e.g, Health Insurance Portability and Accountability Act (HIPPA, North America), General Data Protection Regulation (GDPR, UK and European Union), and Digital Personal Data Protection Act (DPDPA, India). Currently, access to raw data acquired at all sites, and with appropriate ethical review board approval, is facilitated via data contributor and access agreements (DCAs and DAAs, respectively) that are coordinated via KCL with the data available on FlyWheel. Data acquired in India is the lone exception to this due to national regulations on data sharing, which also requires the use of a FlyWheel instance hosted in India. For these sites, summary statistics may be shared. For machine learning model building, the federated learning capabilities of FlyWheel will allow all sites to integrate all data even without physical access to the raw data. It is expected that data will be more broadly accessible via FlyWheel exchange and, where possible, other common repositories, such as the NIH Data Archive.
2.5.3. Image processing to achieve the goals of UNITY
As outcomes, UNITY aims to: 1. Characterize patterns of brain growth spanning birth through early childhood (i.e., birth to 5 years of age) across the participating LMIC and HIC centers, and to identify potential alterations in growth associated with birth injury, maternal and child nutritional (e.g., anemia) status, and other maternal and child health and environmental factors; 2. Explore relationships between neuroimaging measures with concurrent and later cognitive outcome metrics as well as physical growth; and 3. Identify global vs. regionally-specific factors that shape brain growth patterns and neurocognitive outcomes.
To achieve these three primary aims, we will employ adapted or custom-developed image processing tools to the acquired LF-MRI data. As a start, country-specific and global whole-brain templates will be constructed from the high-resolution super-resolution (SR)-reconstructed T2 (and where possible, T1 images) using the antsMultivariateTemplateConstruction2.sh tool that is part of the Advanced Normalization Tools (Avants et al., 2011). Age-specific templates will be calculated corresponding to 3, 6, 9, 12, 18, 24, 36, 48, and 60 months (or appropriate age subsets depending on data collection) using a minimum of 15 male and 15 female children at each time point within each country (i.e., country--specific templates). From these, a global brain template will be calculated from the set of country-specific templates (Fig. 12).
Fig. 12.
(Bottom to top) Creation of the age- and country-specific, and global templates from data generated at each of the clinical cohort sites. At least 15 male and female datasets at each age will be combined to generate age-specific templates within each country. These age-specific templates will then be used to generate a country-specific template. Finally, country-specific templates will be combined into the global ‘world’ template.
2.5.4. Developing custom processing tools
The majority of image processing tools available for MRI have been developed for use with high-field T1-weighted images of the adult brain. Direct translation of these algorithms to pediatric cases, and particularly to early development, are generally impaired by differences in the morphology of the developing brain that changes rapidly over the first year of life and imaging characteristics such as the poor gray/white matter differentiation in the developing brain (Dubois et al., 2021). These challenges impact the ability of existing algorithms to accurately segment pediatric brain structures even on high-spatial-resolution and high signal-to-noise (SNR) images acquired on 3 T MRI systems. Segmentation inaccuracies are magnified in the lower fidelity (i.e., resolution and SNR) acquired on low-field strength systems such as the Hyperfine Swoop scanner. As such, commonly accepted toolkits for skull stripping, tissue segmentation, and registration do not work or are not optimized for low-field pediatric images that have significantly different contrast, quality, and resolution characteristics (for example, Fig. 13).
Fig. 13.
Examples of segmentations on low-field pediatric data with current segmentation tools. (A) Hippocampal segmentation using SynthSeg. (B) Segmentation of the cortex with iBeat. (C) Skull stripping performed using SynthStrip. On each image, white arrows point to examples of regions that were given the wrong tissue label.
To address this problem several image segmentation and skull stripping strategies will be tested (illustrated in Fig. 14) as follows:
Fig. 14.
Flowchart encompassing our overall strategy for the implementation of segmentation tools for the Hyperfine Swoop scanner.
2.5.4.1. Data curation / quality control
Data from multiple clinical sources will be evaluated for completeness, consistency with imaging protocol, motion and imaging artifacts,
2.5.4.2. Ground truth labeling / segmentation
Semi-automated labeling of the training data is first performed to be subsequently fed into the automated deep learning segmentation algorithms. Online segmentation tools, such as the iBEAT (Dai et al., 2013) and SynthSeg (Billot et al., 2023), are used for initial segmentation, followed by manual editing to build these training sets. Initial labels of interest will include the cortex and hippocampus, as well as brain vs. non-brain tissues (skull stripping).
2.5.4.3. Existing method comparison and new algorithm development
We are implementing convolutional neural network-based architectures for the segmentation of cortical and subcortical structures and for skull stripping. These architectures recognize both local and global features and are popular for segmentation tasks (Zhang et al., 2021). For instance, we are experimenting with architectures such as UNet (Ronneberger et al., 2015), VNet (O. C¸ ic¸ek et al., 2016), SegResNet (Milletari et al., 2018), SegResNetVAE (Isensee et al., 2021), nnUNet (Hatamizadeh et al., 2021)and SwinUNetR (Bishop, 1995), to automatically segment the left and right hippocampus structures in 3D MRI. Each network offers distinct advantages in capturing essential local, global, and contextual information from the images. The performance will be measured and summarized by the Dice coefficients, Hausdorff distance and relative volume errors computed between automatically segmented structures on low-field MRI and the paired manually segmented gold standard from high-field MRI.
2.5.4.4. Data challenges
While developing in-house methods to provide improved segmentations over the current state-of-the-art for low-field pediatric images, a public challenge will also be organized for each segmentation task to crowd-source algorithm development. The Medical Image Computing and Computer Aided Intervention (MICCAI 2024) conference will take place for the first time in 2024 on the African continent in Marrakesh, Morocco. It will provide an ideal venue for this challenge, which will be organized in partnership with the conference organizing committee. MRI data and ground truth segmentation will be shared publicly for algorithm training with an independent testing set that will be private and used for algorithm benchmarking.
2.5.4.5. Algorithm benchmarking and ensemble model development
To advance the predictive power of the in-house method as well as the models collected from the challenge, ensemble learning methods (Sagi and Rokach, 2018) will be employed to combine various model predictions. A comparison will be conducted to evaluate the performance of various ensemble methods for different age groups and genders. The best method will be used to perform the output fusion of all the models.
2.5.4.6. Image enhancement
Each deep learning segmentation tool will be validated with and without image enhancement, e.g., super-resolution of the original low-field image, multi-sequence analysis, inhomogeneity correction, etc. We will train dedicated neural networks based on some of the above convolutional neural-network-based architectures to learn the “image quality transfer” mapping from ultra-low-field to high-field data, which can subsequently be applied to super-resolve unseen ultra-low-field scans. We are exploring both the re-training of existing super-resolution methods (e.g. SynthSR) on pediatric scans, as well as the development of dedicated super-resolution models, such as 3D UNets trained on paired empirical ultra-low-field and high-field data. Additional architectures are relevant for super-resolution, including the CycleGAN (Zhu 2017), which can potentially be trained on unpaired ultra-low-field and high-field scans from different populations. We plan to compare the performance of our segmentation tools on original versus super-resolved images, and we expect the latter to yield significantly improved measures over the former. In turn, super-resolution performance is commonly quantified by comparing tissue segmentations and volumes from super-resolved ultra-low-field scans to corresponding high-field MRI; as a result, advances in dedicated tissue segmentation methods can serve to improve the performance of custom super-resolution approaches.
2.5.5. Assessing other domains of child health and their environment
Alongside LF-MRI measures of gross brain anatomy and structure (i.e., total and regional brain volumes), and where and when possible more sensitive measures of tissue microstructure (e.g., quantitative T1 and T2, magnetization transfer ratio, myelin water fraction, and diffusion), multiple domains of contextual child health and family environment will be examined. While specific measures and biological samples (and their time points of collection) will vary by cohort, these will include child anthropometry (length/height, weight, head circumference, upper arm circumference), age-appropriate neurocognitive measures (including the Global Scale of Early Development, GSED, BSID-III or BSID-IV, WPPSI-3, as well as assessments of executive functioning, school readiness, and pre-academic skills, i.e., numeracy and literacy), anemia (via blood hemoglobin, Hb) and/or iron deficiency status, and other general assessments of child health and well-being. Where possible, biosamples including dried blood spots, saliva, hair cortisol, fecal microbiome, and nail clippings will be collected. Whilst individual sites will use these samples for cohort-specific investigations (e.g., genotyping, HIV status, microbiome diversity, specific nutrient deficiencies, and drug exposure), common analyses will include the evaluation of anemia (Hb) status.
