Abstract
The HEALthy Brain and Child Development (HBCD) Study, a multi-site prospective longitudinal cohort study, will examine human brain, cognitive, behavioral, social and emotional development beginning prenatally and planned through early childhood. Many prenatal and early childhood exposures impact both later physical health and development. Moreover, early deficits in physical health, such as growth and vision, are associated with differences in brain development, language and cognitive functioning. For these reasons, the HBCD Study includes measures of early childhood physical health, many of which have clinical relevance, and are applicable for use as both predictors and outcomes. Study measures assess a broad range of physical health domains and include both objective measurement of child growth and health and subjective caregiver report of behaviors and attitudes about constructs known to influence growth and physical development. Lastly, we obtain caregiver report of the child’s routine medical care as well as acute and chronic medical issues. We anticipate that these data will contextualize the impact of child physical growth and health on child brain development and function. In this report we present the rationale for each domain and an overview of the physical health measures included in the current HBCD Study protocol.
Keywords: HBCD, Physical Health, Child Development, Screen Media, Nutrition, Growth, Vision, Physical Activity, Greenspace, Quality of Life, Sleep
1. Introduction
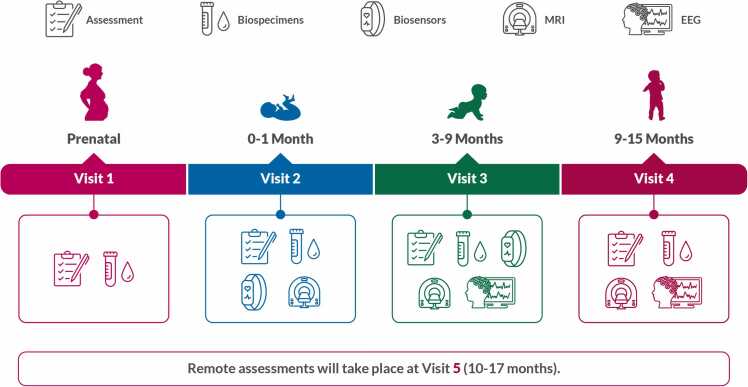
The HEALthy Brain Child Development (HBCD) Study is an unparalleled study that will improve understanding of the environmental, behavioral, social, and genetic factors that affect health and development in infancy and early childhood. As described in the study introduction paper of this special edition, the HBCD Study design includes collection of biospecimens, biosensor, EEG, MRI, observed behavioral, and caregiver reported survey data (Fig. 1). Study visits will start in pregnancy and are planned to continue for up to 10 years after the child participant is born. The jittered design (See Advancing High Quality Longitudinal Data Collection: Implications for Study Design and Recruitment in this Special Issue) will allow for collection of data throughout wide visit windows enabling assessment of developmental trajectories (Fig. 1). The HBCD Study would not be complete without the assessment of physical health, an aspect of one’s life that has direct and profound impacts on development. The breadth of HBCD Study data collection includes assessment of social and environmental risk factors that shape and provide context for children’s physical health. The Physical Health Working Group has developed a battery of assessments that evaluate key components of physical health while accounting for the many other kinds of data included in the protocol and described elsewhere in this special issue.
Fig. 1.
The HBCD Study’s comprehensive protocol includes assessments of children’s physical, cognitive, behavioral, social, and emotional development, plus surveys of parent/caregiver health and well-being. Assessments are distributed across in-person and remote visits during an initial 5-year period. Visits are scheduled at variable intervals designed to capture developmental trajectories. Please note the protocol or procedures for V4 forward are only current as of the time of manuscript submission and may change during piloting.
The physical health domains included in the HBCD Study protocol are as follows: Growth, Nutrition, Sleep, Screen Media Use, Physical Activity and Green Space, Medical History, Vision and Quality of Life. Each domain includes objective or subjective measurements chosen after consideration of developmentally appropriate timing, length and burden to participants, and the likelihood that inclusion would add meaningfully to the understanding of child development (Table 1). Inclusion of instruments occurred after working group members reviewed suggestions provided by subject matter experts within the working group and with approval of the HBCD Study Steering Committee. When scientifically appropriate, the working group opted for shorter versions of validated measures. Measures were then reviewed by the Diversity, Equity and Inclusion working group to ensure language used is inclusive and concepts are appropriate for all, particularly participants previously underrepresented in research. All measures were piloted, allowing for the opportunity to receive feedback from both participants and research staff administering the protocol. We hope the battery of measures chosen will allow for the open-science research community to develop thoughtful scientific questions that take advantage of the opportunity to include physical health data among the other HBCD Study data streams such as EEG, MRI, and child-caregiver interactions. Additionally, we encourage others to consider inclusion of these or comparable measures to harmonize future studies with data collected in this large cohort.
Table 1.
Timing and measure selection of social and environmental exposures in the HBCD Study.
| Construct | Measure |
Visits administered |
|||||
|---|---|---|---|---|---|---|---|
| V1 | V2 | V3 | V4 | V5 | V6 | ||
| Growth | Head circumference | X | X | X | X | ||
| Weight | X | X | X | X | |||
| Length | X | X | X | X | |||
| Nutrition | Food insecurity | X | X | X | X | ||
| PhenX Toolkit | X | X | X | X | |||
| HBCD-specific nutrition survey | X | X | |||||
| Sleep | MRI screener | X | X | ||||
| BISQ | X | ||||||
| PROMIS EC sleep problems | X | X | |||||
| Screen media use | ScreenQ questionnaire | X | X | ||||
| Activity | PROMIS EC Physical Activity | X | X | ||||
| Greenspace | X | X | |||||
| Medical History | Medical history questionnaire | X | X | X | |||
| Vision | Vision screener | X | X | ||||
| Injury Prevention | Safe sleep | X | |||||
| Car seat safety | X | ||||||
| Ingestions | X | ||||||
| Drowning | X | ||||||
V1: Visit 1 (prenatal); V2: Visit 2 (0–30 days); V3: Visit 3 (3–9 months); V4: Visit 3 (9–15 months); V5: Visit 5, remote (10–17 months); V6: Visit 6 (15–48 months); V7: Visit 7 remote (16–50 months).
Please note that the protocol or procedures for V4 and onward are only current as of the time of manuscript submission and may change during piloting.
2. Growth
Growth and physical development through infancy and early childhood represent one of the most objective and standardized measures of overall physical health in the population of children included in HBCD. Being overweight or underweight at birth and throughout childhood often represents poor nutritional status and may have long-term consequences for both physical health and development. Poor growth, resulting from malnutrition, is associated with lower cognitive functioning in early childhood (Pizzol et al., 2021). Recent evidence suggests that being overweight in childhood may also impact cognitive and behavioral development throughout childhood (Suryawan et al., Jun 2022, Li et al., Jun 2018). The longitudinal study design of HBCD strengthens the ability to investigate the impact of early childhood growth characteristics on cognitive and behavioral development. Although low birth weight and small size for gestational age may be independently related to many of the childhood neurocognitive and behavioral outcomes assessed in the study, evidence also suggests that among children with malnutrition in early life, cognitive and developmental deficits may be recovered by middle childhood when nutrition improves (Suryawan et al., 2022). Thus, the HBCD Study has potential to improve understanding of the critical windows in which growth and nutrition impact development and, importantly, opportunities for intervention to promote healthy development.
In addition, the sample size of HBCD will allow for investigation of several prenatal exposures previously associated with low birth weight. Comprehensive assessment of prenatal exposures combined with several growth measurements in early life will allow for enhanced understanding of the impact of these exposures on infant physical health. By design, the HBCD Study cohort will be enriched for children who have been exposed to potentially harmful substances (alcohol, tobacco, cannabis, opioids) before birth, while simultaneously enrolling children born to demographically and socioeconomically matched pregnant individuals who do not report using substances. Prenatal exposure to the aforementioned substances among others have been correlated with low birth weight (Sebastiani et al., Aug 2 2018, Umer et al., May 2023, Hamułka et al., 2018, Chasnoff et al., Jul-Aug 1986, Gouin et al., Apr 2011, Zhao et al., Dec 1 2018). However, most studies of growth in children exposed to substances are small and do not adequately report and control for relevant concurrent exposures such as food insecurity and prenatal stress that may impact the nutritional status of the pregnant person. The breadth of the prenatal assessments and sample size of the HBCD cohort will improve understanding of the impacts of these intersecting exposures on physical health outcomes at birth.
