Abstract
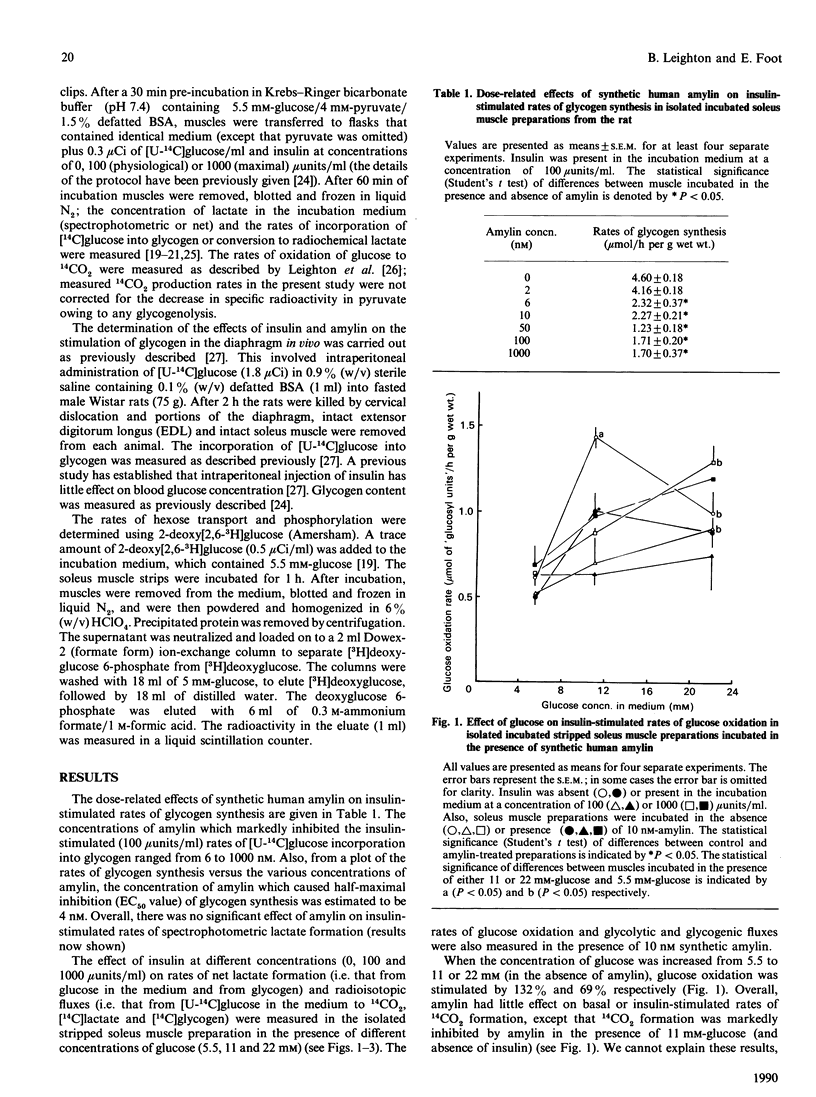
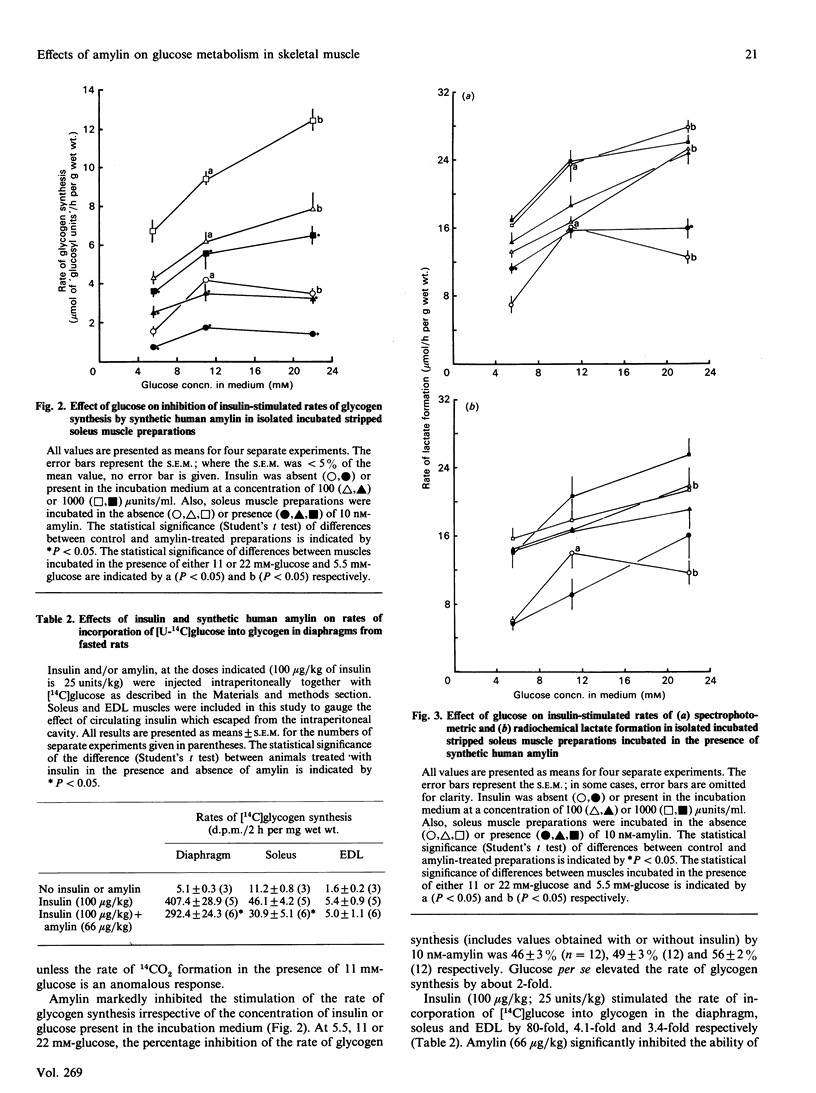
1. The effects of synthetic human amylin on basal and insulin-stimulated (100 and 1000 microunits/ml) rates of lactate formation, glucose oxidation and glycogen synthesis were measured in the isolated rat soleus muscle preparation incubated in the presence of various concentrations of glucose (5, 11 and 22 mM). 2. The rate of glucose utilization was increased by about 2-fold by increasing the glucose concentration from 5 to 22 mM. 3. Synthetic human amylin (10 nM) significantly inhibited (by 46-56%) glycogen synthesis, irrespective of the concentration of insulin or glucose present in the incubation medium. 4. Amylin (10 nM) did not affect insulin-stimulated rates of 2-deoxy[3H]glucose transport and phosphorylation. 5. Intraperitoneal administration of insulin (100 micrograms/kg) to rats in vivo stimulated the rate of [U-14C]glucose incorporation into glycogen in the diaphragm by about 80-fold. This rate was decreased (by 28%) by co-administration of amylin (66 micrograms/kg).
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Challiss R. A., Espinal J., Newsholme E. A. Insulin sensitivity of rates of glycolysis and glycogen synthesis in soleus, stripped soleus, epitrochlearis, and hemi-diaphragm muscles isolated from sedentary rats. Biosci Rep. 1983 Jul;3(7):675–679. doi: 10.1007/BF01172878. [DOI] [PubMed] [Google Scholar]
- Challiss R. A., Lozeman F. J., Leighton B., Newsholme E. A. Effects of the beta-adrenoceptor agonist isoprenaline on insulin-sensitivity in soleus muscle of the rat. Biochem J. 1986 Jan 15;233(2):377–381. doi: 10.1042/bj2330377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A., Edwards C. A., Ostle L. R., Sutton R., Rothbard J. B., Morris J. F., Turner R. C. Localisation of islet amyloid peptide in lipofuscin bodies and secretory granules of human B-cells and in islets of type-2 diabetic subjects. Cell Tissue Res. 1989 Jul;257(1):179–185. doi: 10.1007/BF00221649. [DOI] [PubMed] [Google Scholar]
- Cooper G. J., Day A. J., Willis A. C., Roberts A. N., Reid K. B., Leighton B. Amylin and the amylin gene: structure, function and relationship to islet amyloid and to diabetes mellitus. Biochim Biophys Acta. 1989 Dec 14;1014(3):247–258. doi: 10.1016/0167-4889(89)90220-6. [DOI] [PubMed] [Google Scholar]