The use of psychometric tools, such as the BSID, WPPSI, the NIH Toolbox, and others, in LMIC settings is often challenged by the lack of country-specific translations and populations norms. In most instances, whilst these tools have previously been used widely across the UNITY countries in past studies of child development or of various health interventions (Cromwell et al., 2014, Hanlon et al., 2016, Mal-Ed Network Investigators, 2018, Rasheed et al., 2018), none have been re-normed for the LMIC populations. Thus, comparison of scores across populations is difficult and will be avoided in UNITY. Rather, these tools will be used in a cohort-specific basis to examine developmental outcomes following health interventions where there is a corresponding control group (e.g., the PRIMES, PRISMA, NESHIE, and REVAMP trials) (Semrud-Clikeman et al., 2017).
Retrospective health data (e.g., records of birth weight, gestation duration, etc.) is also challenging in many LMIC settings due to the lack of comprehensive electronic health records. For birth data. all studies in UNITY are pregnancy cohorts, allowing this information to be directly assessed by the study team.
Given the potential role of social equity and gender discrimination on child development (Vlassoff, 2007), a broad range of individual and population measures will be examined. On an individual level, maternal autonomy, decision-making, mental health (e.g., depression), and inter-partner violence will be assessed. In addition, local (to the site) male-female differences in education and expected years of schooling (Local Burden of Disease Educational Attainment, 2020), new-born care seeking (Willis et al., 2009), and workforce participation and unpaid labor. These measures will be directly assessed in study participants, with additional local information derived where possible from existing databases, such as the Institute for Health Metrics and Evaluation (https://www.healthdata.org/).
Finally, broad and location-appropriate factors related to socioeconomic status will also be measured, including, for example, income-to-needs, social standing, housing type and ownership status, cooking fuel type, availability of electricity/lighting/cooling fans, and possession of a cellular phone.
An overview of common assessments is provided in Supplemental Table 1, which includes major domains assessed and assessment instruments.
2.6. Clinical and research training and capacity building
Across many LMIC settings, including many of the clinical cohort sites in UNITY, neuroimaging expertise (in both clinical and research capacities) is nascent. Outside of major tertiary or referral hospitals, access to MRI systems is severely constrained as are the opportunities for training, which feeds a larger human capital challenge with few trained radiologists, radiology technicians, nurses, and other support staff needed to maintain, operate, and read diagnostic images. In many countries, only a handful of radiologists may support a total population of 10–20 million. Beyond healthcare service, this can present challenges in research capacity with respect to ethical IRB considerations for incidental findings and follow-up care. However, given the lack of healthcare access to MRI, it is unsurprising that MRI research is similarly scarce.
Two of the five pillars of UNITY (Fig. 1) deal specifically with capacity building on both clinical and research levels. Whilst the goal of UNITY is not to improve access per se, the provision of ultra-low-field strength scanners and the large, assembled network of clinical and academic health researchers offer a compelling opportunity to increase knowledge and reduce barriers to MRI across Sub-Saharan Africa and South Asia.
2.6.1. Community support
For many of the centers involved in UNITY, there is no formal radiology department and/or radiologist available to perform clinical interpretation of the research scans, such as might be needed to identify or confirm incidental findings. This is further amplified by the general lack of MRI-trained radiologists in many LMICs and regions and, particularly, those familiar with the unique image quality and tissue contrasts provided by low and ultra-low-field MRI. To assist with incidental reporting, as well as general image interpretation and grading, UNITY has aimed to establish a community approach through the use of CollectiveMinds Radiology (www.cmrad.com). CollectiveMinds is an internet cloud-based platform that provides a secure and protected space for UNITY members to upload anonymized images with suspected quality issues, artifacts, or potential clinical abnormalities and elicit community feedback. In this way, clinical sites with greater experience (e.g., Aga Khan University, University of Cape Town, Pretoria University, KCL, University of Bonn) can assist those sites with lesser experience. Through this interaction, UNITY aims to help spread and improve low-field MRI expertise across all sites.
2.6.2. MRI physics education
In addition to building experience with grading low-field images, there is also a focus on improving knowledge of the underlying MRI physics and image analysis principles to expand the research and education capacity across sites. These aims are facilitated through partnership with the International Society for Magnetic Resonance in Medicine (ISMRM) and online lectures and tutorials provided by leading experts and educators from the MRI community. This monthly series encompasses the basic principles of signal generation and localization, contrast mechanisms, acquisition protocols, reconstruction, and image artifacts.
2.6.3. Image analysis training and workshops
Alongside the basic principles of MRI, methods for image analysis are also covered through a series of individual 1:1 and group tutorials. The use of Flywheel further helps to reduce barriers to image processing across sites, allowing UNITY members to first employ and experiment with developed processing tools (gears) before expanding their expertise through the development of new Flywheel “gears” and standalone analysis tools.
3. Discussion
The first 1000 days of a child’s life is an important early window of neurodevelopment during which lifelong patterns of health and cognitive development are established. The human infant brain is unique in its prolonged period of maturation (Dehaene-Lambertz and Spelke, 2015, Gao et al., 2015, Huang et al., 2015). While this protracted timeline enables plasticity and refinement of the infant brain to evolving environmental factors, it also places developing neural systems at risk to adverse conditions, including suboptimal maternal health, poor pregnancy outcomes (e.g., growth restriction, preterm delivery), malnutrition, infection and disease, and other forms of social and environmental adversity (Bick and Nelson, 2017). Given this sensitivity and plasticity, it is hypothesized that maternal and child health interventions, aimed at improving child neurodevelopmental outcomes, will have the greatest impact and efficacy if delivered during this early developmental period (Cusick and Georgieff, 2016, Vir and Suri, 2023). The impact of these interventions may be greatest felt in LMICs, where poor maternal and child health conditions, malnutrition, and other environmental adversities are of high prevalence and severity.
As part of the environmental conditions experienced by infants and young children, social inequality and gender discrimination remains a sociocultural norm across many parts of the world, with severe consequences for female children in certain communities. In parts of India, for example, preference for a male child, female feticide, and higher rates of infant mortality, malnutrition, and physical growth faltering among girls have been reported (Willis, Kumar et al., 2009, Marphatia, Cole et al., 2016). In many parts of the world, female infants and young women often receive differential access to food, healthcare, education, and later employment opportunities. Here, adversity and inequality may not only contribute to poor brain development but is also an outcome of it, perpetuating a vicious intergenerational cycle. The impact of these inequalities on the developing male and female brain, layered on top of pre-existing general socioeconomic and environmental adversities is unknown.
A longstanding challenge in global health studies, however, has been the accurate assessment of the neurodevelopmental implications of these interventions, particularly on the fetal and early infant brain when cognitive assessments may be difficult or impossible to perform. Given that neuroimaging may be more sensitive to the subtle changes in brain structure and function resulting from improved antenatal care, maternal or child nutritional supplementation, or other intervention, and offers more stable predictive ability (particularly in infants and younger children), its complementary use with clinical assessment measures has the potential to help improve early identification of efficacious interventions and refine their implementation across diverse geographies and populations.
The UNITY project aims to contribute to our understanding of how neurodevelopmental is impacted by the myriad of pre and post-natal health, environment, and social equity factors through the characterization of the patterns of structural brain development across multiple high, middle, and low-income settings and populations; and identify early and predictive imaging biomarkers of neurodevelopmental outcome (e.g., later childhood school readiness, language, and numeracy skills, and socioemotional processing). Beyond these direct aims, the project will further contribute to the emerging state-of-the-art of low and ultra-low field MRI (<100mT) and help build access and expand capacity (skills, training, and personnel) for MRI throughout parts of sub-Saharan Africa and south Asia.