To achieve the accurate longitudinal assessment of growth in HBCD participants will have anthropometrics collected at all in-person study visits beginning with the child participant’s first visit at 0–1 months after birth. As of this publication, in-person study visits will occur for child participants at 0–1 month, 3–9 month, 9–15 month, and 15–48 month timepoints. Anthropometric measurements will include length or height, weight, and head circumference. Length measurements are collected via a length board for participants under the age of 2 years and those who cannot stand. Once child participants are able to stand, research staff collect height measurements using a measuring rod. To ensure accurate weight measurements, staff are instructed to weigh infants in a diaper, taring out the diaper weight with a clean dry diaper. Once child participants are old enough to use a standing scale, staff are instructed to have the child undress to a light layer of clothing (ie. shirt, pants, socks, etc.) Research staff will collect each measurement twice and enter them into the data collection system. These measurements will then be averaged and plotted against the World Health Organization’s child growth standards. Validation measures include flagging measurements that are lower than those previously recorded, measurements that are greater than the 97th percentile, and measurements that are less than the 3rd percentile for sex and corrected age. When a data point is flagged the research team member is instructed to re-measure or confirm the out of range value.
To ensure consistent collection of data, HBCD sites each purchased a Seca 354 digital baby scale, Seca 216 measuring rod for children and adults, and Seca 417 mobile measuring board (https://us.secashop.com/). Working group members trained all research staff in accurate measurement of anthropometrics during a hands-on in-person training session prior to launch of the pilot study. The same working group members then conducted a virtual refresher training prior to launch of the main study. This training reinforced proper technique for obtaining measurements and discussion of data obtained during the pilot focused on outlying values that had been recorded and validation measures that had been added. A standard operating procedure was created which detailed the steps for obtaining measurements consistently and accurately, including reminders to calibrate all instruments before use.
3. Nutrition
Nutrition is the basis for physical growth and is essential for brain development (Ottolini et al., 2020). It is especially important during pregnancy and infancy which are crucial periods for the formation of the brain that lays the foundation for development of cognitive, motor, and socioemotional skills during childhood and adulthood (Morton et al., 2022). Many nutrients such as iron, zinc, copper, choline, vitamins A, B and D, cholesterol and lipids are crucial for myelin synthesis, composition, and maintenance. These nutrients and others contribute to neuron proliferation, axon and dendrite growth, synapse formation, pruning, and neuronal apoptosis (Deoni et al., 2018). During the first two years of life there is rapid brain development with high energy requirements, which makes this stage vulnerable to nutritional deficits (Cusick et al., 2021). Long term neurodevelopmental consequences (Morton et al., 2022, Coviello et al., 2018, Suchdev et al., 2017, Marshall et al., 2022) can occur if macronutrients and micronutrients are not available in adequate amounts during sensitive periods of brain development (Morton et al., 2022, Cusick et al., 2021, Mattei and Pietrobelli, 2019, Lockyer et al., 2021, Cusick and Georgieff, 2016).
There is a complex relationship between nutrition, environmental factors, inflammation, genetic heterogeneity, and neurodevelopment. During the first six months of life nutritional intake typically occurs via breast milk or formula. Breastfeeding is associated with multiple benefits, preventing infectious diseases, reducing morbidity and mortality, and influencing both short- and long-term infant development (Deoni et al., 2018, Schneider et al., 2023, Gilbreath et al., 2023) in preterm and term infants (Fan et al., 2023, Gialeli et al., 2023). However, there is emerging data that the societal perceptions of breast feeding being “natural” and that “breast is best” has had negative impacts on the stress and guilt postpartum individuals experience around feeding choices (Korth et al., 2022, Jackson et al., Jan 2024). Increased anxiety and guilt in the immediate postpartum period are correlated with poor infant bonding (Davies et al., 2021, Della Vedova et al., 2023). Later in life, picky eating is more common in children with behavioral and/or neurodevelopmental differences and is associated with increased family stress and social isolation (Fernández de Valderrama Rodríguez et al., Nov 2022, Thorsteinsdottir et al., Jun 25 2021). Therefore, to get a full picture of feeding practices, psychosocial context around feeding and nutritional components of food consumed by children is necessary to better assess the impact of nutritional exposures on developmental outcomes.
Although a thorough assessment of nutritional content, feeding practices, and behaviors is necessary to get a full understanding of the nutritional environment, we were required to balance the depth of instruments with the length and burden on participants in the HBCD Study. As such, in the first two post-partum visits, coinciding with the first 9 months of life, we focused on breastfeeding questions and food security. We selected the PhenX Toolkit breastfeeding protocol for the assessment of feeding practices as it is limited to 3 questions that may be repeated longitudinally and permit assessment of duration and exclusivity of breastfeeding as well as timing and introduction of complementary foods. Food insecurity will be measured at each in person visit after infants are born. The 2-item food insecurity screening questionnaire was chosen due to its widespread use in clinical practice and brevity (Hager et al., 2010). Starting at visit 4 participants are given an HBCD Study-specific survey that combines questions from select nutrition surveys to meet the needs of different stakeholders and researchers likely to be interested in this construct. For relevance to clinical practice and public health policy makers, questions that were common to WIC (Women Infants and Children) offices across the US are used to evaluate the location of food consumption (i.e. home or babysitter’s home), content of typical meals obtained through general dietary recall, amount and frequency of sugary beverages and milk products, behaviors around food (i.e. eating as a family, with the television on, etc.). Infant feeding practices (reasons for discontinuing breastfeeding, combination feeding behaviors, and complementary feeding practices) are assessed with questions selected from the Infant Feeding Practices Study II postnatal questionnaires (Fein et al., 2008). Lastly general perceptions and behaviors around mealtime are assessed with questions from the AAP Bright Futures Nutrition Questionnaire for Children Ages 1–10 (Hagan et al., 2017).
With the inclusion of these nutritional survey assessments, the HBCD Study has the unique potential to combine parental report of dietary habits and growth data with biosamples that may be assessed for specific metabolites or nutritional profiles, neurodevelopmental and behavioral data spanning multiple modalities (imaging, EEG, behavioral assessments, and cognitive testing), and genetic and epigenetic data. We expect this will improve understanding of the complex ways in which nutrition impacts child growth and development.
4. Sleep
Sleep is a crucial biological function. Sleep patterns change substantially between infancy and early childhood. In early infancy, sleep is polyphasic with sleep distributed throughout a 24 hour period (Lokhandwala and Spencer, 2022). Total sleep duration in the newborn period is between 14 and 16 hours a day and falls to 10–12 hours by six months of life (Dias et al., 2018). Naps decrease to once a day in toddlerhood when sleep transitions to a biphasic pattern. By early childhood there is a transition to the adult pattern of sleep which typically occurs exclusively overnight (Lokhandwala and Spencer, 2022). Within these distinct sleep patterns, problems with sleep have been associated with infant/child temperament, maternal history of adversity, and parental stress (Ciciolla et al., Feb 2022, Morales-Muñoz et al., Nov 2020, Hiscock et al., Sep 2008). A multitude of studies have shown that disrupted or inadequate sleep can affect cognition, executive function, mental health, and physical health in children (Lokhandwala and Spencer, 2022).
Few studies have evaluated potential short- and long-term impacts of prenatal substance exposure on infant and child sleep. Data from the Maternal Lifestyle multicenter study utilized parental report of sleep problems through 12 years and found that prenatal tobacco exposure was more strongly associated with sleep problems than exposure to alcohol, marijuana, opiates, or cocaine (Stone et al., 2010). Similarly, an Australian community-based cohort study identified associations between prenatal maternal smoking and sleep problems through 14 years (O'Callaghan et al., 6 2019). In contrast, prenatal alcohol exposure is associated with sleep problems through 3 years; however, this relationship is almost fully explained by family and environmental factors (Lund and Ystrom, 2022). Assessments of prenatal stress, environment, and substance exposure together with longitudinal measures of child sleep in HBCD will enable evaluation of how these important factors interact with one another and ultimately influence childhood developmental and behavioral outcomes.
