Abstract
OBJECTIVE:
To characterize the outcomes of children with community acquired pneumonia (CAP) across 41 United States hospitals and evaluate factors associated with potentially unnecessary admissions.
METHODS:
We performed a cross-sectional study of patients with CAP from 41 United States pediatric hospitals and evaluated clinical outcomes using a composite ordinal severity outcome: mild-discharged (discharged from the emergency department), mild-admitted (hospitalized without other interventions), moderate (provision of intravenous fluids, supplemental oxygen, broadening of antibiotics, complicated pneumonia, and presumed sepsis) or severe (ICU, positive-pressure ventilation, vasoactive infusion, chest drainage, extracorporeal membrane oxygenation, severe sepsis, or death). Our primary outcome was potentially unnecessary admissions (ie, mild-admitted). Among mild-discharged and mild-admitted patients, we constructed a generalized linear mixed model for mild-admitted severity and assessed the role of fixed (demographics and clinical testing) and random effects (institution) on this outcome.
RESULTS:
Of 125 180 children, 68.3% were classified as mild-discharged, 6.6% as mild-admitted, 20.6% as moderate and 4.5% as severe. Among admitted patients (n = 39 692), 8321 (21%) were in the mild-admitted group, with substantial variability in this group across hospitals (median 19.1%, interquartile range 12.8%–28.4%). In generalized linear mixed models comparing mild-admitted and mild-discharge severity groups, hospital had the greatest contribution to model variability compared to all other variables.
CONCLUSIONS:
One in 5 hospitalized children with CAP do not receive significant interventions. Among patients with mild disease, institutional variation is the most important contributor to predict potentially unnecessary admissions. Improved prognostic tools are needed to reduce potentially unnecessary hospitalization of children with CAP.
Pediatric community-acquired pneumonia (CAP) is among the most common infectious diseases worldwide. In resource-rich countries, pediatric CAP occurs in ~1.5 per 1000 children annually.1 CAP is the fifth most common and second most costly reason for pediatric hospitalization in the United States.2 Approximately one-half of children <5 years of age with CAP are hospitalized.3
Studies of pediatric CAP often use outcomes such as hospital length of stay (LOS) or revisit to medical care that are multifaceted and lack granularity and objectivity.4–6 Hospitalization, for example, represents individual and institutional norms, in addition to clinical features, and may overestimate disease severity among children with CAP.7 However, factors influencing LOS may be more related to institutional practice patterns or social determinants and unrelated to severity.8–10 Without objective, clinically meaningful outcome measures, studies of pediatric CAP can be difficult to interpret, limiting their generalizability and applicability. As such, the 2011 Infectious Diseases Society of America or Pediatric Infectious Diseases Society pediatric CAP guideline emphasizes the need for objective outcome measures that can be “standardized, measured, and compared” as a key area for future research.11
The use of a standardized, interventions-based, outcome measure for pediatric CAP may provide opportunities to better describe and identify groups that require further investigation. In particular, some children who are admitted with CAP, particularly those not receiving any major medical interventions, may not substantially benefit from hospitalization. In a single-center prospective cohort study of 1142 children presenting to the emergency department (ED) with suspected CAP, 40% of those hospitalized were discharged within 24 hours.12 Of those, 72% did not require oxygen, 85% did not require intravenous fluids, and none required more invasive respiratory support. Hospitalization may, therefore, be unwarranted for many children, exposing them to nosocomial infections, unnecessary testing and treatment, medical errors, time lost from school or work, anxiety, and cost.13,14 Given the risks associated with hospitalization, greater efforts are needed to identify potentially unnecessary hospitalizations for CAP in which children require few, or no, meaningful interventions. The use of an objective, outcomes-based severity measure may be beneficial to identify children who do not require admission and are amenable to earlier deescalation of care and less testing and interventions.
In this study, we sought to describe outcomes of pediatric CAP outcomes among children admitted to pediatric hospitals and to evaluate the role of institutional variation of care among patients with CAP having potentially unnecessary hospitalizations, defined as hospitalizations without the receipt of major medical interventions.
METHODS
Data Source
We performed a multicenter cross-sectional study using data abstracted from the Pediatric Health Information System (PHIS), an administrative database that contains ED, inpatient, ambulatory surgery, and observation data from geographically diverse children’s hospitals in the United States affiliated with the Children’s Hospital Association (Overland Park, KS). Data are deidentified, but unique patient identifiers facilitate longitudinal tracking.15 The Children’s Hospital Association and member hospitals jointly ensure data quality and integrity.16 We included 41 hospitals with complete data during the studied timeframe. The study was designated as exempt by our institutional review board.