UNITY partners aim to create a unique neuroimaging resource. Namely, a harmonized dataset of brain morphometry and microstructure (assessed via volumetric structural imaging, tissue relaxometry, and magnetization transfer imaging) acquired on the same scanner platform (Hyperfine Swoop) from multiple child populations and settings where current MRI access is sparse and past MRI neuroimaging studies are few or non-existent. These data will be paired with concurrent assessments of child cognitive development (principally the GSED (McCray et al., 2023), but complemented as necessary by traditional tools such as the BSID (Milne, McDonald, and Comino, 2012), the Mullen Scales of Early Learning (E.M. 1995), and the Malawi Developmental Assessment Tool (Gladstone et al., 2010)), as well as rich demographic, socioeconomic, prenatal health, birth history, and home environment information. Data collection includes cohorts of healthy children as well as targeted health risks and conditions, including preterm birth, maternal anemia, and neonatal hypoxic-ischemic encephalopathy, in diverse geographic, cultural, and social environments and contexts. This rich and varied dataset will provide novel insight into potential factors that shape neurodevelopment, and how these factors may vary in importance and impact by region, country, and continent. The needs of the individual cohorts provide the impetus and priority for the neuroimaging methods. This unique collaboration between the clinical, physics, and engineering groups is meant to accelerate the development of important methods custom-tailored to the relevant age group and cohort.
In addition to scientific endpoints, The UNITY network will help build research and clinical capacity across sites where MRI research and/or clinical usage is nascent. Here the aim is to build local, regional, and national collaborations with links to international societies such that growth and capacity building occur from the ground up. While UNITY will not overcome all obstacles that prevent the wide and ubiquitous adoption of MRI - for example, the lack of equipment maintenance support and service that has often led to “equipment graveyards” (De Maria et al., 2022), our connected network with support from major academic societies can lend pressure to major equipment vendors and facilitate local problem solving.
The UNITY project is based on open science, with the goal of rapid sharing of data with the broad global health community. However, it must be recognized that some countries consider neuroimaging data in the same manner as human biosamples, with analogous regulatory concerns and restrictions. Further, unrestricted access is challenging for sensitive pediatric cohorts who have not given their consent for data sharing. Thus, the network has adopted a “share what you can, when you can” approach in line with funder (the Bill & Melinda Gates Foundation) principles and policies.
4. Conclusion
The UNITY project will provide new information on the patterns of neurodevelopment, their potential differences, and the factors that shape them across a unique set of child populations from varied low- and middle-income settings while driving development access to low and ultra-low magnetic field MRI.
Role of the funding source
Funding for the various aspects and individual study cohorts that comprise UNITY has been provided by the Bill & Melinda Gates Foundation, The Wellcome Trust, Wellcome LEAP, and the Maudsley NHS Foundation Trust. These funders played no role in the direct administration, research activities, or the presentation of the results. BMGF did develop the overall neuroimaging strategy into which UNITY fits and, as such, helped shape individual project goals, organize and facilitate communication across and between sites and hubs, and host workshops and meetings for the UNITY network. S Deoni from the Bill & Melinda Gates Foundation led the writing effort of this paper on behalf of the larger UNITY network.
Declaration of interests
The authors declare the following competing interests:
H Frail, R O’Halloran, F Padormo, M Poorman, J Rogers, L Sacolick, K Siddiqui, R Teixeira, M Traughber are employees of Hyperfine.io
W Hollander, T. Karaulanov, and C Weiant are employees of CaliberMRI.
C Akgun, and P Velasco are employees of Flywheel.io
S Deoni is a Senior Program Officer at the Bill & Melinda Gates Foundation.
Funding
Funding for this work includes the Bill & Melinda Gates Foundation, the NIHR Maudsley Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London, and through a Wellcome Trust Investigator Award (096646/Z/11/Z) and a Wellcome Trust Strategic Award (104943/Z/14/Z).
CRediT authorship contribution statement
F. Abate: Involved in study design, supervision, and data collection. A. Adu-Amankwah: Involved in study design, supervision, and data collection. KA Ae-Ngibise: Involved in study design, supervision, and data collection. F Agbokey: Involved in study design, supervision, and data collection. VA Agyemang: Involved in study design, supervision, and data collection. CT Agyemang: Involved in study design, supervision, and data collection. C. Akgun: Involved in study design, supervision, and data collection. J. Ametepe: Involved in study design, supervision, and data collection. T. Arichi: Involved in study design, supervision, and data collection. KP Asante: Involved in study design, supervision, and data collection. S. Balaji: Involved in study design, supervision, and data collection. L. Baljer: Involved in study design, supervision, and data collection. PJ Basser: Involved in study design, supervision, and data collection. J. Beauchemin: Involved in study design, supervision, and data collection. C. Bennallick: Involved in study design, supervision, and data collection. Y. Berhane: Involved in study design, supervision, and data collection. Y. Boateng-Mensah: Involved in study design, supervision, and data collection. NJ Bourke: Involved in study design, supervision, and data collection. L. Bradford: Involved in study design, supervision, and data collection. MMK Bruchhage: Involved in study design, supervision, and data collection. R.Cano Lorente: Involved in study design, supervision, and data collection. P. Cawley: Involved in study design, Involved in study design, supervision, and data collection, and data collection. M. Cercignani: Involved in study design, supervision, and data collection. V. D Sa: Involved in study design, supervision, and data collection. A.de Canha: Involved in study design, supervision, and data collection. N.de Navarro: Involved in study design, supervision, and data collection. DC Dean: Involved in study design, supervision, and data collection. J. Delarosa: Involved in study design, supervision, and data collection. KA Donald: Involved in study design, supervision, and data collection. A. Dvorak: Involved in study design, supervision, and data collection. AD Edwards: Involved in study design, supervision, and data collection. D. Field: Involved in study design, supervision, and data collection. H. Frail: Involved in study design, supervision, and data collection. B. Freeman: Involved in study design, supervision, and data collection. T. George: Involved in study design, supervision, and data collection. J. Gholam: Involved in study design, supervision, and data collection. J. Guerrero-Gonzalez: Involved in study design, supervision, and data collection. JV Hajnal: Involved in study design, supervision, and data collection. R. Haque: Involved in study design, supervision, and data collection. W. Hollander: Involved in study design, supervision, and data collection. Z. Hoodbhoy: Involved in study design, supervision, and data collection. M. Huentelman: Involved in study design, supervision, and data collection. SK Jafri: Involved in study design, supervision, and data collection. DK Jones: Involved in study design, supervision, and data collection. F. Joubert: Involved in study design, supervision, and data collection. T. Karaulanov: Involved in study design, supervision, and data collection. MP Kasaro: Involved in study design, supervision, and data collection. S. Knackstedt: Involved in study design, supervision, and data collection. S. Kolind: Involved in study design, supervision, and data collection. B. Koshy: Involved in study design, supervision, and data collection. R. Kravitz: Involved in study design, supervision, and data collection. S.Lecurieux Lafayette: Involved in study design, supervision, and data collection. AC Lee: Involved in study design, supervision, and data collection. B. Lena: Involved in study design, supervision, and data collection. N. Lepore: Involved in study design, supervision, and data collection. M. Linguraru: Involved in study design, supervision, and data collection. E. Ljungberg: Involved in study design, supervision, and data collection. Z. Lockart: Involved in study design, supervision, and data collection. E. Loth: Involved in study design, supervision, and data collection. P. Mannam: Involved in study design, supervision, and data collection. KM Masemola: Involved in study design, supervision, and data collection. R. Moran: Involved in study design, supervision, and data collection. D. Murphy: Involved in study design, supervision, and data collection. FL Nakwa: Involved in study design, supervision, and data collection. V. Nankabirwa: Involved in study design, supervision, and data collection. CA Nelson: Involved in study design, supervision, and data collection. K. North: Involved in study design, supervision, and data collection. S. Nyame: Involved in study design, supervision, and data collection. R. O Halloran: Involved in study design, supervision, and data collection. J. O'Muircheartaigh: Involved in study design, supervision, and data collection. BF Oakley: Involved in study design, supervision, and data collection. H. Odendaal: Involved in study design, supervision, and data collection. CM Ongeti: Involved in study design, supervision, and data collection. D. Onyango: Involved in study design, supervision, and data collection. SA Oppong: Involved in study design, supervision, and data collection. F. Padormo: Involved in study design, supervision, and data collection. D. Parvez: Involved in study design, supervision, and data collection. T. Paus: Involved in study design, supervision, and data collection. MS Pepper: Involved in study design, supervision, and data collection. KS Phiri: Involved in study design, supervision, and data collection. M. Poorman: Involved in study design, supervision, and data collection. JE Ringshaw: Involved in study design, supervision, and data collection. J. Rogers: Involved in study design, supervision, and data collection. M. Rutherford: Involved in study design, supervision, and data collection. H. Sabir: Involved in study design, supervision, and data collection. L. Sacolick: Involved in study design, supervision, and data collection. M. Seal: Involved in study design, supervision, and data collection. ML Sekoli: Involved in study design, supervision, and data collection. T. Shama Involved in study design, supervision, and data collection. K. Siddiqui: Involved in study design, supervision, and data collection. N. Sindano: Involved in study design, supervision, and data collection. MB Spelke: Involved in study design, supervision, and data collection. PE Springer: Involved in study design, supervision, and data collection. FE Suleman: Involved in study design, supervision, and data collection. PC Sundgren: Involved in study design, supervision, and data collection. R.Teixeira: Involved in study design, supervision, and data collection. W. Terekegn: Involved in study design, supervision, and data collection. M. Traughber: Involved in study design, supervision, and data collection. MG Tuuli: Involved in study design, supervision, and data collection. J.van Rensburg: Involved in study design, supervision, and data collection. F. Váša: Involved in study design, supervision, and data collection. S. Velaphi: Involved in study design, supervision, and data collection. P. Velasco: Involved in study design, supervision, and data collection. IM Viljoen: Involved in study design, supervision, and data collection. M. Vokhiwa: Involved in study design, supervision, and data collection. A. Webb: Involved in study design, supervision, and data collection. C. Weiant: Involved in study design, supervision, and data collection. N. Wiley: Involved in study design, supervision, and data collection. P. Wintermark: Involved in study design, supervision, and data collection. K. Yibetal: Involved in study design, supervision, and data collection. SCL Deoni: Involved in study design and manuscript writing and editing. SCR Williams: Involved in study design, supervision, data collection, funding, manuscript writing and editing.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: H Frail, R O’Halloran, F Padormo, M Poorman, J Rogers, L Sacolick, K Siddiqui, R Teixeira, M Traughber are employees of Hyperfine.io W Hollander, T. Karaulanov, and C Weiant are employees of CaliberMRI. C Akgun, and P Velasco are employees of Flywheel.io
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.dcn.2024.101397.
Appendix A. Supplementary material
Supplementary material
Data Availability
Data collected as part of the UNITY network will be made available to researchers from the academic communities at varying levels of granularity depending on site-specific IRB approvals. For some sites, full access to individual raw and processed data will be provided, whilst for others, owing to national policies (e.g., those located in India) may only be able to provide de-identified composite values (e.g., regional volumes, mean relaxometry measures, etc.). The Bill & Melinda Gates Foundation is committed to open access and broad data availability as permitted.
References
- Anderson P.J., Burnett A. Assessing developmental delay in early childhood - concerns with the Bayley-III scales. Clin. Neuropsychol. 2017;31:371–381. doi: 10.1080/13854046.2016.1216518. [DOI] [PubMed] [Google Scholar]
- Anjos T., Altmae S., Emmett P., Tiemeier H., Closa-Monasterolo R., Luque V., Wiseman S., Perez-Garcia M., Lattka E., Demmelmair H., Egan B., Straub N., Szajewska H., Evans J., Horton C., Paus T., Isaacs E., van Klinken J.W., Koletzko B., Campoy C., Nutrimenthe Research Group Nutrition and neurodevelopment in children: focus on NUTRIMENTHE project. Eur. J. Nutr. 2013;52:1825–1842. doi: 10.1007/s00394-013-0560-4. [DOI] [PubMed] [Google Scholar]
- Ashburner J., Friston K.J. Voxel-based morphometry--the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Avants B.B., Tustison N.J., Wu J., Cook P.A., Gee J.C. An open source multivariate framework for n-tissue segmentation with evaluation on public data. Neuroinformatics. 2011;9:381–400. doi: 10.1007/s12021-011-9109-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bick J., Nelson C.A. Early experience and brain development. Wiley Inter. Rev. Cogn. Sci. 2017;8 doi: 10.1002/wcs.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billot B., Greve D.N., Puonti O., Thielscher A., Van Leemput K., Fischl B., Dalca A.V., Iglesias J.E., Adni SynthSeg: Segmentation of brain MRI scans of any contrast and resolution without retraining. Med Image Anal. 2023;86 doi: 10.1016/j.media.2023.102789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop C.M. university press; Oxford: 1995. Neural networks for pattern recognition. [Google Scholar]