Acknowledging the importance of sleep patterns and problems across childhood, this working group aimed to balance the importance of measuring sleep and sleep problems with the burden to participants. As such, we chose to measure key pieces of sleep safety, sleep patterns and sleep problems throughout the protocol but decided against measuring each of those constructs at every time point. We aimed to include the most critical data at each time frame: infancy, toddlerhood, and early childhood. To do so, sleep measures in infancy (visits 2 and 3) focus on safe sleep practices. Sleep patterns are measured during the transition from infancy to toddlerhood (visit 4) and thereafter we will measure perceived sleep problems and impacts on daily function.
4.1. Safe sleep practices
Sleep-related deaths are a leading cause of infant mortality in the US, with significant racial and ethnic disparities in infant mortality due to sleep-related causes (Prevention UCfDCa, 2023, Müller-Nordhorn et al., Jun 11 2021). The American Academy of Pediatrics recommends a safe sleep environment to reduce these risks, including supine positioning, use of a firm non-inclined sleep surface, room sharing without bed sharing, human milk feeding, and avoidance of exposures to nicotine, alcohol, marijuana, and opioids (Moon et al., 2022). Due to the time frame during which the earliest HBCD Study visits were piloted and sleep questions were introduced into the study protocol, a validated questionnaire about sleep was not included in the first (0–1 month) infant study visit protocol. However, questions asked in preparation for MRI scanning, to allow sites to mimic the home sleep environment and facilitate natural sleep during the MRI scan, provided a novel opportunity to glean information about sleep routines and safe sleep practices during the first weeks of the infant’s life. Specific questions are provided in the Supplement, including, “In what position does your child typically sleep?” and “Does your child sleep with any sleep aids?”. The data collected with these MRI preparation questions will be publicly released alongside other HBCD Study data.
4.2. Sleep patterns
Infant sleep patterns will be measured with the Brief Infant Sleep Questionnaire – Revised (BISQ) (Sadeh, Jun 2004, Sadeh et al., Mar 2009, Mindell et al., Nov 2019). The BISQ, published in 2004, is a freely-available instrument (www.babysleep.com/BISQ/) that measures sleep patterns, sleep ecology, and parental perceptions of sleep in children 0–36 months of age (Sadeh, Jun 2004, Sadeh et al., Mar 2009). A recent revision (BISQ-R) includes a wider array of sleep behaviors and outcomes and was validated against both actigraphy and daily sleep logs. It is available in more than 20 languages and a norm-referenced scoring system was recently published (Mindell et al., 2019). The 19 scored questions from the BISQ-R will be administered during the 9–15 month HBCD Study visit (visit 4).
4.3. Sleep problems
At subsequent visits, caregivers will complete the newly established 8-item PROMIS Early Childhood Parent Report of Sleep Problems (www.healthmeasures.net). Validated from 1 to 5 years, this shorter form allows for assessment throughout childhood of sleep disturbances and sleep related impairment (Lai et al., 2022). Inclusion of this measure in the longitudinal study of early childhood development has the potential to add to the current understanding of the impact of sleep differences on growth and development. These data coupled with the biosensor data (See Pini et al., 2024) will provide a multimodal assessment of infant and child sleeping patterns and behaviors across early childhood.
5. Screen media use
There is growing interest in the impact of screen media use on children, particularly as it relates to neurobehavioral and cognitive development. In late childhood and adolescence, increased screen time has been associated with poor school performance, increased risk taking behaviors, poor emotional regulation, depression, and suicide (Fors and Barch, 2019, Boers et al., Jul 15 2019, Twenge et al., 2018, Fischer et al., 2011, Goldfield et al., 2016). However, current knowledge suggests that problematic screen use begins much earlier than adolescence, and may be influenced by several social and familial factors. Increased screen exposure in early life is correlated with decreased vocabulary and language development (Sundqvist et al., 2023, Hammrich et al., 2023, Zimmerman et al., 2007, Chonchaiya and Pruksananonda, 2008). There is also increasing evidence that increased screen time is associated with decreased cognitive function and social emotional skills in preschoolers (Schwarzer et al., 2022, Madigan et al., 2019). Furthermore, early screen use is associated with continued use throughout childhood. Sundqvist et al. recently demonstrated that screen media behavior as early as 9 months may “set the stage” for continued heavy use throughout childhood, such that use at 9 months was correlated with use at 2 years, and 2 year use was predictive of use at 5 years (Sundqvist et al., 2023). These findings are complemented by other studies which demonstrate that preventing use when children are 2 years may improve rates of problematic screen use later in childhood (Shawcroft et al., 2023). Like most childhood exposures, screen use is correlated with both parenting behaviors and family demographics (Hammrich et al., 2023, Shawcroft et al., 2023, Swit et al., 2023).
To assess screen media use in the HBCD Study, we include the ScreenQ questionnaire in each in person visit starting at 9 months of age. The ScreenQ was developed to assess the 4 domains of screen media use addressed by current AAP guidelines: access to screens, frequency of use, content of media and co-viewing (whether media is viewed with adults) (Hutton et al., 2020a). This 15-question parental survey takes less than 5 minutes to complete. Higher scores have been correlated with decreased integrity of white matter tracts in areas related to language and literacy as well as cortical thickness in areas supporting visual processing in preschool children (Hutton et al., 2020a, Hutton et al., 2020b). Longitudinal use of the ScreenQ in the HBCD Study will represent one of the largest studies of early childhood screen behavior to date and allow for examination of associations between screen use trajectories, childhood well-being, behavior and brain development.
6. Green space and physical activity
Childhood physical activity promotes child development in virtually every domain. Physical activity has both short-term benefits (improved mood, better quality sleep, decreased stress) and long-term benefits (increased bone density and muscle strength; lower risk of obesity, Type 2 diabetes and heart disease). Importantly, physical activity in children is inversely correlated with screen time use (see above for measurement of screen media use). Motor skills in preschoolers are positively associated with vigorous physical activity and negatively associated with screen time (Webster et al., 2019). In children, physical activity has direct benefits beyond physical health. Toddlers who meet the American Academy of Pediatrics goals for screen time (<1 hour a day) and physical activity (>3 hours a day) tend to show better executive function than their counterparts who have more screen time and/or less physical activity (McMath et al., 2023).
Design of the built environment, defined by the American Academy of Pediatrics as “the man-made structures that comprise the neighborhoods and communities where people live, work, learn, and recreate,” is closely associated with physical and mental health (Bole et al., 2024). Access to green space facilitates physical activity and is associated with many health benefits including lower rates of depression, obesity, and hypertension (Cox et al., 2017, Galvez et al., 2010). Emerging data suggest that in children, access to green spaces and playgrounds is associated with reduced inattention and hyperactivity along with improved cognitive outcomes and school performance (Bole et al., 2024). The importance of green space to health during pregnancy is also increasingly recognized (Tiako et al., 2021a, Tiako et al., 2021b). For example, a recent study reported associations between increased tree coverage and lower rates of postpartum depression (Sun et al., 2023). However, little is known about how people use green space during and after pregnancy, or whether access to green space translates to increased physical activity and improved health in the children. Across the HBCD Study, in which both urban and rural participants will be enrolled, use of green space for socialization and physical activity during and after pregnancy is expected to differ, as are family perceptions of the safety of using such spaces for recreation.
A Japanese birth cohort study of children born from 2007 to 2012 evaluated relationships between frequency of outdoor play and screen time exposure during the toddler years and neurodevelopment measured at 4 years (Sun et al., 2023). Increased screen time was associated with both poor communication and daily living skills, and frequency of outdoor play mediated the relationship between screen time and daily living skills. Further, frequency of outdoor play was associated with improved socialization skills (Oswald et al., 2020). More research is needed to further elucidate the associations between screen time, physical activity, green space, and physical and cognitive development in all children, and particularly among children born in high-risk environments and those with prenatal substance exposure. The HBCD Study will provide critical contributions to understanding of these relationships as well as potential opportunities for interventions to improve childhood neurobehavioral and health outcomes.
As shown in Table 1 physical activity will be measured with the PROMIS Parent Proxy Physical Activity Short Form (www.healthmeasures.net). A validated measure of green space access does not exist. Greenspace access and use will be assessed with four questions about frequency of use, accessibility, types of activities, and factors that limit use of green space for recreation and socialization, specifically for parents of young children (See Supplemental Material). An additional key research opportunity provided by the HBCD Study is geocoding of residential addresses of study participants, which will then be linked to data including participant-reported physical activity and green space use.