Patient Inclusion
We included children 90 days to 18 years of age with a diagnosis of CAP who presented to a PHIS hospital ED from January 1, 2016 to March 31, 2020. Consistent with previous studies of pediatric CAP, we used 90 days as the lower limit of age to avoid including neonatal pneumonia, which may be because of distinct organisms and have a different management approach compared with older infants.12,17 For similar reasons, we did not include direct admissions to the hospital. We identified patients with a diagnosis of CAP using a previously validated algorithm of primary International Classification of Disease (ICD) discharge diagnosis codes,18 which we cross-walked to ICD, 10th Revision, Clinical Modification codes (ICD-10-CM) using general equivalence mappings19 (Supplementary Table 4). The validity of using general equivalence mappings to convert diagnosis codes for pneumonia has been previously demonstrated in pneumonia research with high accuracy.20 The present list of diagnosis codes has been previously used in research on pediatric complicated pneumonia21 and chest radiography use.22 We excluded clinic, ambulatory surgery, or ‘other’ encounters, and patients with complex chronic conditions (CCC). CCC were defined as medical conditions expected to last >12 months and to require specialty pediatric care and/or hospitalization in a tertiary care center by using encounter-level diagnoses.23 The CCC algorithm uses all available diagnosis and procedure codes to identify those with medical complexity and includes conditions such as tracheostomy status and cystic fibrosis. Because the goal of our investigation was to evaluate CAP outcomes in mostly healthy children, we excluded patients with CCCs given the distinct risk factors, predisposition, and microbiology of pneumonia in many of these patients.24
Data Acquisition
For each encounter, demographics included age at admission, sex, race, ethnicity, day of presentation, geographic region by United States census region, and primary payer status. Race and ethnicity was classified as a composite variable of White non-Hispanic, Black non-Hispanic, Hispanic, and Asian (including multiracial).25,26 Day of presentation was classified as end of week (Friday and Saturday) versus all other days. We used this definition to identify potential differences in care related to primary care physician follow up. Diagnostic testing included performance of blood culture, complete blood count, C-reactive protein, procalcitonin, respiratory pathogen panel, respiratory culture, chest radiography, chest ultrasound, or chest computed tomography on the first day (defined as day 0 or 1 within PHIS) of hospital encounter. Procalcitonin was assessed using orders for calcitonin (which included procalcitonin). Medication information included use of intravenous fluids (including electrolyte solutions with or without dextrose, Ringers’ lactate, and hyperalimentation) and use and timing (ie, day of administration) of antibiotics. Antibiotic prescribing data in PHIS is only available for orders placed during hospitalization (ED or inpatient) and not for outpatient visits. Interventions examined included endotracheal intubation, oxygen administration, positive-pressure ventilation including noninvasive (ie, high-flow nasal cannula [HFNC], continuous positive airway pressure, or bilevel positive airway pressure) and invasive (ie, mechanical ventilation), and extracorporeal membrane oxygenation. Additionally, we acquired diagnosis codes, procedure codes, disposition status (admission, ICU admission, or ED discharge), revisits, and in-hospital mortality.
Severity Outcome
We classified encounters using an ordinal pneumonia severity measure derived from previous prospective work.12,17 Severity tiers were labeled as mild-discharged, mild-admitted, moderate, and severe (Table 1).12 Mild-discharged patients were discharged from the ED and did not have any readmission within 7 days. Mild-admitted patients were admitted on the index visit or a revisit within 7 days but did not have any additional indicators of severity contained within the moderate or severe groups. Patients with moderate disease were hospitalized and required intravenous fluid, oxygen, broadening of antibiotics, had a complicated pneumonia, or presumed sepsis. Patients with severe disease required the ICU, positive-pressure ventilation, vasoactive infusion, chest drainage, extracorporeal membrane oxygenation, had severe sepsis, or in-hospital mortality. We used discharge ICD diagnosis codes to identify patients with complicated CAP, mechanical ventilation, systemic inflammatory response syndrome or sepsis, and severe sepsis.27 HFNC has substantial variability in application, lack of clear indications for use, and limited delivery of positive pressure to the lower airways; therefore, we did not include HFNC as a positive-pressure modality in our outcome measure. Additionally, it was not used in the original risk stratification criteria which we adapted here.12,17 Diagnosis and procedure codes used to identify interventions and clinical testing are provided in Supplemental Tables 4–6. We also evaluated revisits within 7 days of the index visit. For final category designation, encounters were assigned to the highest level of severity for which criteria were met.
TABLE 1.
Application of the Ordinal Pneumonia Severity Measure to PHIS Database
| Criteria | Criteria Adaptation to PHIS |
|---|---|
| Mild-Discharged | |
| Discharged from ED | No admission, and No revisit leading to readmission <7 d from index visit |
| Mild-Admitted | |
| Hospitalized but not meeting any further criteria | Admitted from index visit, or Admitted from revisit and No additional criteria met from the moderate or severe groups |
| Moderate | |
| Hospitalized with bolus or IV fluids | Hospitalized, and Provision of any IV fluids |
| Hospitalized with oxygen | Hospitalized, and Provision of supplemental oxygen |
| Hospitalized with broadening of antibiotics from an aminopenicillin | Hospitalized, and Provision of an aminopenicillin on day 1 and Provision of any other antibiotic through the parenteral or oral route starting after day 1 OR Hospitalized, and Provision of an aminopenicillin and another oral/intravenous antibiotic on day 1 and Discontinuation of the aminopenicillin after day 1 with continuation of another oral or intravenous antibiotic after day 1 |
| Hospitalized with a complicated pneumonia | Hospitalized, and Presence of an additional diagnosis code for that encounter (see Supplemental Table 4) |
| Hospitalized with presumed sepsis | Hospitalized, and Presence of a SIRS or sepsis diagnosis code (see Supplemental Table 4) Provision of any IV fluids |
| Severe | |
| ICU admission | Dedicated ICU flag within PHIS |
| Positive-pressure ventilation | Dedicated mechanical ventilation flag in PHIS, OR Procedure of endotracheal intubation, OR Provision of CPAP or BiPAP, OR Concomitant ICD-10 code “Z99.11” (Dependence on ventilator status) |
| Vasoactive infusion | Provision of any of the following parenteral medications: epinephrine, norepinephrine, dopamine, milrinone, dobutamine, phenylephrine, or ephedrine |
| Chest drainage | Performance of any drainage procedure of the chest, lung, pleura, bronchus or pericardium (see Supplemental Table 5) |
| ECMO | Dedicated ECMO flag within PHIS |
| Severe sepsis | Presence of an additional diagnosis code for that encounter (see Supplemental Table 4) |
| Death | Dedicated mortality flag within PHIS |
CPAP, continuous positive airway pressure; BiPAP, bilevel positive airway pressure; ECMO, extracorporeal membrane oxygenation; IV, intravenous.