- Black M.M., Walker S.P., Fernald L.C.H., Andersen C.T., DiGirolamo A.M., Lu C., McCoy D.C., Fink G., Shawar Y.R., Shiffman J., Devercelli A.E., Wodon Q.T., Vargas-Baron E., Grantham-McGregor S., Committee Lancet Early Childhood Development Series Steering Early childhood development coming of age: science through the life course. Lancet. 2017;389:77–90. doi: 10.1016/S0140-6736(16)31389-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black R.E., Allen L.H., Bhutta Z.A., Caulfield L.E., de Onis M., Ezzati M., Mathers C., Rivera J., Maternal, and Group Child Undernutrition Study Maternal and child undernutrition: global and regional exposures and health consequences. Lancet. 2008;371:243–260. doi: 10.1016/S0140-6736(07)61690-0. [DOI] [PubMed] [Google Scholar]
- Black R.E., Victora C.G., Walker S.P., Bhutta Z.A., Christian P., de Onis M., Ezzati M., Grantham-McGregor S., Katz J., Martorell R., Uauy R., Maternal, and Group Child Nutrition Study Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013;382:427–451. doi: 10.1016/S0140-6736(13)60937-X. [DOI] [PubMed] [Google Scholar]
- Bottomley P.A., Foster T.H., Argersinger R.E., Pfeifer L.M. A review of normal tissue hydrogen NMR relaxation times and relaxation mechanisms from 1–100 MHz: dependence on tissue type, NMR frequency, temperature, species, excision, and age. Med Phys. 1984;11:425–448. doi: 10.1118/1.595535. [DOI] [PubMed] [Google Scholar]
- Bottomley P.A., Hardy C.J., Argersinger R.E., Allen-Moore G. A review of 1H nuclear magnetic resonance relaxation in pathology: are T1 and T2 diagnostic? Med Phys. 1987;14:1–37. doi: 10.1118/1.596111. [DOI] [PubMed] [Google Scholar]
- Bugada M.C., Kline J.E., Parikh N.A. Microstructural Measures of the Inferior Longitudinal Fasciculus Predict Later Cognitive and Language Development in Infants Born With Extremely Low Birth Weight. J. Child Neurol. 2021;36:981–989. doi: 10.1177/08830738211019862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvaratskhelia N., Rurua N., Vadachkoria S.G. Biomedical and Psychosocial Determinants of Early Neurodevelopment After Preterm Birth. Glob. Pedia Health. 2023;10 doi: 10.1177/2333794X231160366. 2333794×231160366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Local Burden of Disease Educational Attainment, Collaborators Mapping disparities in education across low- and middle-income countries. Nature. 2020;577:235–238. doi: 10.1038/s41586-019-1872-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasundaram, P., and I.D. Avulakunta. 2023. Bayley Scales Of Infant and Toddler Development.' in, StatPearls (Treasure Island (FL) with ineligible companies. Disclosure: Indirapriya Darshini Avulakunta declares no relevant financial relationships with ineligible companies.). [PubMed]
- Bedi K.S., Bhide P.G. Effects of environmental diversity on brain morphology. Early Hum. Dev. 1988;17:107–143. [PubMed] [Google Scholar]
- Bethlehem R.A.I., Seidlitz J., White S.R., Vogel J.W., Anderson K.M., Adamson C., Adler S., Alexopoulos G.S., Anagnostou E., Areces-Gonzalez A., Astle D.E., Auyeung B., Ayub M., Bae J., Ball G., Baron-Cohen S., Beare R., Bedford S.A., Benegal V., Beyer F., Blangero J., Blesa Cabez M., Boardman J.P., Borzage M., Bosch-Bayard J.F., Bourke N., Calhoun V.D., Chakravarty M.M., Chen C., Chertavian C., Chetelat G., Chong Y.S., Cole J.H., Corvin A., Costantino M., Courchesne E., Crivello F., Cropley V.L., Crosbie J., Crossley N., Delarue M., Delorme R., Desrivieres S., Devenyi G.A., Di Biase M.A., Dolan R., Donald K.A., Donohoe G., Dunlop K., Edwards A.D., Elison J.T., Ellis C.T., Elman J.A., Eyler L., Fair D.A., Feczko E., Fletcher P.C., Fonagy P., Franz C.E., Galan-Garcia L., Gholipour A., Giedd J., Gilmore J.H., Glahn D.C., Goodyer I.M., Grant P.E., Groenewold N.A., Gunning F.M., Gur R.E., Gur R.C., Hammill C.F., Hansson O., Hedden T., Heinz A., Henson R.N., Heuer K., Hoare J., Holla B., Holmes A.J., Holt R., Huang H., Im K., Ipser J., Jack C.R., Jr., Jackowski A.P., Jia T., Johnson K.A., Jones P.B., Jones D.T., Kahn R.S., Karlsson H., Karlsson L., Kawashima R., Kelley E.A., Kern S., Kim K.W., Kitzbichler M.G., Kremen W.S., Lalonde F., Landeau B., Lee S., Lerch J., Lewis J.D., Li J., Liao W., Liston C., Lombardo M.V., Lv J., Lynch C., Mallard T.T., Marcelis M., Markello R.D., Mathias S.R., Mazoyer B., McGuire P., Meaney M.J., Mechelli A., Medic N., Misic B., Morgan S.E., Mothersill D., Nigg J., Ong M.Q.W., Ortinau C., Ossenkoppele R., Ouyang M., Palaniyappan L., Paly L., Pan P.M., Pantelis C., Park M.M., Paus T., Pausova Z., Paz-Linares D., Pichet Binette A., Pierce K., Qian X., Qiu J., Qiu A., Raznahan A., Rittman T., Rodrigue A., Rollins C.K., Romero-Garcia R., Ronan L., Rosenberg M.D., Rowitch D.H., Salum G.A., Satterthwaite T.D., Schaare H.L., Schachar R.J., Schultz A.P., Schumann G., Scholl M., Sharp D., Shinohara R.T., Skoog I., Smyser C.D., Sperling R.A., Stein D.J., Stolicyn A., Suckling J., Sullivan G., Taki Y., Thyreau B., Toro R., Traut N., Tsvetanov K.A., Turk-Browne N.B., Tuulari J.J., Tzourio C., Vachon-Presseau E., Valdes-Sosa M.J., Valdes-Sosa P.A., Valk S.L., van Amelsvoort T., Vandekar S.N., Vasung L., Victoria L.W., Villeneuve S., Villringer A., Vertes P.E., Wagstyl K., Wang Y.S., Warfield S.K., Warrier V., Westman E., Westwater M.L., Whalley H.C., Witte A.V., Yang N., Yeo B., Yun H., Zalesky A., Zar H.J., Zettergren A., Zhou J.H., Ziauddeen H., Zugman A., Zuo X.N., Aibl Brain R., Initiative Alzheimer's Disease Neuroimaging, Investigators Alzheimer's Disease Repository Without Borders, Calm Team, C. A. N. Cam, Ccnp, Cobre, cVeda, Enigma Developmental Brain Age Working Group, Project Developing Human Connectome, FinnBrain, Study Harvard Aging Brain, Imagen, Kne, Aging Mayo Clinic Study of, Nspn, Pond, Prevent-Ad Research Group, Vetsa. Bullmore E.T., Alexander-Bloch A.F. Brain charts for the human lifespan. Nature. 2022;604:525–533. [Google Scholar]
- Bick J., Nelson C.A. Early Adverse Experiences and the Developing Brain. Neuropsychopharmacology. 2016;41:177–196. doi: 10.1038/npp.2015.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawley P., Padormo F., Cromb D., Almalbis J., Marenzana M., Teixeira R., Unity Consortium, Uus A., O'Muircheartaigh J., Williams S.C.R., Counsell S.J., Arichi T., Rutherford M.A., Hajnal J.V., Edwards A.D. Development of neonatal-specific sequences for portable ultralow field magnetic resonance brain imaging: a prospective, single-centre, cohort study. EClinicalMedicine. 2023;65 doi: 10.1016/j.eclinm.2023.102253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawley P.A., Nosarti C., Edwards A.D. In-unit neonatal magnetic resonance imaging-new possibilities offered by low-field technology. J. Perinatol. 2022;42:843–844. doi: 10.1038/s41372-022-01401-w. [DOI] [PubMed] [Google Scholar]
- Cooper Rebecca, Hayes Rebecca A., Corcoran Mary, Sheth Kevin N., Arnold Thomas Campbell, Stein Joel M., Glahn David C., Jalbrzikowski Maria. Bridging the gap: improving correspondence between low-field and high-field magnetic resonance images in young people. Front. Neurol. 2024;15 doi: 10.3389/fneur.2024.1339223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R.W. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cromwell E.A., Dube Q., Cole S.R., Chirambo C., Dow A.E., Heyderman R.S., Van Rie A. Validity of US norms for the Bayley Scales of Infant Development-III in Malawian children. Eur. J. Paediatr. Neurol. 2014;18:223–230. doi: 10.1016/j.ejpn.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusick S.E., Georgieff M.K. The Role of Nutrition in Brain Development: The Golden Opportunity of the "First 1000 Days. ", J. Pedia. 2016;175:16–21. doi: 10.1016/j.jpeds.2016.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y., Shi F., Wang L., Wu G., Shen D. iBEAT: A toolbox for infant brain magnetic resonance image processing. Neuroinformatics. 2013;11:211–225. doi: 10.1007/s12021-012-9164-z. [DOI] [PubMed] [Google Scholar]