7. Vision
The majority of maturation of the visual system occurs after birth and requires visual stimuli. Rapid development of the visual system aids in continued neurodevelopment (Atkinson et al., 2011). Differences in visual development are common. Up to 1 in 5 infants under a year of age are diagnosed with an ocular disorder (Mansukhani et al., 2023) and 6.2 % of infants and children between 6 and 71 months of age exhibit at least one eye abnormality (Repka et al., 2012). In addition, the prevalence of refractive errors, including myopia, hyperopia, and astigmatism, have been shown to be as high as 50 % in children between 5 and 17 years of age (Kleinstein et al., 2003). One recent Canadian study reported undetected visual problems in 10.7 % of kindergarteners using a short vision screener in schools (Nishimura et al., 2020). Receipt of screening is associated with social determinants of health, and about 40 % of children in the United States do not receive routine vision screening (Chauhan et al., Jan 2023, Ambrosino et al., 2023).
Some populations are at higher risk for visual differences. For instance, in-utero opioid or substance exposure is associated with both decreased visual acuity and increased oculomotor abnormalities during early childhood (McGlone et al., Sep 2009, Walhovd et al., Mar 2015, Melinder et al., Dec 2013, Gill et al., 2003, Spiteri Cornish et al., Jul 2013, Auger et al., Dec 2020, Yen and Davis, 2022). However, incidence rates of reported visual abnormalities and ages of assessment vary widely across the literature, limiting conclusions. The available studies used disparate approaches to sampling, including evaluation of children with neonatal abstinence syndrome who received pharmacologic treatment or were rehospitalized and recruited from ophthalmology programs. These variations further limit assessment of both incidence and the natural history of these abnormalities. Perhaps most importantly, no data about associations between these ophthalmologic findings and MRI, EEG, and developmental/behavioral outcomes are available.
Assessment of visual acuity and visual processing in the HBCD Study will significantly advance our understanding of the factors important for typical visual development and the effects of visual differences on typical development. The HBCD Study currently plans to screen infants for visual and oculomotor problems during in-person visits at 9–15 months and at 15–48 months using the Welch Allyn Spot Vision Screener (Hill-Rom Holdings, Inc., Chicago, IL). The Spot Vision Screener is an instrument-based vision screener, which means that it captures an image of the eyes and then uses the image to measure refractive error and ocular misalignment. The device requires no direct patient contact, screens for a variety of ophthalmologic disorders in a few seconds and can be used in patients as young as 6 months. The Spot Vision Screener has been reported to have specificity and sensitivity as high as 93.4 % and 91.7 %, respectively (Misra et al., 2021).
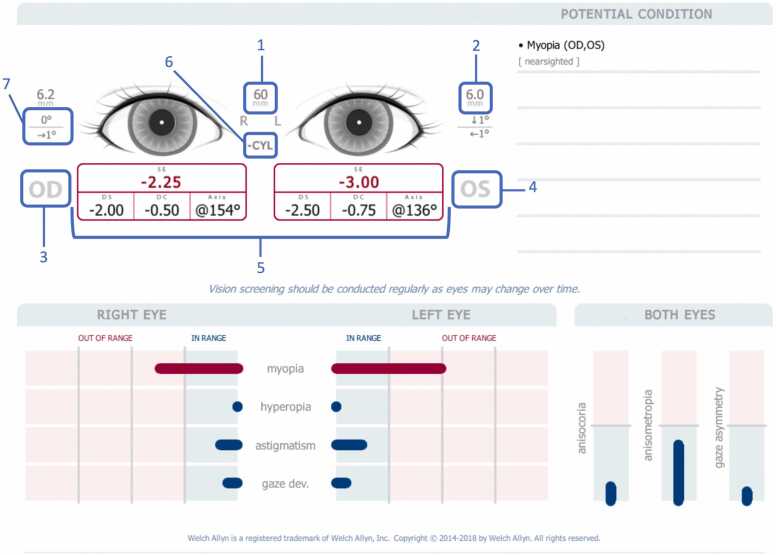
HBCD Study personnel have been trained to use the device by the device customer representatives and working group members, Data retrieval after the screening will take about two minutes and provide easy to understand results (See Fig. 2 and Table 2). Study participants who do not pass the screening will be referred for clinical ophthalmologic care. Data about outcomes of these referrals will be collected at subsequent study visits. Thus, results of this screening will not only be valuable for the HBCD Study but are expected to provide important information to the participants and their families.
Fig. 2.
Vision Screener output. 1: Pupillary Distance; 2: Pupil Size Indicator; 3: Right Eye (OD); 4: Left eye (OS); 5: Complete refraction SE - Spherical equivalent, DS – Sphere, DC – Cylinder, Axis – Axis; 6: Cylinder Convention; 7: Alignment Indicator Degree: vertical or horizontal.
Table 2.
Visual Screener measurements and definitions.
| Definitions | |
|---|---|
| Refractive Measurements | Consists of Sphere, Cylinder and Axis |
| Sphere [DS] | Measures the power of the eye for myopia and hyperopia |
| Cylinder [DC] | Measures the shape of the cornea |
| Axis | Measurable location of astigmatism |
| Spherical Equivalent [SE] | Equal to the sum of the value of the sphere and half of the cylinder |
| Pupillary Distance [PD] | Distance between the centers of the pupils in each eye |
| Cylinder Convention | There are two different conventions for indicating the amount of cylinder: "plus cylinder notation" and "minus cylinder notation" |
| OD [oculus dexter] | Right eye |
| OS [oculus sinister] | Left eye |
| Binocular | Both eyes |
| Monocular | One eye |
| Myopia | Near-sightedness |
| Hyperopia | Far-sightedness |
| Anisometropia | Unequal refractive power |
| Astigmatism | Blurred vision, eye structure problem |
| Anisocoria | Pupil size deviations |
| Strabismus | Eye misalignment, Gaze |
8. Quality of life
Quality of life (QOL) has recently emerged as an essential health outcome. Although QOL includes physical health, it also encompasses emotional, psychological, and social well-being. In a longitudinal study of child development such as HBCD, QOL measures may serve as some of the most clinically meaningful and important outcomes in assessing the relationship between overall wellbeing and numerous developmental and behavioral outcomes. As a working group comprised of many clinicians, we felt it important to include a measure of quality of life in this domain to continue to encourage clinical researchers to consider a broader view of “health” when examining physical health outcomes. In adults, life satisfaction and QOL have repeatedly been associated with treatment efficacy, illness complications and mortality (Phyo et al., 6 2020). In children, QOL has been repeatedly correlated with physical health such that those with chronic or acute illness have lower quality of life (Varni et al., 2001). Additionally, developmental delays in both cognitive and motor domains have been associated with lower QOL ratings in children as young as 4 (Redondo-Tébar et al., Dec 2021, Ncube et al., Mar 2018). Furthermore, children with lower language ability demonstrate a decline in QOL trajectories between early childhood and early adolescence (Le et al., 2021). Studies have yet to use early childhood QOL as a predictor of later outcomes, likely because of the lack of longitudinal studies including these measurements. As such, the inclusion of QOL in the HBCD Study protocol is an important step toward improving understanding of the drivers of early childhood QOL. It will also allow for investigation of developmental trajectories of early childhood QOL and of relationships between QOL and other developmental outcomes. We selected the PedsQL 4.0 as a measure of early childhood QOL (Varni et al., 2001). The advantages of this measure include validation in ages as young as 2 years and across several cultures. This widely validated instrument will allow for comparison of HBCD findings to many other studies. Importantly the PedsQL dimensional scales allowing for the evaluation of different aspects of life functioning. Internal consistency reliability for the total score, physical health and psychosocial health summary scores range from α=0.8–0.9 (Varni et al., 2001). Lastly, the ability to include child reported QOL as early as 5 years alongside parent reported QOL using the same scale has potential to allow for overlap and comparison between child and caregiver reported QOL during later study visits.
9. Medical history
Physical health as captured by medical history will be a critical contextual variable in analyses of HBCD Study data including assessment of brain growth and neurocognitive development. The impact of physical health on growth and development begins in utero and continues throughout childhood. Preterm birth alone represents 12 per 100 live births across the United States (Khan et al., 2023) and is associated with differences in neurodevelopment in early childhood (Shaw et al., 2023, Ryan et al., 2023). Furthermore, chronic illnesses, hospitalizations and injuries in childhood have been associated with differences in development (Dipnall et al., 2023, Fardell et al., 2023). Thus, in the HBCD Study we seek to longitudinally capture participants’ medical history using a questionnaire that includes history of acute and chronic illnesses, injuries and hospitalizations, interactions with the health care system, medication exposure, use of assistive devices, vaccination status and frequency of common symptoms throughout the study time period.