Statistical Analysis
As our aim was to identify children potentially not requiring hospitalization, our primary outcome of interest was the mild-admitted subgroup, representing children hospitalized but not receiving any major medical interventions or meeting moderate or severe criteria. We evaluated the proportion of patients in each severity stratum by hospital.
To evaluate hospital-level effects associated with variation in care for patients with mild-admitted CAP among patients with mild disease severity, we limited our analysis to patients with mild-discharged and mild-admitted severity. We constructed a generalized linear mixed model for an outcome of mild-admitted disease, considering clinical testing (including blood testing and chest radiography) and demographics (such as payor status and age) with hospital as a random effect. From the random-effects model, we calculated χ2 statistics for the fixed and random effects to identify their relative importance to the model. This model was fit in 3 ways: first, including clinical tests as individual binary variables (performance of procalcitonin, C-reactive protein, blood culture, payor status, respiratory viral panel, chest radiography, and complete blood count); second, including a single binary variable representing the performance of any of the tests; and third, including the count of the number of tests performed. For the model with the best fit (defined as the maximal negative log-likelihood ratio), we evaluated the contribution of each hospitals’ individual intercept against a fixed intercept of the logistic model. Analyses were performed by using the lme4 package in R, version 4.1.0 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
We identified 125 180 patients during the 51 month period (Fig 1). The median age was 3.8 years (interquartile range [IQR]; 1.9–7.0 years), and 52% of patients were males (Table 2). Most patients (82%) received a chest radiograph, and 19% had a blood culture obtained. When limited to hospitalized patients, 87% received antibiotics, and the median LOS was 3 days (IQR 1–4 days). Antibiotic use among admitted patients by principal diagnosis is provided in Supplemental Table 7.
FIGURE 1.
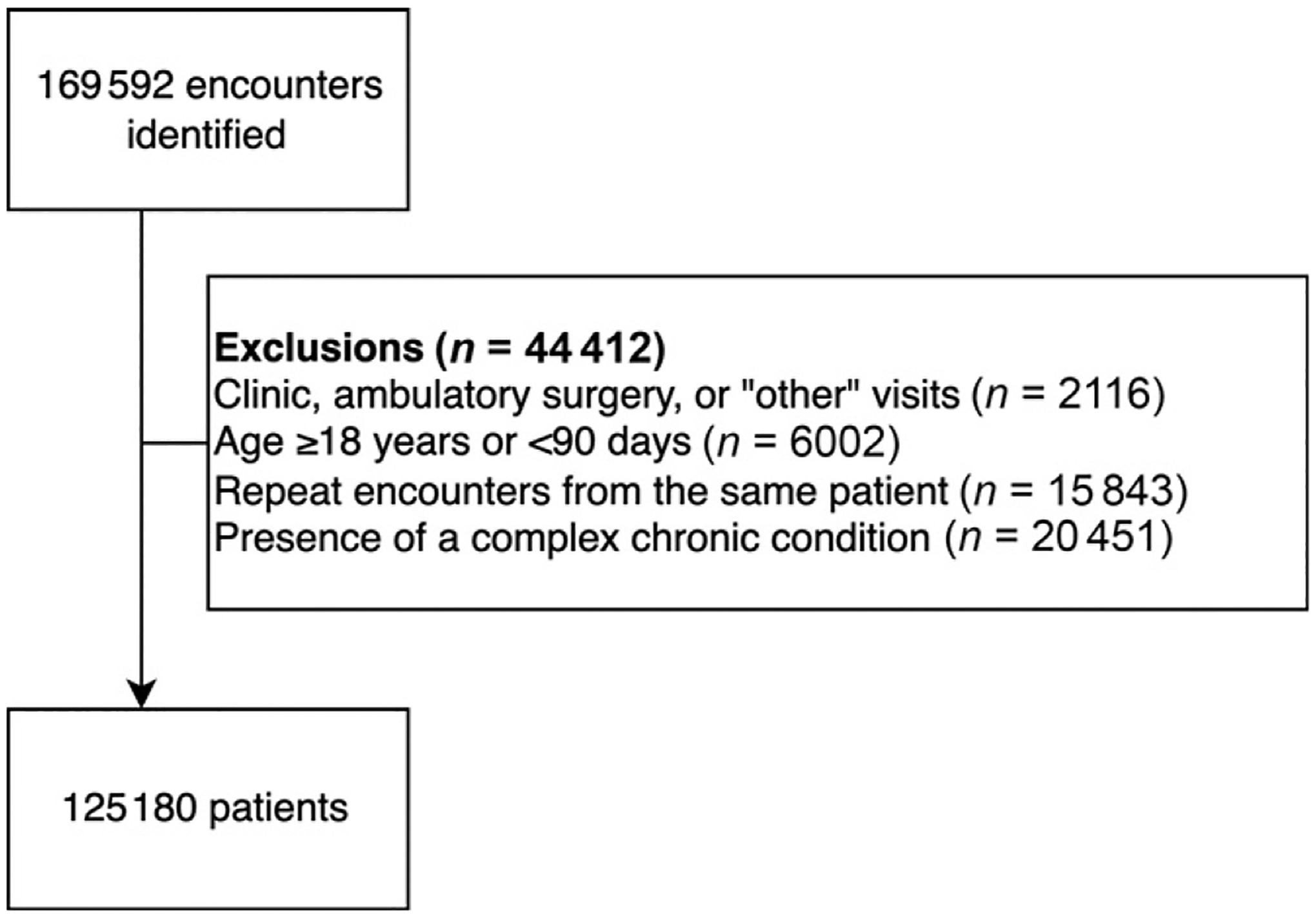
Flow diagram of study cohort.
TABLE 2.