- De Maria C., Diaz Lantada A., Jamsa T., Pecchia L., Ahluwalia A. Biomedical engineering in low- and middle-income settings: analysis of current state, challenges and best practices. Health Technol. (Berl. ) 2022;12:643–653. doi: 10.1007/s12553-022-00657-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean D.C., 3rd, Dirks H., O'Muircheartaigh J., Walker L., Jerskey B.A., Lehman K., Han M., Waskiewicz N., Deoni S.C. Pediatric neuroimaging using magnetic resonance imaging during non-sedated sleep. Pedia Radio. 2014;44:64–72. doi: 10.1007/s00247-013-2752-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene-Lambertz G., Spelke E.S. The Infancy of the Human Brain. Neuron. 2015;88:93–109. doi: 10.1016/j.neuron.2015.09.026. [DOI] [PubMed] [Google Scholar]
- Deoni S.C.L., Bruchhage M.M.K., Beauchemin J., Volpe A., D'Sa V., Huentelman M., Williams S.C.R. Accessible pediatric neuroimaging using a low field strength MRI scanner. Neuroimage. 2021;238 doi: 10.1016/j.neuroimage.2021.118273. [DOI] [PubMed] [Google Scholar]
- Deoni S.C.L., O'Muircheartaigh J., Ljungberg E., Huentelman M., Williams S.C.R. Simultaneous high-resolution T2 -weighted imaging and quantitative T2 mapping at low magnetic field strengths using a multiple TE and multi-orientation acquisition approach. Magn. Reson Med. 2022;88:1273–1281. doi: 10.1002/mrm.29273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deoni S.C.L., Medeiros P., Deoni A.T., Burton P., Beauchemin J., Sa V.D., Boskamp E., By S., McNulty C., Mileski W., Welch B.E., Huentelman M. Development of a mobile low-field MRI scanner. Sci. Rep. 2022;12:5690. doi: 10.1038/s41598-022-09760-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois J., Alison M., Counsell S.J., Hertz-Pannier L., Huppi P.S., Benders Mjnl. MRI of the Neonatal Brain: A Review of Methodological Challenges and Neuroscientific Advances. J. Magn. Reson Imaging. 2021;53:1318–1343. doi: 10.1002/jmri.27192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak A.V., Kumar D., Zhang J., Gilbert G., Balaji S., Wiley N., Laule C., Moore G.R.W., MacKay A.L., Kolind S.H. The CALIPR framework for highly accelerated myelin water imaging with improved precision and sensitivity. Sci. Adv. 2023;9 doi: 10.1126/sciadv.adh9853. eadh9853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B. FreeSurfer. Neuroimage. 2012;62:774–781. doi: 10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald E., Hor K., Drake A.J. Maternal influences on fetal brain development: The role of nutrition, infection and stress, and the potential for intergenerational consequences. Early Hum. Dev. 2020;150 doi: 10.1016/j.earlhumdev.2020.105190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherman V.J., Murungi J., Bale C., Dickinson S., Chen X., Namiiro F., Nankunda J., Pollack L.M., Laleau V., Kim M.O., Allison D.B., Ginsburg A.S., Braima de Sa A., Nankabirwa V. Breastfeeding and Once-Daily Small-Volume Formula Supplementation to Prevent Infant Growth Impairment. Pediatrics. 2023 doi: 10.1542/peds.2023-062228. [DOI] [PubMed] [Google Scholar]
- Gao W., Alcauter S., Smith J.K., Gilmore J.H., Lin W. Development of human brain cortical network architecture during infancy. Brain Struct. Funct. 2015;220:1173–1186. doi: 10.1007/s00429-014-0710-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge R., Yu Y., Qi Y.X., Fan Y.N., Chen S., Gao C., Haas S.S., New F., Boomsma D.I., Brodaty H., Brouwer R.M., Buckner R., Caseras X., Crivello F., Crone E.A., Erk S., Fisher S.E., Franke B., Glahn D.C., Dannlowski U., Grotegerd D., Gruber O., Hulshoff Pol H.E., Schumann G., Tamnes C.K., Walter H., Wierenga L.M., Jahanshad N., Thompson P.M., Frangou S., and Enigma Lifespan Working Group Normative modelling of brain morphometry across the lifespan with CentileBrain: algorithm benchmarking and model optimisation. Lancet Digit Health. 2024;6:e211–e221. doi: 10.1016/S2589-7500(23)00250-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgieff M.K. The role of iron in neurodevelopment: fetal iron deficiency and the developing hippocampus. Biochem Soc. Trans. 2008;36:1267–1271. doi: 10.1042/BST0361267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladstone M., Lancaster G.A., Umar E., Nyirenda M., Kayira E., van den Broek N.R., Smyth R.L. The Malawi Developmental Assessment Tool (MDAT): the creation, validation, and reliability of a tool to assess child development in rural African settings. PLoS Med. 2010;7 doi: 10.1371/journal.pmed.1000273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon C., Medhin G., Worku B., Tomlinson M., Alem A., Dewey M., Prince M. Adapting the Bayley Scales of infant and toddler development in Ethiopia: evaluation of reliability and validity. Child Care Health Dev. 2016;42:699–708. doi: 10.1111/cch.12371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- A. Hatamizadeh, V. Nath, Y. Tang, D. Yang, H.R. Roth, and D. Xu. 2021. Swin unetr: Swin transformers for semantic segmentation of brain tumors in mri images. In International MICCAI Brainlesion Workshop, edited by C. S. Springer, Jr., 272–284. Springer.
- Huang H., Shu N., Mishra V., Jeon T., Chalak L., Wang Z.J., Rollins N., Gong G., Cheng H., Peng Y., Dong Q., He Y. Development of human brain structural networks through infancy and childhood. Cereb. Cortex. 2015;25:1389–1404. doi: 10.1093/cercor/bht335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ic¸ek O.C., Abdulkadir A., Lienkamp S.S., Brox T., Ronneberger O. International conference on medical image computing and computer-assisted intervention. Springer; Munich, Germany: 2016. 3d u-net: learning dense volumetric segmentation from sparse annotation; pp. 424–432. [Google Scholar]
- Iglesias J.E., Billot B., Balbastre Y., Tabari A., Conklin J., Gilberto Gonzalez R., Alexander D.C., Golland P., Edlow B.L., Fischl B., Initiative Alzheimer's Disease Neuroimaging Joint super-resolution and synthesis of 1 mm isotropic MP-RAGE volumes from clinical MRI exams with scans of different orientation, resolution and contrast. Neuroimage. 2021;237 doi: 10.1016/j.neuroimage.2021.118206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias J.E., Billot B., Balbastre Y., Magdamo C., Arnold S.E., Das S., Edlow B.L., Alexander D.C., Golland P., Fischl B. SynthSR: A public AI tool to turn heterogeneous clinical brain scans into high-resolution T1-weighted images for 3D morphometry. Sci. Adv. 2023;9 doi: 10.1126/sciadv.add3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isensee F., Jaeger P.F., Kohl S.A.A., Petersen J., Maier-Hein K.H. nnU-Net: a self-configuring method for deep learning-based biomedical image segmentation. Nat. Methods. 2021;18:203–211. doi: 10.1038/s41592-020-01008-z. [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Beckmann C.F., Behrens T.E., Woolrich M.W., Smith S.M. Fsl. Neuroimage. 2012;62:782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Keenan K.E., Stupic K.F., Russek S.E., Mirowski E. MRI-visible liquid crystal thermometer. Magn. Reson Med. 2020;84:1552–1563. doi: 10.1002/mrm.28224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackes N.K., Golm D., Sarkar S., Kumsta R., Rutter M., Fairchild G., Mehta M.A., Sonuga-Barke E.J.S., E. R. A. Young Adult Follow-up team Early childhood deprivation is associated with alterations in adult brain structure despite subsequent environmental enrichment. Proc. Natl. Acad. Sci. USA. 2020;117:641–649. doi: 10.1073/pnas.1911264116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansson J., Kallen K., Eklof E., Serenius F., Aden U., Stjernqvist K. The ability of Bayley-III scores to predict later intelligence in children born extremely preterm. Acta Paediatr. 2021;110:3030–3039. doi: 10.1111/apa.16037. [DOI] [PubMed] [Google Scholar]