9.1. Birth outcomes
Pregnancy complication, delivery mode, birth outcomes and length of stay will be obtained by participant report at V2 as described elsewhere in this edition (Gurka et al., 2024). Subsequent medical history assessments will occur at V4 and are anticipated to be completed at each in person visit following V4. The constructs assessed are described below:
9.2. Well child care
Participants are asked at V4 about if they have a place they take their children for routine care, and general vaccine behaviors with two questions from the parental attitudes about childhood vaccines survey (PACV) (Opel et al., 2013) that establish if parents have ever delayed or decided against a vaccine recommended by their medical provider. Participant burden prevented inclusion of the full PACV survey. Given the lack of understanding about long term impacts of SARS-COV-2 on health and development, we include specific questions related to COVID infection and vaccination behaviors.
9.3. Acute and specialty care
At V4, participants are asked about whether the child has received any emergency care, required an overnight inpatient stay or surgical procedure and if so, they are asked to report the diagnosis driving the medical care. Lastly families will report if they have ever been told their child has a chronic illness. Common chronic disease including but not limited to Trisomy 21, asthma, obesity are listed out specifically, and other chronic diseases will be captured in an “other” category to allow for coding of common outcomes. Again, given the lack of understanding of the impact of COVID, more in depth questions about COVID infection and hospitalization are included.
9.4. Dental health
Participants are asked when their child’s first tooth erupted, and number of teeth at the V4 visit. They are also asked about access to routine dental care, and if their child has had mouth injuries. Teeth brushing behaviors are assessed with the questions “On average, how many times are the child’s teeth brushed per day?” and “Does an adult help brush your child’s teeth?” Lastly, presence and duration of thumb sucking and/or pacifier use is reported by participants. These questions, coupled with the nutrition questions may be used to assess risk factors for dental caries, the most common chronic disease in childhood (Colak et al., 2011). Dental health and caries have been associated with growth delays, behavior problems and lower quality of life (Gomersall et al., 2024) Additionally, early tooth maturation, thought to be a biomarker of advanced aging, has been associated with early life stress (McDermott et al., 2021). Therefore, the inclusion of these questions will allow for assessment of the relationship between dental health and developmental outcomes.
9.5. Medications
V4 will also include questions about prescribed and over the counter medication exposure, frequency, and indication. These will be populated using ontology boxes to allow for some flexibility but consistency between participants.
9.6. Injury prevention
We anticipate that these data may be used to assess patterns in injury risk so we also include 1–2 questions about developmentally appropriate injury prevention behaviors each time the medical history questionnaire is administered.
9.7. Assistive devices and services
To complement the data collected by the social and environmental determinants working group, we ask about the access to and use of health insurance, and early intervention services. Additionally, parents will report use of common assistive devices such as hearing aids, glasses, and orthopedic braces.
In summary, we present the battery of assessments included in the evaluation of physical health and development in the HBCD Study as it stands at the time of this publication. Details of the study protocol, particularly the visits that will take place as the participants get older, are subject to change as these future visits are piloted and refined. Nevertheless, the constructs described above will be evaluated throughout the study, and include both well-established moderators of development such as nutrition, growth, and sleep as well as more novel assessments such as vision, greenspace and screen media use in a large longitudinal cohort. We expect that the data gathered will be used to elucidate trajectories of both typical development and atypical childhood health and development and aid in the identification of modifiable factors that promote healthy development across childhood.
CRediT authorship contribution statement
Sara B. DeMauro: Writing – review & editing, Writing – original draft, Methodology, Conceptualization. Misha Sisodia: Writing – review & editing, Writing – original draft. Stepahnie Merhar: Writing – original draft. Jessie R. Maxwell: Writing – original draft. Lisa S. Scott: Writing – review & editing, Writing – original draft, Methodology. Myriam Peralta-Carcelen: Writing – original draft. Bailey Garner: Writing – original draft. Leigh-Anne Cioffredi: Writing – review & editing, Writing – original draft, Methodology, Conceptualization.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Data/Processes/Plans/Concepts (select as appropriate) used in the preparation of this article were obtained from the Healthy Brain and Child Development (HBCD) Study (https://hbcdstudy.org/). This is a multisite, longitudinal study designed to recruit over 7000 families and follow them from pregnancy to early childhood. The HBCD Study is supported by the National Institutes of Health and additional federal partners under award numbers U01DA055352, U01DA055353, U01DA055366, U01DA055365, U01DA055362, U01DA055342, U01DA055360, U01DA055350, U01DA055338, U01DA055355, U01DA055363, U01DA055349, U01DA055361, U01DA055316, U01DA055344, U01DA055322, U01DA055369, U01DA055358, U01DA055371, U01DA055359, U01DA055354, U01DA055370, U01DA055347, U01DA055357, U01DA055367, U24DA055325, U24DA055330. A full list of supporters is available at https://hbcdstudy.org/about/federal-partners/. A listing of participating sites and a complete listing of the study investigators can be found at https://hbcdstudy.org/study-sites/. HBCD consortium investigators designed and implemented the study and/or provided data but did not necessarily participate in the analysis or writing of this report. This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIH or HBCD consortium investigators
Declaration of Interests
Outside of the abovementioned NIH funding, the authors have no conflict of interests to disclose. No AI software was used in the preparation of this manuscript
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.dcn.2024.101414.
Appendix A. Supplementary material
Supplementary material
References
- Ambrosino C., Dai X., Antonio Aguirre B., Collins M.E. Pediatric and school-age vision screening in the United States: rationale, components, and future directions. Children. 2023;10(3):490. doi: 10.3390/children10030490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson J., Braddick O., Riva D., Njiokiktjien C., Bulgheroni S. Linked brain development for vision, visual attention and visual cognition in typical development and in developmental disorders. Brain Lesion Localization and Developmental Functions: Frontal Lobes, Limbic System. Visuocognitive Syst. 2011:247–270. [Google Scholar]
- Auger N., Rhéaume M.A., Low N., Lee G.E., Ayoub A., Luu T.M. Impact of prenatal exposure to opioids, cocaine, and cannabis on eye disorders in children. J. Addict. Med. 2020;14(6):459–466. doi: 10.1097/adm.0000000000000621. [DOI] [PubMed] [Google Scholar]
- Boers E., Afzali M.H., Newton N., Conrod P. Association of screen time and depression in adolescence. JAMA Pedia. 2019 doi: 10.1001/jamapediatrics.2019.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bole A., Bernstein A., White M.J. SECTION ON MINORITY HEALTH E, INCLUSION Heard Garris Nia MD M, FAAP Brown Kimberly MD, FAAP Chomilo Nathan MD, FAAP Jones Nathaniel MD Rodriguez Patricia MD, FAAP Walker Valencia MD, FAAP Onyema-Melton Ngozi. The built environment and pediatric health. Pediatrics. 2024;153(1) e2023064773. [Google Scholar]
- Chasnoff I.J., Burns K.A., Burns W.J., Schnoll S.H. Prenatal drug exposure: effects on neonatal and infant growth and development. Neurobehav. Toxicol. Teratol. 1986;8(4):357–362. [PubMed] [Google Scholar]
- Chauhan M.Z., Elhusseiny A.M., Samarah E.S., Rook B.S., Sallam A.B., Phillips P.H. Five-year trends in pediatric vision screening and access in the United States. Ophthalmology. Jan 2023;130(1):120–122. doi: 10.1016/j.ophtha.2022.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chonchaiya W., Pruksananonda C. Television viewing associates with delayed language development. Acta Paediatr. 2008;97(7):977–982. doi: 10.1111/j.1651-2227.2008.00831.x. [DOI] [PubMed] [Google Scholar]