Demographics and clinical characteristics of study cohort
| Variable | Number (%) or Median (IQR) N = 125 180 |
|---|---|
| Demographics | |
| Age, years | 3.8 (1.9–7.0) |
| Male sex | 65 402 (52.2) |
| Race and ethnicity | |
| Non-Hispanic White | 44 170 (35.3) |
| Non-Hispanic Black | 25 092 (20.0) |
| Hispanic | 35 477 (28.3) |
| All others | 17 271 (13.8) |
| Missing | 3170 (2.5) |
| Geographic region | |
| Midwest | 29 758 (23.8) |
| Northeast | 14 266 (11.4) |
| South | 48 810 (39.0) |
| West | 32 346 (25.8) |
| Primary source of payment | |
| Public | 74 539 (59.5) |
| Private | 41 834 (33.4) |
| Other or unknown | 8807 (7.0) |
| Friday or Saturday encounter | 33 872 (27.1) |
| Diagnostic testing | |
| Chest radiography | 102 875 (82.2) |
| Chest computed tomography | 1083 (0.9) |
| Chest ultrasonography | 1862 (1.5) |
| Blood culture | 23 458 (18.7) |
| Complete blood count | 34 203 (27.3) |
| C-reactive protein | 18 575 (14.8) |
| Procalcitonin | 2974 (2.4) |
| Viral testing | 21 390 (17.1) |
| Respiratory culture | 490 (0.4) |
| Treatment | |
| Any intravenous fluid | 30 042 (24.0) |
| Intravenous fluid for ≥2 d | 11 979 (9.6) |
| Any supplementary oxygen | 15 450 (12.3) |
| Supplementary oxygen for ≥2 d | 1499 (7.4) |
| High flow nasal cannula | 1411 (1.1) |
| CPAP or BiPAP | 2901 (2.3) |
| Given antibioticsa | 65 062 (52.0) |
| Disposition | |
| Admitted on initial visit | 37 154 (29.7) |
| Admission length of stay, daysb | 3 (2–4) |
| ICU length of stay, daysc | 2 (1–3) |
| Any revisit | 7530 (6.0) |
| Revisit leading to admission | 2948 (2.4) |
Defined as ordering of oral or intravenous antibiotics at any time during encounter, not including antibiotics prescribed at discharge.
Among admitted patients, taking the longer admission from the index or revisit (where applicable) per encounter.
Among patients admitted to ICU, taking the longer admission from the index or revisit (where applicable) per encounter.
By using the ordinal severity outcome, 85 488 patients (68.3%) were classified as mild-discharged, 8321 (6.6%) as mild-admitted, 25 781 (20.6%) as moderate, and 5590 (4.5%) as severe. Demographics by severity tier are provided in Supplemental Table 8. Among patients with moderate CAP, 17 434 (67.6%) had 1 moderate criterion, 7550 (29.3%) had 2 criteria, 751 (2.9%) had 3, and 46 (0.2%) had 4. Of these, intravenous fluid use was the only defining criteria in 44.3%, followed by supplemental oxygen in 20.0% (Table 3).
TABLE 3.
Severity Outcomes of Included Patients With the Moderate and Severe Classification
| Criteria | N (% of all included patients) | Only Meeting Criteria Within Groupa |
|---|---|---|
| Moderate | ||
| Hospitalized with any intravenous fluid | 23 386 (18.7) | 11 422 (44.3) |
| Hospitalized with oxygen | 14741 (11.8) | 5148 (20.0) |
| Hospitalized with broadening of antibiotics from an aminopenicillin | 1760 (1.4) | 249 (1.0) |
| Hospitalized with a complicated pneumonia | 3909 (3.1) | 585 (2.3) |
| Hospitalized with presumed sepsis | 305 (0.2) | 30 (0.1) |
| Severe | ||
| ICU admission | 3973 (3.2) | 1695 (30.3) |
| Positive-pressure ventilation | 2901 (2.3) | 794 (14.2) |
| Vasoactive infusion | 458 (0.4) | 155 (2.7) |
| Chest drainage | 923 (0.7) | 595 (10.6) |
| ECMO | 10 (0.0) | 0 (0.0) |
| Severe sepsis | 85 (0.1) | 22 (0.4) |
| Death | 8 (0.0) | 0 (0.0) |
Encounters in these groups may satisfy more than one criterion. In the severe group, some encounters may also satisfy criteria in the moderate group.
This column represents the proportion of the population who had each outcome as the only outcome to classify them into the severity group, with proportions as a column percentage within the group.
Role of Institution in Potentially Unnecessary Hospitalizations
Twenty-one percent of hospitalized patients were classified with ‘mild-admitted’ severity. There was substantial variability in the proportion of children with mild-admitted CAP among the 41 PHIS hospitals (median 19.1%, IQR 12.8% to 28.4%; Fig 2). When evaluating the association of laboratory testing and demographic factors with mild-admitted CAP in the cohort limited to mild-admitted and mild-discharged patients, the largest amount of variability in the model was attributed to the random intercepts per hospital. The model including binary indicators for each individual test provided the best fit (smallest log-likelihood) (Fig 3). When inspecting this model, we identified the greatest random variability was in the intercept term, indicating that within-hospital variability had the greatest contribution toward mild-admitted CAP (Supplemental Fig 4). The model including the number of tests exhibited the same variation in the intercept term, but additionally exhibited substantial random variation across hospitals in the effect of the number of tests on the likelihood of mild-admitted CAP. For nearly all hospitals, more tests corresponded with a higher likelihood of admission, but the magnitude of this effect varied considerably.
FIGURE 2.