- Cooper G. J., Leighton B., Dimitriadis G. D., Parry-Billings M., Kowalchuk J. M., Howland K., Rothbard J. B., Willis A. C., Reid K. B. Amylin found in amyloid deposits in human type 2 diabetes mellitus may be a hormone that regulates glycogen metabolism in skeletal muscle. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7763–7766. doi: 10.1073/pnas.85.20.7763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper G. J., Willis A. C., Clark A., Turner R. C., Sim R. B., Reid K. B. Purification and characterization of a peptide from amyloid-rich pancreases of type 2 diabetic patients. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8628–8632. doi: 10.1073/pnas.84.23.8628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crettaz M., Prentki M., Zaninetti D., Jeanrenaud B. Insulin resistance in soleus muscle from obese Zucker rats. Involvement of several defective sites. Biochem J. 1980 Feb 15;186(2):525–534. doi: 10.1042/bj1860525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrikson R. L., Meredith S. C. Amino acid analysis by reverse-phase high-performance liquid chromatography: precolumn derivatization with phenylisothiocyanate. Anal Biochem. 1984 Jan;136(1):65–74. doi: 10.1016/0003-2697(84)90307-5. [DOI] [PubMed] [Google Scholar]
- Howard C. F., Jr Longitudinal studies on the development of diabetes in individual Macaca nigra. Diabetologia. 1986 May;29(5):301–306. doi: 10.1007/BF00452067. [DOI] [PubMed] [Google Scholar]
- Johnson K. H., Stevens J. B. Light and electron microscopic studies of islet amyloid in diabetic cats. Diabetes. 1973 Feb;22(2):81–90. doi: 10.2337/diab.22.2.81. [DOI] [PubMed] [Google Scholar]
- Jue T., Rothman D. L., Tavitian B. A., Shulman R. G. Natural-abundance 13C NMR study of glycogen repletion in human liver and muscle. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1439–1442. doi: 10.1073/pnas.86.5.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanatsuka A., Makino H., Ohsawa H., Tokuyama Y., Yamaguchi T., Yoshida S., Adachi M. Secretion of islet amyloid polypeptide in response to glucose. FEBS Lett. 1989 Dec 18;259(1):199–201. doi: 10.1016/0014-5793(89)81527-3. [DOI] [PubMed] [Google Scholar]
- Kreutter D., Orena S. J., Andrews K. M. Suppression of insulin-stimulated glucose transport in L6 myocytes by calcitonin gene-related peptide. Biochem Biophys Res Commun. 1989 Oct 16;164(1):461–467. doi: 10.1016/0006-291x(89)91742-7. [DOI] [PubMed] [Google Scholar]
- Laury M. C., Penicaud L., Ktorza A., Benhaiem H., Bihoreau M. T., Picon L. In vivo insulin secretion and action in hyperglycemic rat. Am J Physiol. 1989 Aug;257(2 Pt 1):E180–E184. doi: 10.1152/ajpendo.1989.257.2.E180. [DOI] [PubMed] [Google Scholar]