- Ciciolla L., Addante S., Quigley A., Erato G., Fields K. Infant sleep and negative reactivity: the role of maternal adversity and perinatal sleep. Infant Behav. Dev. Feb 2022;66 doi: 10.1016/j.infbeh.2021.101664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colak H., Dülgergil C., Dalli M. American academy on pediatric dentistry. policy on early childhood caries (ECC): classifications, consequences, and preventive strategies. Pedia Dent. 2011;30:40–43. [PubMed] [Google Scholar]
- Coviello C., Keunen K., Kersbergen K.J., et al. Effects of early nutrition and growth on brain volumes, white matter microstructure, and neurodevelopmental outcome in preterm newborns. Pediatr. Res. 2018;83(1):102–110. doi: 10.1038/pr.2017.227. [DOI] [PubMed] [Google Scholar]
- Cox D.T., Shanahan D.F., Hudson H.L., et al. Doses of nearby nature simultaneously associated with multiple health benefits. Int. J. Environ. Res. Public Health. 2017;14(2):172. doi: 10.3390/ijerph14020172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusick S.E., Barks A., Georgieff M.K. Springer; 2021. Nutrition and Brain Development. Sensitive Periods of Brain Development and Preventive Interventions; pp. 131–165. [Google Scholar]
- Cusick S.E., Georgieff M.K. The role of nutrition in brain development: the golden opportunity of the “first 1000 days”. J. Pediatr. 2016;175:16–21. doi: 10.1016/j.jpeds.2016.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies S.M., Silverio S.A., Christiansen P., Fallon V. Maternal-infant bonding and perceptions of infant temperament: the mediating role of maternal mental health. J. Affect. Disord. 2021;282:1323–1329. doi: 10.1016/j.jad.2021.01.023. [DOI] [PubMed] [Google Scholar]
- Della Vedova A.M., Santoniccolo F., Sechi C., Trombetta T. Perinatal depression and anxiety symptoms, parental bonding and dyadic sensitivity in mother–baby interactions at three months post-partum. Int. J. Environ. Res. Public Health. 2023;20(5):4253. doi: 10.3390/ijerph20054253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deoni S., Dean D., III, Joelson S., O'Regan J., Schneider N. Early nutrition influences developmental myelination and cognition in infants and young children. Neuroimage. 2018;178:649–659. doi: 10.1016/j.neuroimage.2017.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias C.C., Figueiredo B., Rocha M., Field T. Reference values and changes in infant sleep-wake behaviour during the first 12 months of life: a systematic review. J. Sleep. Res. 2018;27(5) doi: 10.1111/jsr.12654. [DOI] [PubMed] [Google Scholar]
- Dipnall J.F., Lyons J., Lyons R.A., et al. Impact of an injury hospital admission on childhood academic performance: a Welsh population-based data linkage study. Inj. Prev. 2023 doi: 10.1136/ip-2023-045027. [DOI] [PubMed] [Google Scholar]
- Fan Y., McMath A.L., Donovan S.M. Review on the impact of milk oligosaccharides on the brain and neurocognitive development in early life. Nutrients. 2023;15(17):3743. doi: 10.3390/nu15173743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fardell J.E., Hu N., Wakefield C.E., et al. Impact of hospitalizations due to chronic health conditions on early child development. J. Pediatr. Psychol. 2023 doi: 10.1093/jpepsy/jsad025. jsad025. [DOI] [PubMed] [Google Scholar]
- Fein S.B., Labiner-Wolfe J., Shealy K.R., Li R., Chen J., Grummer-Strawn L.M. Infant feeding practices study II: study methods. Pediatrics. 2008;122(Suppl 2):S28–S35. doi: 10.1542/peds.2008-1315c. [DOI] [PubMed] [Google Scholar]
- Fernández de Valderrama Rodríguez A., Ochoa Sangrador C., Pedrón Giner C., Sánchez Hernández J. Psychological and social impact on parents of children with feeding difficulties. Pedia (Engl. Ed.) 2022;97(5):317–325. doi: 10.1016/j.anpede.2022.09.004. [DOI] [PubMed] [Google Scholar]
- Fischer P., Greitemeyer T., Kastenmüller A., Vogrincic C., Sauer A. The effects of risk-glorifying media exposure on risk-positive cognitions, emotions, and behaviors: a meta-analytic review. Psychol. Bull. 2011;137(3):367. doi: 10.1037/a0022267. [DOI] [PubMed] [Google Scholar]
- Fors P.Q., Barch D.M. Differential relationships of child anxiety and depression to child report and parent report of electronic media use. Child Psychiatry Hum. Dev. 2019;50:907–917. doi: 10.1007/s10578-019-00892-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvez M.P., Pearl M., Yen I.H. Childhood obesity and the built environment: a review of the literature from 2008-2009. Curr. Opin. Pediatr. 2010;22(2):202. doi: 10.1097/MOP.0b013e328336eb6f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gialeli G., Panagopoulou O., Liosis G., Siahanidou T. Potential epigenetic effects of human milk on infants’ neurodevelopment. Nutrients. 2023;15(16):3614. doi: 10.3390/nu15163614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbreath D., Hagood D., Alatorre-Cruz G.C., Andres A., Downs H., Larson-Prior L.J. Effects of early nutrition factors on baseline neurodevelopment during the first 6 months of life: an EEG study. Nutrients. 2023;15(6):1535. doi: 10.3390/nu15061535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill A.C., Oei J., Lewis N.L., Younan N., Kennedy I., Lui K. Strabismus in infants of opiate-dependent mothers. Acta Paediatr. 2003;92(3):379–385. [PubMed] [Google Scholar]
- Goldfield G.S., Murray M., Maras D., et al. Screen time is associated with depressive symptomatology among obese adolescents: a HEARTY study. Eur. J. Pediatr. 2016;175:909–919. doi: 10.1007/s00431-016-2720-z. [DOI] [PubMed] [Google Scholar]
- Gomersall J.C., Slack-Smith L., Kilpatrick N., Muthu M.S., Riggs E. Interventions with pregnant women, new mothers and other primary caregivers for preventing early childhood caries. Cochrane Database Syst. Rev. 2024;5(5) doi: 10.1002/14651858.CD012155.pub3. Cd012155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouin K., Murphy K., Shah P.S. Effects of cocaine use during pregnancy on low birthweight and preterm birth: systematic review and metaanalyses. Am. J. Obstet. Gynecol. 2011;204(4) doi: 10.1016/j.ajog.2010.11.013. 340.e1-12. [DOI] [PubMed] [Google Scholar]
- Gurka K.K., Burris H.H., Ciciolla L., Coles C.D., Massey S.H., Newman S., Rajagopalan V., Smith L.M., Zilverstand A., Bandoli G. HBCD pregnancy exposures including substances workgroup. Assessment of maternal health and behavior during pregnancy in the HEALthy Brain and Child Development Study: Rationale and approach. Dev. Cogn. Neurosci. 2024 [Google Scholar]
- Hagan J.F., Shaw J.S., Duncan P.M. Bright futures: Guidelines for health supervision of infants, children, and adolescents: Pocket guide. (No Title). 2017;
- Hager E.R., Quigg A.M., Black M.M., et al. Development and validity of a 2-item screen to identify families at risk for food insecurity. Pediatrics. 2010;126(1):e26–e32. doi: 10.1542/peds.2009-3146. [DOI] [PubMed] [Google Scholar]
- Hammrich C.M., Götz S., Daseking M., Weyers S. Does the association between preschool media use and language difficulties at school entry vary by first language of the child and parental education? Children. 2023;10(12):1848. doi: 10.3390/children10121848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamułka J., Zielińska M.A., Chądzyńska K. The combined effects of alcohol and tobacco use during pregnancy on birth outcomes. Rocz. Panstw. Zakl. Hig. 2018;69(1):45–54. [PubMed] [Google Scholar]
- Hiscock H., Bayer J.K., Hampton A., Ukoumunne O.C., Wake M. Long-term mother and child mental health effects of a population-based infant sleep intervention: cluster-randomized, controlled trial. Pediatrics. 2008;122(3):e621–e627. doi: 10.1542/peds.2007-3783. [DOI] [PubMed] [Google Scholar]
- Hutton J.S., Dudley J., Horowitz-Kraus T., DeWitt T., Holland S.K. Associations between screen-based media use and brain white matter integrity in preschool-aged children. JAMA Pediatr. 2020;174(1) doi: 10.1001/jamapediatrics.2019.3869. e193869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton J.S., Huang G., Sahay R.D., DeWitt T., Ittenbach R.F. A novel, composite measure of screen-based media use in young children (ScreenQ) and associations with parenting practices and cognitive abilities. Pediatr. Res. 2020;87(7):1211–1218. doi: 10.1038/s41390-020-0765-1. [DOI] [PubMed] [Google Scholar]