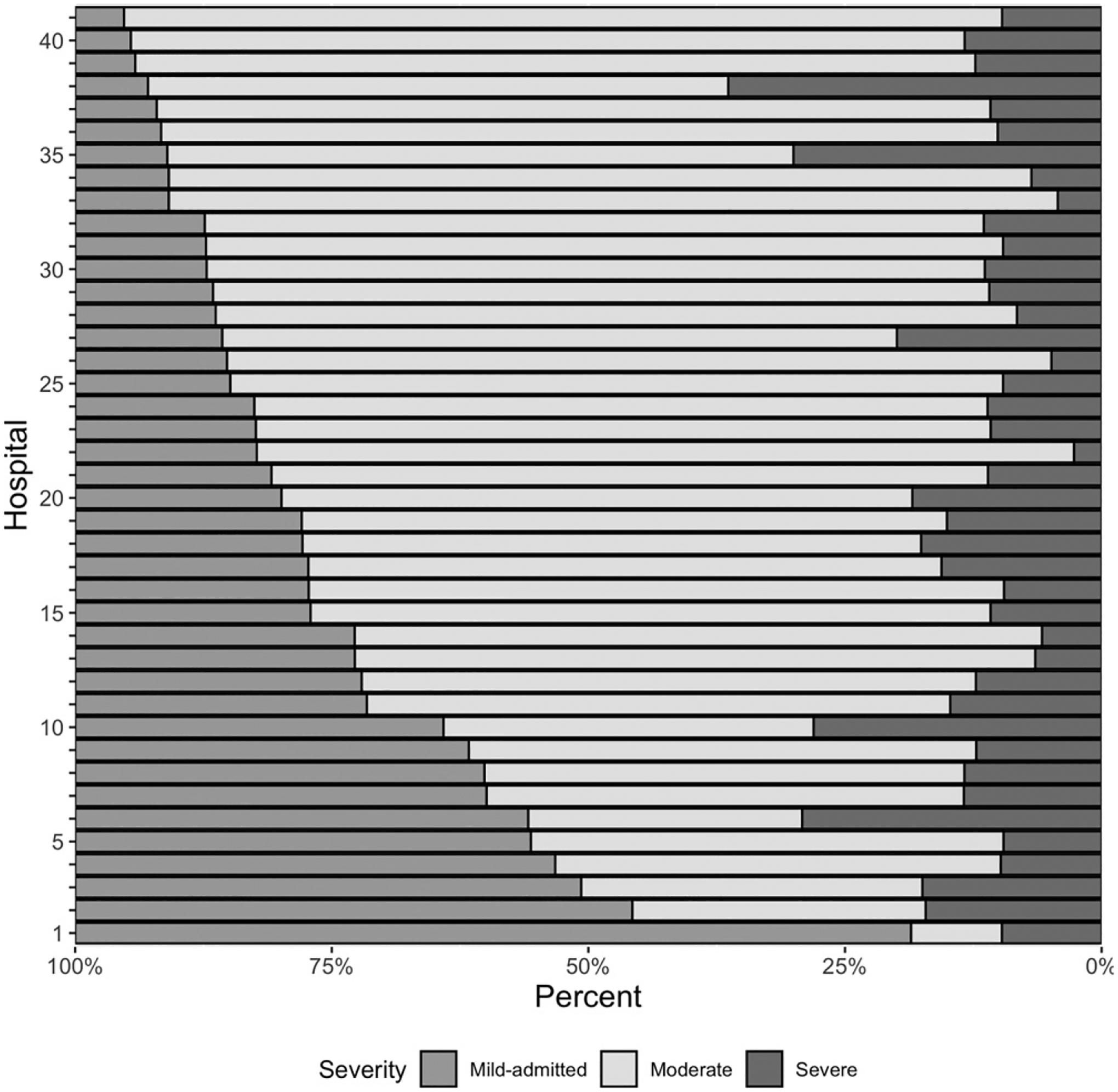
Institutional variability of disease severity classification among 41 included hospitals. Hospitals are ordered on the basis of the proportion of patients meeting “mild-admitted” severity criteria.
FIGURE 3.
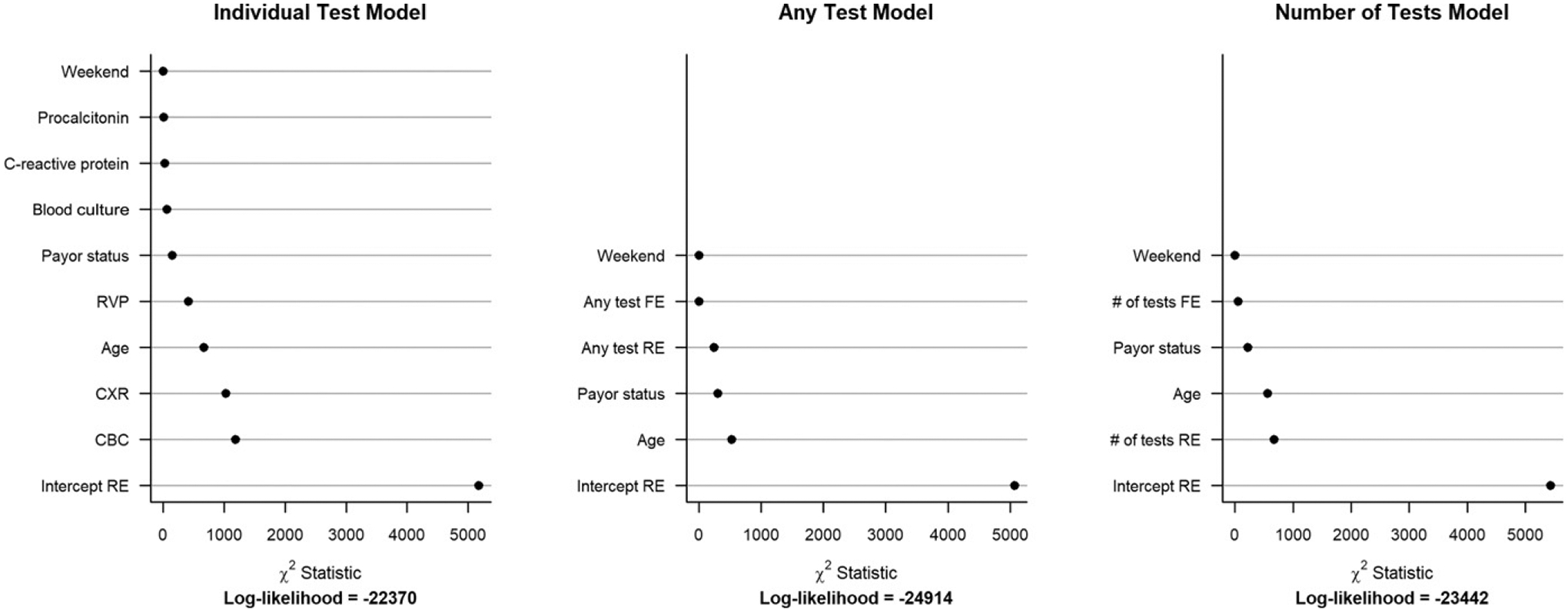
Relative importance of variables in random effects models (assessed by the χ2 test for fixed and random effects) which incorporate (A) individual tests, (B) a dichotomous variable of “any” test, and (C) an integer value of the number of effects among the cohort of patients with mild-admitted and mild-discharged disease severity. χ2: Relative importance of variables in mixed effects logistic regression models, as measured by χ2 test statistics. The models include as fixed effects either binary indicators for each test (“individual test” model), a binary variable indicating administration of any test (“any test” model), or the number of tests administered (“number of tests” model). FE, fixed effect; RE, random effect.