- McCarthy-Jones S., Oestreich L.K.L., Lyall A.E., Kikinis Z., Newell D.T., Savadjiev P., Shenton M.E., Kubicki M., Pasternak O., Whitford T.J., , and Bank Australian Schizophrenia Research Childhood adversity associated with white matter alteration in the corpus callosum, corona radiata, and uncinate fasciculus of psychiatrically healthy adults. Brain Imaging Behav. 2018;12:449–458. doi: 10.1007/s11682-017-9703-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy D.C., Peet E.D., Ezzati M., Danaei G., Black M.M., Sudfeld C.R., Fawzi W., Fink G. Early Childhood Developmental Status in Low- and Middle-Income Countries: National, Regional, and Global Prevalence Estimates Using Predictive Modeling. PLoS Med. 2016;13 doi: 10.1371/journal.pmed.1002034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCray G., McCoy D., Kariger P., Janus M., Black M.M., Chang S.M., Tofail F., Eekhout I., Waldman M., van Buuren S., Khanam R., Sazawal S., Nizar A., Schonbeck Y., Zongo A., Brentani A., Zhang Y., Dua T., Cavallera V., Raikes A., Weber A.M., Bromley K., Baqui A., Dutta A., Nisar I., Detmar S.B., Anago R., Mercadante P., Jiang F., Kaur R., Hepworth K., Rubio-Codina M., Kembou S.N., Ahmed S., Lancaster G.A., Gladstone M. The creation of the Global Scales for Early Development (GSED) for children aged 0-3 years: combining subject matter expert judgements with big data. BMJ Glob. Health. 2023;8 doi: 10.1136/bmjgh-2022-009827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer J.S., Erickson-Owens D.A., Deoni S.C.L., Dean Iii D.C., Tucker R., Parker A.B., Joelson S., Mercer E.N., Collins J., Padbury J.F. The Effects of Delayed Cord Clamping on 12-Month Brain Myelin Content and Neurodevelopment: A Randomized Controlled Trial. Am. J. Perinatol. 2022;39:37–44. doi: 10.1055/s-0040-1714258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milletari F., Navab N., Ahmadi S.-A. Fourth international conference on 3D vision (3DV). IEEE. IEEE; 2018. V-net: Fully convolutional neural networks for volumetric medical image segmentation; pp. 565–571. [Google Scholar]
- Milne S., McDonald J., Comino E.J. The use of the Bayley Scales of Infant and Toddler Development III with clinical populations: a preliminary exploration. Phys. Occup. Ther. Pedia. 2012;32:24–33. doi: 10.3109/01942638.2011.592572. [DOI] [PubMed] [Google Scholar]
- Mullen E.M. American Guidance Services, Inc; Circle Pines, MN: 1995. Mullen Scales of Early Learning. [Google Scholar]
- Nicolaou L., Ahmed T., Bhutta Z.A., Bessong P., Kosek M., Lima A.A.M., Shrestha S., Chandyo R., Mduma E.R., Murray-Kolb L., Morgan B., Grigsby M.R., Checkley W., Maled Network Investigators Factors associated with head circumference and indices of cognitive development in early childhood. BMJ Glob. Health. 2020;5 doi: 10.1136/bmjgh-2020-003427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogbole G.I., Adeyomoye A.O., Badu-Peprah A., Mensah Y., Nzeh D.A. Survey of magnetic resonance imaging availability in West Africa. Pan Afr. Med J. 2018;30:240. doi: 10.11604/pamj.2018.30.240.14000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendergast L.L., Schaefer B.A., Murray-Kolb L.E., Svensen E., Shrestha R., Rasheed M.A., Scharf R.J., Kosek M., Vasquez A.O., Maphula A., Costa H., Rasmussen Z.A., Yousafzai A., Tofail F., Seidman J.C., Mal-Ed Network Investigators . Vol. 33. 2018. Assessing development across cultures: Invariance of the Bayley-III Scales Across Seven International MAL-ED sites; pp. 604–614. (Sch Psychol Q). [DOI] [PubMed] [Google Scholar]
- Rasheed Muneera A., Pham Sofia, Memon Uzma, Siyal Saima, Obradović Jelena, Yousafzai Aisha K. Adaptation of the Wechsler Preschool and Primary Scale of Intelligence-III and lessons learned for evaluating intelligence in low-income settings. Int. J. Sch. Educ. Psychol. 2018;6:197–207. [Google Scholar]
- Ronneberger O., Fischer P., Brox T. Medical Image Computing and Computer-Assisted Intervention–MICCAI. Springer; Munich, Germany: 2015. U-net: Convolutional networks for biomedical image segmentation; pp. 234–241. [Google Scholar]
- Rutherford S., Fraza C., Dinga R., Kia S.M., Wolfers T., Zabihi M., Berthet P., Worker A., Verdi S., Andrews D., Han L.K., Bayer J.M., Dazzan P., McGuire P., Mocking R.T., Schene A., Sripada C., Tso I.F., Duval E.R., Chang S.E., Penninx B.W., Heitzeg M.M., Burt S.A., Hyde L.W., Amaral D., Wu Nordahl C., Andreasssen O.A., Westlye L.T., Zahn R., Ruhe H.G., Beckmann C., Marquand A.F. Charting brain growth and aging at high spatial precision. Elife. 2022;11 doi: 10.7554/eLife.72904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabir H., Kipfmueller F., Bagci S., Dresbach T., Grass T., Nitsch-Felsecker P., Pantazis C., Schmitt J., Schroeder L., Mueller A. Feasibility of bedside portable MRI in neonates and children during ECLS. Crit. Care. 2023;27:134. doi: 10.1186/s13054-023-04416-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagi Omer, Rokach Lior. Interdisciplinary Reviews: Data Mining and Knowledge Discovery. Wiley; 2018. Ensemble learning: A survey. [Google Scholar]
- Sarracanie M., LaPierre C.D., Salameh N., Waddington D.E.J., Witzel T., Rosen M.S. Low-Cost High-Performance MRI. Sci. Rep. 2015;5:15177. doi: 10.1038/srep15177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C.A., Rasband W.S., Eliceiri K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonhaut L., Perez M., Armijo I., Maturana A. Comparison between Ages & Stages Questionnaire and Bayley Scales, to predict cognitive delay in school age. Early Hum. Dev. 2020;141 doi: 10.1016/j.earlhumdev.2019.104933. [DOI] [PubMed] [Google Scholar]
- Semrud-Clikeman Margaret, Romero Regilda Anne A., Prado Elizabeth L., Shapiro Elsa G., Bangirana Paul, John Chandy C. Selecting measures for the neurodevelopmental assessment of children in low- and middle-income countries. Child Neuropsychol. 2017;23:761–802. doi: 10.1080/09297049.2016.1216536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar K., Pivik R.T., Johnson S.L., van Ommen B., Demmer E., Murray R. Environmental Forces that Shape Early Development: What We Know and Still Need to Know. Curr. Dev. Nutr. 2018;2 doi: 10.3945/cdn.117.001826. nzx002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J., Ma S., Hofer E., Patel Y., Vosberg D.E., Tilley S., Roshchupkin G.V., Sousa A.M.M., Jian X., Gottesman R., Mosley T.H., Fornage M., Saba Y., Pirpamer L., Schmidt R., Schmidt H., Carrion-Castillo A., Crivello F., Mazoyer B., Bis J.C., Li S., Yang Q., Luciano M., Karama S., Lewis L., Bastin M.E., Harris M.A., Wardlaw J.M., Deary I.E., Scholz M., Loeffler M., Witte A.V., Beyer F., Villringer A., Armstrong N.J., Mather K.A., Ames D., Jiang J., Kwok J.B., Schofield P.R., Thalamuthu A., Trollor J.N., Wright M.J., Brodaty H., Wen W., Sachdev P.S., Terzikhan N., Evans T.E., Adams Hhhh, Ikram M.A., Frenzel S., Auwera-Palitschka S.V., Wittfeld K., Bulow R., Grabe H.J., Tzourio C., Mishra A., Maingault S., Debette S., Gillespie N.A., Franz C.E., Kremen W.S., Ding L., Jahanshad N., Consortium Enigma, Sestan N., Pausova Z., Seshadri S., Paus T., Charge Working Group neuro Global and Regional Development of the Human Cerebral Cortex: Molecular Architecture and Occupational Aptitudes. Cereb. Cortex. 2020;30:4121–4139. doi: 10.1093/cercor/bhaa035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shroff M., Griffiths P., Adair L., Suchindran C., Bentley M. Maternal autonomy is inversely related to child stunting in Andhra Pradesh, India. Matern Child Nutr. 2009;5:64–74. doi: 10.1111/j.1740-8709.2008.00161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sizonenko S.V., Babiloni C., Sijben J.W., Walhovd K.B. Brain imaging and human nutrition: which measures to use in intervention studies? Adv. Nutr. 2013;4:554–556. doi: 10.3945/an.113.004283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolovic N., Selvam S., Srinivasan K., Thankachan P., Kurpad A.V., Thomas T. Catch-up growth does not associate with cognitive development in Indian school-age children. Eur. J. Clin. Nutr. 2014;68:14–18. doi: 10.1038/ejcn.2013.208. [DOI] [PubMed] [Google Scholar]