- Jackson L., Fallon V., Harrold J.A., De Pascalis L. Psychosocial predictors of post-natal anxiety and depression: Using Structural Equation Modelling to investigate the relationship between pressure to breastfeed, health care professional support, post-natal guilt and shame, and post-natal anxiety and depression within an infant feeding context. Matern Child Nutr. 2024;20(1) doi: 10.1111/mcn.13558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S.S., Vaughan A.S., Harrington K., et al. US County–Level Variation in Preterm Birth Rates, 2007-2019. JAMA Netw. Open. 2023;6(12) doi: 10.1001/jamanetworkopen.2023.46864. e2346864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinstein R.N., Jones L.A., Hullett S., et al. Refractive error and ethnicity in children. Arch. Ophthalmol. 2003;121(8):1141–1147. doi: 10.1001/archopht.121.8.1141. [DOI] [PubMed] [Google Scholar]
- Korth C.X., Keim S.A., Crerand C.E., Jackson J.L. New Mothers’ Perceptions of Pressure to Breastfeed. MCN Am. J. Matern. Child Nurs. 2022;47(3):160. doi: 10.1097/NMC.0000000000000814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai J.S., Blackwell C.K., Tucker C.A., Jensen S.E., Cella D. Measuring PROMIS® physical activity and sleep problems in early childhood. J. Pedia Psychol. 2022;47(5):534–546. doi: 10.1093/jpepsy/jsac028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le H.N.D., Mensah F., Eadie P., et al. Health-related quality of life of children with low language from early childhood to adolescence: results from an Australian longitudinal population-based study. J. Child Psychol. Psychiatry. 2021;62(3):349–356. doi: 10.1111/jcpp.13277. [DOI] [PubMed] [Google Scholar]
- Li N., Yolton K., Lanphear B.P., Chen A., Kalkwarf H.J., Braun J.M. Impact of early-life weight status on cognitive abilities in children. Obesity (Silver Spring) 2018;26(6):1088–1095. doi: 10.1002/oby.22192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockyer F., McCann S., Moore S.E. Breast milk micronutrients and infant neurodevelopmental outcomes: a systematic review. Nutrients. 2021;13(11):3848. doi: 10.3390/nu13113848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokhandwala S., Spencer R.M.C. Relations between sleep patterns early in life and brain development: a review. Dev. Cogn. Neurosci. 2022;56 doi: 10.1016/j.dcn.2022.101130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund I.O., Ystrom E. Prenatal alcohol exposure and child sleep problems: a family-based quasi-experimental study. JCPP Adv. 2022;2(4) doi: 10.1002/jcv2.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madigan S., Browne D., Racine N., Mori C., Tough S. Association between screen time and children’s performance on a developmental screening test. JAMA Pediatr. 2019;173(3):244–250. doi: 10.1001/jamapediatrics.2018.5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansukhani S.A., Bothun C.E., Xu T.T., et al. Incidence and distribution of ocular disorders in the first year of life. J. aapos. 2023;27(2):80.e1–80.e5. doi: 10.1016/j.jaapos.2023.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall N.E., Abrams B., Barbour L.A., et al. The importance of nutrition in pregnancy and lactation: lifelong consequences. Am. J. Obstet. Gynecol. 2022;226(5):607–632. doi: 10.1016/j.ajog.2021.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattei D., Pietrobelli A. Micronutrients and brain development. Curr. Nutr. Rep. 2019;8:99–107. doi: 10.1007/s13668-019-0268-z. [DOI] [PubMed] [Google Scholar]
- McDermott C.L., Hilton K., Park A.T., et al. Early life stress is associated with earlier emergence of permanent molars. Proc. Natl. Acad. Sci. USA. 2021;118(24) doi: 10.1073/pnas.2105304118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlone L., Mactier H., Weaver L.T. Drug misuse in pregnancy: losing sight of the baby? Arch. Dis. Child. 2009;94(9):708–712. doi: 10.1136/adc.2008.156851. [DOI] [PubMed] [Google Scholar]
- McMath A.L., Iwinski S., Shen S., Bost K.F., Donovan S.M., Khan N.A. Adherence to screen time and physical activity guidelines is associated with executive function in US toddlers participating in the STRONG Kids 2 birth cohort study. J. Pediatr. 2023;252:22–30. doi: 10.1016/j.jpeds.2022.08.026. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melinder A., Konijnenberg C., Sarfi M. Deviant smooth pursuit in preschool children exposed prenatally to methadone or buprenorphine and tobacco affects integrative visuomotor capabilities. Addiction. 2013;108(12):2175–2182. doi: 10.1111/add.12267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindell J.A., Gould R.A., Tikotzy L., Leichman E.S., Walters R.M. Norm-referenced scoring system for the Brief Infant Sleep Questionnaire - Revised (BISQ-R) Sleep. Med. 2019;63:106–114. doi: 10.1016/j.sleep.2019.05.010. [DOI] [PubMed] [Google Scholar]
- Misra N., Khanna R.C., Mettla A.L., Marmamula S., Keeffe J.E. Agreement and diagnostic accuracy of vision screening in preschool children between vision technicians and spot vision screener. Indian J. Ophthalmol. 2021;69(1):117–121. doi: 10.4103/ijo.IJO_1740_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon R.Y., Carlin R.F., Hand I. Syndrome TFoSID, Fetus tCo, Newborn. Evidence base for 2022 updated recommendations for a safe infant sleeping environment to reduce the risk of sleep-related infant deaths. Pediatrics. 2022;150(1) doi: 10.1542/peds.2022-057991. [DOI] [PubMed] [Google Scholar]
- Morales-Muñoz I., Nolvi S., Virta M., Karlsson H., Paavonen E.J., Karlsson L. The longitudinal associations between temperament and sleep during the first year of life. Infant Behav. Dev. 2020;61 doi: 10.1016/j.infbeh.2020.101485. [DOI] [PubMed] [Google Scholar]
- Morton S.U., Leyshon B.J., Tamilia E., et al. A role for data science in precision nutrition and early brain development. Front. Psychiatry. 2022;13 doi: 10.3389/fpsyt.2022.892259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Nordhorn J., Neumann K., Keil T., Willich S.N., Binting S. State-level trends in sudden unexpected infant death and immunization in the United States: an ecological study. BMC Pediatr. 2021;21(1):274. doi: 10.1186/s12887-021-02733-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ncube B.L., Perry A., Weiss J.A. The quality of life of children with severe developmental disabilities. J. Intellect. Disabil. Res. 2018;62(3):237–244. doi: 10.1111/jir.12460. [DOI] [PubMed] [Google Scholar]
- Nishimura M., Wong A., Dimaras H., Maurer D. Feasibility of a school-based vision screening program to detect undiagnosed visual problems in kindergarten children in Ontario. Cmaj. 2020;192(29):E822–E831. doi: 10.1503/cmaj.191085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan F., O'Callaghan M., Scott J.G., Najman J., Al Mamun A. Effect of maternal smoking in pregnancy and childhood on child and adolescent sleep outcomes to 21 years: a birth cohort study. BMC Pediatr. 2019;19(1):70. doi: 10.1186/s12887-019-1439-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opel D.J., Taylor J.A., Zhou C., Catz S., Myaing M., Mangione-Smith R. The relationship between parent attitudes about childhood vaccines survey scores and future child immunization status: a validation study. JAMA Pediatr. 2013;167(11):1065–1071. doi: 10.1001/jamapediatrics.2013.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald T.K., Rumbold A.R., Kedzior S.G., Moore V.M. Psychological impacts of “screen time” and “green time” for children and adolescents: a systematic scoping review. PLoS One. 2020;15(9) doi: 10.1371/journal.pone.0237725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottolini K.M., Andescavage N., Keller S., Limperopoulos C. Nutrition and the developing brain: the road to optimizing early neurodevelopment: a systematic review. Pediatr. Res. 2020;87(2):194–201. doi: 10.1038/s41390-019-0508-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phyo A.Z.Z., Freak-Poli R., Craig H., et al. Quality of life and mortality in the general population: a systematic review and meta-analysis. BMC Public Health. 2020;20(1):1596. doi: 10.1186/s12889-020-09639-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pini N., Fifer W.P., Oh J., Nebeker C., Croff J.M., Smith B.A. Remote Data collection of infant activity and sleep patterns via wearable sensors in the HEALthy Brain and Child Development Study (HBCD) Dev. Cogn. Neurosci. 2024 [Google Scholar]
- Pizzol D., Tudor F., Racalbuto V., Bertoldo A., Veronese N., Smith L. Systematic review and meta-analysis found that malnutrition was associated with poor cognitive development. Acta Paediatr. 2021;110(10):2704–2710. doi: 10.1111/apa.15964. [DOI] [PubMed] [Google Scholar]
- Prevention UCfDCa. By the Numbers. 2023. 〈https://safetosleep.nichd.nih.gov/about/by-the-numbers〉.