DISCUSSION
In this multicenter cross-sectional study of administrative data, we describe the distribution of severity among children with CAP presenting to pediatric hospitals and identify institutional variation in the prevalence of patients with mild-admitted disease. Most (70%) children presenting to the ED with CAP have mild disease. One in 5 (21%) hospitalized children met criteria for mild-admitted CAP, not receiving major medical interventions (eg, intravenous fluids, supplemental oxygen) or having diagnoses requiring hospitalization (eg, septic shock). Although concerns of low-value care may exist across the severity spectrum, it is likely that some in the mild-admitted CAP group may represent potentially unnecessary hospitalizations. Among admitted patients, institutional factors had the greatest contribution to our outcome of mild-admitted disease severity.
The distribution of disease severity described in this study is similar to other work evaluating pneumonia severity in children. In our study, 21% of admitted patients were classified in the mild-admitted category of our ordinal outcome. Although factors driving admission for this subgroup are likely multifaceted and include physical examination features, diagnostic testing results, or social factors, some of these hospitalizations may have been potentially unnecessary. Additionally, some admitted patients initially with mild disease may have progressed to more severe disease states during their hospitalization. In the previous single-center prospective study that first used the ordinal severity outcome, 62.9% of 1128 patients had mild disease, 32.9% developed moderate disease (equivalent to mild-admitted and moderate in this study), and 4.3% severe disease.12 Among hospitalized patients in this study, 40% had an admission without major medical interventions (equivalent to the mild-admitted group in this study), suggesting a potentially unnecessary hospitalization. Given the variability demonstrated between institutions in these potentially unnecessary admissions, it is not surprising that we found differences in our multicenter study compared to that single-site study. Our reported prevalence of severe CAP is consistent with data from a 2006 nationally representative study of hospitalized children with CAP, which reported that 2.7% to 4.8% of patients with CAP had local complications, such as empyema, and 3.1% to 3.4% of patients experienced systemic complications, such as acute respiratory failure.28 Another 3-site prospective cohort study from 2010 to 2012 found that mechanical ventilation, shock, or death occurred among 7.6% of children hospitalized with CAP.29 The low rate of mortality observed in our study corresponds with longitudinal United States data suggesting a gradually decreasing rate of mortality from pediatric pneumonia over time.30
We found that certain outcomes within each severity class occurred more frequently than others. Because some of the outcomes included in the moderate severity category may be arbitrary (eg, intravenous fluids), our findings may underestimate the prevalence of potentially unnecessary hospitalizations as there are likely patients in this group who received these interventions but did not require them. Use of intravenous fluid was the largest driver of moderate classification, followed by use of oxygen. However, there is considerable debate about the role of intravenous fluids in patients with mild dehydration, with concerns of its minimal benefit, and in some cases risks of iatrogenic harm from hypotonic fluids, and resultant interventions (such unnecessary laboratory testing and correction of their abnormalities) that are representative of low-value care.31 As such, it is possible that our moderate severity class includes some patients who may not have truly required intravenous fluids and would otherwise be classified as ‘mild-admitted.’
Most children who present to the ED with CAP can likely be managed at home, with almost 70% of children in this multicenter cohort discharged without a return visit warranting hospitalization. Overall rates of revisit for pneumonia were low (6%) similar to previous single-center work demonstrating a rate of 4.5%,32 with fewer than one-half of revisits in our study requiring admission. As such, our findings confirm that few children with CAP managed in the outpatient setting undergo clinical deterioration and that most can be safely cared for in the community.
An important and concerning finding was the extensive variability in the proportion of patients identified to have mild-admitted severity (ie, hospitalization without major interventions) across hospitals. Further work is needed to identify the reasons for admission for this group and identify the reasons for this variation (eg, concern for deterioration, results of diagnostic testing, or social factors), because there is likely opportunity to decrease hospital use for this large group of children. In conjunction with improved prediction models for disease severity, additional work is necessary to improve implementation, as suggested by the broad variability between patients meeting mild-admitted disease severity criteria within each institution. For example, although greater testing was associated with a lower odd of classification into mild-admitted disease severity, there was substantial variability at the hospital-level intercepts for these outcomes, suggesting broad institutional differences with respect to testing that may be suitable for targeted multiinstitutional quality improvement efforts.