- Spencer J.P., Forbes S.H., Naylor S., Singh V.P., Jackson K., Deoni S., Tiwari M., Kumar A. Poor air quality is associated with impaired visual cognition in the first two years of life: A longitudinal investigation. Elife. 2023;12 doi: 10.7554/eLife.83876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squires J., Bricker D. Paul H. Brookes Publishing Co., Inc; Baltimore: 2009. Ages & Stages Questionnaires®, Third Edition (ASQ®-3): A Parent-Completed Child Monitoring System. [Google Scholar]
- Stupic K.F., Ainslie M., Boss M.A., Charles C., Dienstfrey A.M., Evelhoch J.L., Finn P., Gimbutas Z., Gunter J.L., Hill D.L.G., Jack C.R., Jackson E.F., Karaulanov T., Keenan K.E., Liu G., Martin M.N., Prasad P.V., Rentz N.S., Yuan C., Russek S.E. A standard system phantom for magnetic resonance imaging. Magn. Reson Med. 2021;86:1194–1211. doi: 10.1002/mrm.28779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason M.E., Hect J., Waller R., Manning J.H., Stacks A.M., Beeghly M., Boeve J.L., Wong K., van den Heuvel M.I., Hernandez-Andrade E., Hassan S.S., Romero R. Prenatal neural origins of infant motor development: Associations between fetal brain and infant motor development. Dev. Psychopathol. 2018;30:763–772. doi: 10.1017/S095457941800072X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran T.D., Holton S., Nguyen H., Fisher J. Physical growth: is it a good indicator of development in early childhood in low- and middle-income countries? BMC Pedia. 2019;19:276. doi: 10.1186/s12887-019-1654-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu D., Goyal M.S., Dworkin J.D., Kampondeni S., Vidal L., Biondo-Savin E., Juvvadi S., Raghavan P., Nicholas J., Chetcuti K., Clark K., Robert-Fitzgerald T., Satterthwaite T.D., Yushkevich P., Davatzikos C., Erus G., Tustison N.J., Postels D.G., Taylor T.E., Small D.S., Shinohara R.T. Automated analysis of low-field brain MRI in cerebral malaria. Biometrics. 2023;79:2417–2429. doi: 10.1111/biom.13708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesel L., Nimako K., Jones R.M., Munson M., Little S., Njogu H., Njuru I., Ogolla T., Kimenju G., Wegner M.N., Rajasekharan S., Pearson N., Langer A. Implementing the INTERGROWTH-21st gestational dating and fetal and newborn growth standards in peri-urban Nairobi, Kenya: Provider experiences, uptake and clinical decision-making. PLoS One. 2019;14 doi: 10.1371/journal.pone.0213388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vir S.C., Suri S. Young Child Undernutrition: Crucial to Prioritize Nutrition Interventions in the First 1000 Days of Life. Indian J. Pedia. 2023 doi: 10.1007/s12098-023-04732-4. [DOI] [PubMed] [Google Scholar]
- Vlassoff C. Gender differences in determinants and consequences of health and illness. J. Health Popul Nutr. 2007;25:47–61. [PMC free article] [PubMed] [Google Scholar]
- Vohr B.R., Poggi Davis E., Wanke C.A., Krebs N.F. Neurodevelopment: The Impact of Nutrition and Inflammation During Preconception and Pregnancy in Low-Resource Settings. Pediatrics. 2017;139:S38–S49. doi: 10.1542/peds.2016-2828F. [DOI] [PubMed] [Google Scholar]
- Walker S.P., Chang S.M., Powell C.A., Simonoff E., Grantham-McGregor S.M. Effects of psychosocial stimulation and dietary supplementation in early childhood on psychosocial functioning in late adolescence: follow-up of randomised controlled trial. BMJ. 2006;333:472. doi: 10.1136/bmj.38897.555208.2F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedderburn C.J., Subramoney S., Yeung S., Fouche J.P., Joshi S.H., Narr K.L., Rehman A.M., Roos A., Ipser J., Robertson F.C., Groenewold N.A., Gibb D.M., Zar H.J., Stein D.J., Donald K.A. Neuroimaging young children and associations with neurocognitive development in a South African birth cohort study. Neuroimage. 2020;219 doi: 10.1016/j.neuroimage.2020.116846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijeakumar S., Kumar A., Delgado Reyes L.M., Tiwari M., Spencer J.P. Early adversity in rural India impacts the brain networks underlying visual working memory. Dev. Sci. 2019;22 doi: 10.1111/desc.12822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis J.R., Kumar V., Mohanty S., Singh P., Singh V., Baqui A.H., Awasthi S., Singh J.V., Santosham M., Darmstadt G.L. Gender differences in perception and care-seeking for illness of newborns in rural Uttar Pradesh, India. J. Health Popul Nutr. 2009;27:62–71. doi: 10.3329/jhpn.v27i1.3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X., Ferradal S.L., Sliva D.D., Dunstan J., Carruthers C., Sanfilippo J., Zuk J., Zollei L., Boyd E., Gagoski B., Ou Y., Grant P.E., Gaab N. Functional Connectivity in Infancy and Toddlerhood Predicts Long-Term Language and Preliteracy Outcomes. Cereb. Cortex. 2021 doi: 10.1093/cercor/bhab230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Zhao C., Liu X., Wei Q., Luo S., Guo S., Zhang J., Wang X., Scherpbier R.W. Inequality in early childhood neurodevelopment in six poor rural counties of China: a decomposition analysis. Int J. Equity Health. 2017;16:212. doi: 10.1186/s12939-017-0691-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Tian Y., Kong Y., Zhong B., Fu Y. Residual Dense Network for Image Restoration. IEEE Trans. Pattern Anal. Mach. Intell. 2021;43:2480–2495. doi: 10.1109/TPAMI.2020.2968521. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Muller H.G., Zhu C., Chen Y., Wang J.L., O'Muircheartaigh J., Bruchhage M., Deoni S., Resonance Consortium Network evolution of regional brain volumes in young children reflects neurocognitive scores and mother's education. Sci. Rep. 2023;13:2984. doi: 10.1038/s41598-023-29797-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zugman A., Alliende L.M., Medel V., Bethlehem R.A.I., Seidlitz J., Ringlein G., Arango C., Arnatkeviciute A., Asmal L., Bellgrove M., Benegal V., Bernardo M., Billeke P., Bosch-Bayard J., Bressan R., Busatto G.F., Castro M.N., Chaim-Avancini T., Compte A., Costanzi M., Czepielewski L., Dazzan P., de la Fuente-Sandoval C., Di Forti M., Diaz-Caneja C.M., Maria Diaz-Zuluaga A., Du Plessis S., Duran F.L.S., Fittipaldi S., Fornito A., Freimer N.B., Gadelha A., Gama C.S., Garani R., Garcia-Rizo C., Gonzalez Campo C., Gonzalez-Valderrama A., Guinjoan S., Holla B., Ibanez A., Ivanovic D., Jackowski A., Leon-Ortiz P., Lochner C., Lopez-Jaramillo C., Luckhoff H., Massuda R., McGuire P., Miyata J., Mizrahi R., Murray R., Ozerdem A., Pan P.M., Parellada M., Phahladira L., Ramirez-Mahaluf J.P., Reckziegel R., Reis Marques T., Reyes-Madrigal F., Roos A., Rosa P., Salum G., Scheffler F., Schumann G., Serpa M., Stein D.J., Tepper A., Tiego J., Ueno T., Undurraga J., Undurraga E.A., Valdes-Sosa P., Valli I., Villarreal M., Winton-Brown T.T., Yalin N., Zamorano F., Zanetti M.V., Veda c, Winkler A.M., Pine D.S., Evans-Lacko S., Crossley N.A. Country-level gender inequality is associated with structural differences in the brains of women and men. Proc. Natl. Acad. Sci. USA. 2023;120 doi: 10.1073/pnas.2218782120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
Data collected as part of the UNITY network will be made available to researchers from the academic communities at varying levels of granularity depending on site-specific IRB approvals. For some sites, full access to individual raw and processed data will be provided, whilst for others, owing to national policies (e.g., those located in India) may only be able to provide de-identified composite values (e.g., regional volumes, mean relaxometry measures, etc.). The Bill & Melinda Gates Foundation is committed to open access and broad data availability as permitted.