- Redondo-Tébar A., Ruiz-Hermosa A., Martínez-Vizcaíno V., Martín-Espinosa N.M., Notario-Pacheco B., Sánchez-López M. Health-related quality of life in developmental coordination disorder and typical developing children. Res. Dev. Disabil. 2021;119 doi: 10.1016/j.ridd.2021.104087. [DOI] [PubMed] [Google Scholar]
- Repka M.X., Friedman D.S., Katz J., Ibironke J., Giordano L., Tielsch J.M. The prevalence of ocular structural disorders and nystagmus among preschool-aged children. J. aapos. 2012;16(2):182–184. doi: 10.1016/j.jaapos.2011.12.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan M.A., Murray D.M., Dempsey E.M., Mathieson S.R., Livingstone V., Boylan G.B. Neurodevelopmental outcome of low-risk moderate to late preterm infants at 18 months. Front. Pediatr. 2023;11 doi: 10.3389/fped.2023.1256872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeh A. A brief screening questionnaire for infant sleep problems: validation and findings for an Internet sample. Pediatrics. 2004;113(6):e570–e577. doi: 10.1542/peds.113.6.e570. [DOI] [PubMed] [Google Scholar]
- Sadeh A., Mindell J.A., Luedtke K., Wiegand B. Sleep and sleep ecology in the first 3 years: a web-based study. J. Sleep. Res. 2009;18(1):60–73. doi: 10.1111/j.1365-2869.2008.00699.x. [DOI] [PubMed] [Google Scholar]
- Schneider N., Hartweg M., O’Regan J., et al. Impact of a nutrient formulation on longitudinal myelination, cognition, and behavior from birth to 2 years: a randomized clinical trial. Nutrients. 2023;15(20):4439. doi: 10.3390/nu15204439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzer C., Grafe N., Hiemisch A., Kiess W., Poulain T. Associations of media use and early childhood development: cross-sectional findings from the LIFE Child study. Pediatr. Res. 2022;91(1):247–253. doi: 10.1038/s41390-021-01433-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastiani G., Borrás-Novell C., Casanova M.A., et al. The effects of alcohol and drugs of abuse on maternal nutritional profile during pregnancy. Nutrients. 2018;10(8) doi: 10.3390/nu10081008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw R.J., Givrad S., Poe C., Loi E.C., Hoge M.K., Scala M. Neurodevelopmental, mental health, and parenting issues in preterm infants. Children. 2023;10(9):1565. doi: 10.3390/children10091565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shawcroft J., Blake H., Gonzalez A., Coyne S.M. Structures for screens: longitudinal associations between parental media rules and problematic media use in early childhood. 2023; [DOI] [PMC free article] [PubMed]
- Spiteri Cornish K., Hrabovsky M., Scott N.W., Myerscough E., Reddy A.R. The short- and long-term effects on the visual system of children following exposure to maternal substance misuse in pregnancy. Am. J. Ophthalmol. 2013;156(1):190–194. doi: 10.1016/j.ajo.2013.02.004. [DOI] [PubMed] [Google Scholar]
- Stone K.C., LaGasse L.L., Lester B.M., et al. Sleep problems in children with prenatal substance exposure: the Maternal Lifestyle study. Arch. Pedia Adolesc. Med. 2010;164(5):452–456. doi: 10.1001/archpediatrics.2010.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchdev P., Boivin M., Forsyth B., Georgieff M., Guerrant R., Nelson 3rd C. Assessment of neurodevelopment, nutrition, and inflammation from fetal life to adolescence in low-resource settings. Pediatrics. 2017;139(Suppl 1):S23–S37. doi: 10.1542/peds.2016-2828E. [DOI] [PubMed] [Google Scholar]
- Sun Y., Molitor J., Benmarhnia T., et al. Association between urban green space and postpartum depression, and the role of physical activity: a retrospective cohort study in Southern California. Lancet Reg. Health–Am. 2023;21 doi: 10.1016/j.lana.2023.100462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundqvist A., Barr R., Heimann M., Birberg-Thornberg U., Koch F.S. A longitudinal study of the relationship between children's exposure to screen media and vocabulary development. Acta Paediatr. 2023 doi: 10.1111/apa.17047. [DOI] [PubMed] [Google Scholar]
- Suryawan A., Jalaludin M.Y., Poh B.K., et al. Malnutrition in early life and its neurodevelopmental and cognitive consequences: a scoping review. Nutr. Res. Rev. 2022;35(1):136–149. doi: 10.1017/s0954422421000159. [DOI] [PubMed] [Google Scholar]
- Swit C.S., Coyne S.M., Shawcroft J., et al. Problematic media use in early childhood: the role of parent-child relationships and parental wellbeing in families in New Zealand and the United States. J. Child. Media. 2023;17(4):443–466. doi: 10.1080/17482798.2023.2230321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorsteinsdottir S., Olsen A., Olafsdottir A.S. Fussy eating among children and their parents: associations in parent-child dyads, in a sample of children with and without neurodevelopmental disorders. Nutrients. 2021;13(7) doi: 10.3390/nu13072196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiako M.J.N., McCarthy C., Meisel Z.F., Elovitz M.A., Burris H.H., South E. Association between low urban neighborhood greenness and hypertensive disorders of pregnancy. Am. J. Perinatol. 2021 doi: 10.1055/s-0041-1733786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiako M.J.N., South E., Shannon M.M., et al. Urban residential tree canopy and perceived stress among pregnant women. Environ. Res. 2021;201 doi: 10.1016/j.envres.2021.111620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twenge J.M., Joiner T.E., Rogers M.L., Martin G.N. Increases in depressive symptoms, suicide-related outcomes, and suicide rates among US adolescents after 2010 and links to increased new media screen time. Clin. Psychol. Sci. 2018;6(1):3–17. [Google Scholar]
- Umer A., Watson E., Lilly C., et al. Substance exposure and adverse neonatal outcomes: a population-based cohort study. J. Pediatr. 2023;256:70–76. doi: 10.1016/j.jpeds.2022.11.040. [DOI] [PubMed] [Google Scholar]
- Varni J.W., Seid M., Kurtin P.S. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care. 2001;39(8):800–812. doi: 10.1097/00005650-200108000-00006. [DOI] [PubMed] [Google Scholar]
- Walhovd K.B., Bjørnebekk A., Haabrekke K., et al. Child neuroanatomical, neurocognitive, and visual acuity outcomes with maternal opioid and polysubstance detoxification. Pedia Neurol. 2015;52(3):326–332. doi: 10.1016/j.pediatrneurol.2014.11.008. e1-3. [DOI] [PubMed] [Google Scholar]
- Webster E.K., Martin C.K., Staiano A.E. Fundamental motor skills, screen-time, and physical activity in preschoolers. J. Sport Health Sci. 2019;8(2):114–121. doi: 10.1016/j.jshs.2018.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen E., Davis J.M. The immediate and long-term effects of prenatal opioid exposure. Front Pedia. 2022;10 doi: 10.3389/fped.2022.1039055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Liu Q., Cao S., et al. A meta-analysis of selective serotonin reuptake inhibitors (SSRIs) use during prenatal depression and risk of low birth weight and small for gestational age. J. Affect Disord. 2018;241:563–570. doi: 10.1016/j.jad.2018.08.061. [DOI] [PubMed] [Google Scholar]
- Zimmerman F.J., Christakis D.A., Meltzoff A.N. Associations between media viewing and language development in children under age 2 years. J. Pediatr. 2007;151(4):364–368. doi: 10.1016/j.jpeds.2007.04.071. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material