Among patients admitted to the hospital, severe outcomes of CAP were infrequent in previously healthy children (3.5% overall, or 14.1% of admitted patients). The relatively low proportion of patients with severe disease in our study is consistent with previous work.28–30 Because severe outcomes are rare but high stakes for children with CAP, there is a need for predictive tools to help identify these “needles in the haystack” without overuse of medical resources, which also has associated harms. There are several examples of successful risk models for other high-stakes conditions, such as traumatic brain injury,33 intraabdominal injury,34 and appendicitis,35 that identify patients at low risk of severe disease to limit unnecessary diagnostic testing and hospitalization. Similarly, models to risk stratify patients with CAP could identify patients at low risk of severe outcomes to allow for targeted use of diagnostic testing and treatment to better identify the cohort of patients who may not require hospitalization.
Our findings are subject to limitations. First, in our efforts to adapt the ordinal outcome to the PHIS data set, certain modifications were made to fit with the constraints of available data, such as an inability to distinguish between bolus versus continuous intravenous fluids. The rationale behind treatment decisions or LOS cannot be ascertained within the PHIS data set. Similarly, our outcome of interest was potentially unnecessary admissions, but a more granular evaluation of patient records beyond what may be performed from an administrative data set would be needed to definitively identify such encounters. For example, the identification of children admitted for intravenous antibiotics because of failure of outpatient therapy or intolerance to oral antibiotics would be identified in this study as a failure of outpatient antibiotics. A more accurate classification of this patient, however, would require in-depth chart review. Importantly, PHIS does not have vital signs (such as respiratory rate) or laboratory results. This may explain some of the study findings, because patients may have been admitted for observation because of tachypnea or respiratory distress without need for further intervention. However, if these patients did not require escalation of care, which can be deduced from our results, this identifies a population for improved prognostic tools to assist ED clinicians in making admission decisions. Similarly, we were unable to identify potential social factors associated with hospitalization, such as presence of primary care physician and distance to the hospital from this data set. Institutional factors (such as the routine administration of broad-spectrum antibiotics) may have had some impact on severity classification, although the number of patients for whom broadening of antibiotics occurred as the sole factor moderate severity group was low (1.0%). With respect to clinical testing performed in the ED, we did not have detailed information with respect to the exact timing of events, because PHIS only provides the date of each service. Finally, our population was derived from previous work identifying ICD-9 diagnosis codes for CAP; however, work in other disease states has suggested that the conversion of ICD-9 to ICD-10 codes can sometimes be incomplete.36 Finally, our inclusion was based on ICD-10 codes that were converted from previously validated ICD-9 codes. Previous work has corroborated the validity of this type of inclusion, as does the high rate of antibiotic use among children with suspected bacterial pneumonia among those admitted to the hospital.20
CONCLUSIONS
In this multicenter cross-sectional study, we modified a previously developed composite outcome and applied it to a multicenter data set to identify cohorts of children with CAP. These outcomes suggest that a large proportion children admitted with CAP have mild disease and do not require any intravenous fluids or supplemental oxygen. A portion of these patients may, therefore, not require hospitalization. There was broad institutional-level variation with respect to the association of testing, demonstrating the need for improved quality improvement methods to reduce this potentially unnecessary variation in care. Given the uncommon occurrence of severe outcomes, predictive models to risk stratify patients with CAP could allow for targeted use of diagnostic testing and treatment of those that are at most risk, while minimizing potentially unnecessary hospitalizations and resource use in those at low risk.
Supplementary Material
FUNDING:
No external funding.
Footnotes
CONFLICT OF INTEREST DISCLOSURES: The authors have indicated they have no conflicts of interest relevant to this article to disclose.
REFERENCES
- 1.Harris M, Clark J, Coote N, et al. ; British Thoracic Society Standards of Care Committee. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011;66(Suppl 2):ii1–ii23 [DOI] [PubMed] [Google Scholar]
- 2.Keren R, Luan X, Localio R, et al. ; Pediatric Research in Inpatient Settings (PRIS) Network. Prioritization of comparative effectiveness research topics in hospital pediatrics. Arch Pediatr Adolesc Med. 2012;166(12): 1155–1164 [DOI] [PubMed] [Google Scholar]
- 3.McAllister DA, Liu L, Shi T, et al. Global, regional, and national estimates of pneumonia morbidity and mortality in children younger than 5 years between 2000 and 2015: a systematic analysis. Lancet Glob Health. 2019;7(1):e47–e57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomson J, Ambroggio L, Murtagh Kurowski E, et al. Hospital outcomes associated with guideline-recommended antibiotic therapy for pediatric pneumonia. J Hosp Med. 2015;10(1): 13–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sulley S, Ndanga M. Pediatric pneumonia: An analysis of cost & outcome influencers in the United States. Int J Pediatr Adolesc Med. 2019;6(3):79–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.St Peter SD, Tsao K, Spilde TL, et al. Thoracoscopic decortication vs tube thoracostomy with fibrinolysis for empyema in children: a prospective, randomized trial. J Pediatr Surg. 2009;44(1):106–111, discussion 111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Florin TA, Brokamp C, Mantyla R, et al. Validation of the Pediatric Infectious Diseases Society-Infectious Diseases Society of America severity criteria in children with community-acquired pneumonia. Clin Infect Dis. 2018;67(1): 112–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harman JS, Kelleher KJ. Pediatric length of stay guidelines and routine practice: the case of Milliman and Robertson. Arch Pediatr Adolesc Med. 2001;155(8): 885–890 [DOI] [PubMed] [Google Scholar]
- 9.Straney L, Clements A, Alexander J, Slater A; ANZICS Paediatric Study Group. Quantifying variation of paediatric length of stay among intensive care units in Australia and New Zealand. Qual Saf Health Care. 2010;19(6):e5–e5 [DOI] [PubMed] [Google Scholar]
- 10.Goodwin JS, Lin YL, Singh S, Kuo YF. Variation in length of stay and outcomes among hospitalized patients attributable to hospitals and hospitalists. J Gen Intern Med. 2013;28(3):370–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bradley JS, Byington CL, Shah SS, et al. ; Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53(7):e25–e76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Florin T, Ambroggio L, Lorenz D, et al. Development and internal validation of a prediction model to risk stratify children with suspected community-acquired pneumonia. Clin Infect Dis. 2020; (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Synhorst DC, Johnson MB, Bettenhausen JL, et al. Room costs for common pediatric hospitalizations and cost-reducing quality initiatives. Pediatrics. 2020;145(6):e20192177. [DOI] [PubMed] [Google Scholar]
- 14.Florin TA, Byczkowski T, Gerber JS, Ruddy R, Kuppermann N. Diagnostic testing and antibiotic use in young children with community-acquired pneumonia in the United States, 2008–2015. J Pediatric Infect Dis Soc. 2020;9(2):248–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fletcher DM. Achieving data quality. How data from a pediatric health information system earns the trust of its users. J AHIMA. 2004;75(10):22–26 [PubMed] [Google Scholar]
- 16.Mongelluzzo J, Mohamad Z, Ten Have TR, Shah SS. Corticosteroids and mortality in children with bacterial meningitis. JAMA. 2008;299(17):2048–2055 [DOI] [PubMed] [Google Scholar]
- 17.Florin TA, Ambroggio L, Brokamp C, et al. Biomarkers and disease severity in children with community-acquired pneumonia. Pediatrics. 2020;145(6): e20193728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams DJ, Shah SS, Myers A, et al. Identifying pediatric community-acquired pneumonia hospitalizations: Accuracy of administrative billing codes. JAMA Pediatr. 2013;167(9):851–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.ICD-10 | CMS Available at: https://www.cms.gov/Medicare/Coding/ICD10/index. Accessed October 18, 2020
- 20.Smithee RB, Markus TM, Soda E, et al. Pneumonia hospitalization coding changes associated with transition from the 9th to 10th revision of international classification of diseases. Health Serv Res Manag Epidemiol. 2020;7:2333392820939801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gross CJ, Porter JJ, Lipsett SC, Monuteaux MC, Hirsch AW, Neuman MI. Variation in management and outcomes of children with complicated pneumonia. Hosp Pediatr. 2021;11(3):207–214 [DOI] [PubMed] [Google Scholar]
- 22.Geanacopoulos AT, Porter JJ, Monuteaux MC, Lipsett SC, Neuman MI. Trends in chest radiographs for pneumonia in emergency departments. Pediatrics. 2020;145(3):e20192816. [DOI] [PubMed] [Google Scholar]
- 23.Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14(1): 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leyenaar JK, Lagu T, Shieh M-S, Pekow PS, Lindenauer PK. Variation in resource utilization for the management of uncomplicated community-acquired pneumonia across community and children’s hospitals. J Pediatr. 2014; 165(3):585–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurz B, Rozas LW. The concept of race in research: using composite variables. Ethn Dis. 2007;17(3):560–567 [PubMed] [Google Scholar]
- 26.Sen M, Wasow O. Race as a bundle of sticks: Designs that estimate effects of seemingly immutable characteristics. Annu Rev Polit Sci. 2016;19: 499–522 [Google Scholar]
- 27.Scott HF, Brilli RJ, Paul R, et al. ; Improving Pediatric Sepsis Outcomes (IPSO) Collaborative Investigators. Evaluating pediatric sepsis definitions designed for electronic health record extraction and multicenter quality improvement. Crit Care Med. 2020; 48(10):e916–e926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee GE, Lorch SA, Sheffler-Collins S, Kronman MP, Shah SS. National hospitalization trends for pediatric pneumonia and associated complications. Pediatrics. 2010;126(2):204–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams DJ, Zhu Y, Grijalva CG, et al. Predicting severe pneumonia outcomes in children. Pediatrics. 2016;138(4): e20161019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dowell SF, Kupronis BA, Zell ER, Shay DK. Mortality from pneumonia in children in the United States, 1939 through 1996. N Engl J Med. 2000;342(19):1399–1407 [DOI] [PubMed] [Google Scholar]
- 31.Rooholamini SN, Jennings B, Zhou C, et al. Effect of a quality improvement bundle to standardize the use of intravenous fluids for hospitalized pediatric patients: a stepped-wedge, cluster randomized clinical trial. JAMA Pediatr. 2022;176(1):26–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ambroggio L, Herman H, Fain E, Huang G, Florin TA. Clinical risk factors for revisits for children with community-acquired pneumonia. Hosp Pediatr. 2018;8(11): 718–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuppermann N, Holmes JF, Dayan PS, et al. ; Pediatric Emergency Care Applied Research Network (PECARN). Identification of children at very low risk of clinically-important brain injuries after head trauma: a prospective cohort study. Lancet. 2009;374(9696):1160–1170 [DOI] [PubMed] [Google Scholar]
- 34.Holmes JF, Lillis K, Monroe D, et al. ; Pediatric Emergency Care Applied Research Network (PECARN). Identifying children at very low risk of clinically important blunt abdominal injuries. Ann Emerg Med. 2013;62(2):107–116.e2 [DOI] [PubMed] [Google Scholar]
- 35.Kharbanda AB, Taylor GA, Fishman SJ, Bachur RG. A clinical decision rule to identify children at low risk for appendicitis. Pediatrics. 2005;116(3):709–716 [DOI] [PubMed] [Google Scholar]
- 36.Tian Y, Ingram ME, Raval MV. A pitfall of using general equivalence mappings to estimate national trends of surgical utilization for pediatric patients. J Pediatr Surg. 2020;55(12): 2602–2607 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