- Le Marchand-Brustel Y., Freychet P. Regulation of glycogen synthase activity in the isolated mouse soleus muscle. Effect of insulin, epinephrine, glucose and anti-insulin receptor antibodies. Biochim Biophys Acta. 1981 Sep 18;677(1):13–22. [PubMed] [Google Scholar]
- Leighton B., Budohoski L., Lozeman F. J., Challiss R. A., Newsholme E. A. The effect of prostaglandins E1, E2 and F2 alpha and indomethacin on the sensitivity of glycolysis and glycogen synthesis to insulin in stripped soleus muscles of the rat. Biochem J. 1985 Apr 1;227(1):337–340. doi: 10.1042/bj2270337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton B., Challiss R. A., Lozeman F. J., Newsholme E. A. Effects of dexamethasone treatment on insulin-stimulated rates of glycolysis and glycogen synthesis in isolated incubated skeletal muscles of the rat. Biochem J. 1987 Sep 1;246(2):551–554. doi: 10.1042/bj2460551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton B., Cooper G. J. Pancreatic amylin and calcitonin gene-related peptide cause resistance to insulin in skeletal muscle in vitro. Nature. 1988 Oct 13;335(6191):632–635. doi: 10.1038/335632a0. [DOI] [PubMed] [Google Scholar]
- Leighton B., Dimitriadis G. D., Parry-Billings M., Lozeman F. J., Newsholme E. A. Effects of aging on the responsiveness and sensitivity of glucose metabolism to insulin in the incubated soleus muscle isolated from Sprague-Dawley and Wistar rats. Biochem J. 1989 Jul 15;261(2):383–387. doi: 10.1042/bj2610383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton B., Foot E. A., Cooper G. G., King J. M. Calcitonin gene-related peptide-1 (CGRP-1) is a potent regulator of glycogen metabolism in rat skeletal muscle. FEBS Lett. 1989 Jun 5;249(2):357–361. doi: 10.1016/0014-5793(89)80658-1. [DOI] [PubMed] [Google Scholar]
- Leighton B., Kowalchuk J. M., Challiss R. A., Newsholme E. A. Circadian rhythm in sensitivity of glucose metabolism to insulin in rat soleus muscle. Am J Physiol. 1988 Jul;255(1 Pt 1):E41–E45. doi: 10.1152/ajpendo.1988.255.1.E41. [DOI] [PubMed] [Google Scholar]
- Leighton B., Lozeman F. J., Vlachonikolis I. G., Challiss R. A., Pitcher J. A., Newsholme E. A. Effects of adenosine deaminase on the sensitivity of glucose transport, glycolysis and glycogen synthesis to insulin in muscles of the rat. Int J Biochem. 1988;20(1):23–27. doi: 10.1016/0020-711x(88)90005-5. [DOI] [PubMed] [Google Scholar]
- Lillioja S., Mott D. M., Howard B. V., Bennett P. H., Yki-Järvinen H., Freymond D., Nyomba B. L., Zurlo F., Swinburn B., Bogardus C. Impaired glucose tolerance as a disorder of insulin action. Longitudinal and cross-sectional studies in Pima Indians. N Engl J Med. 1988 May 12;318(19):1217–1225. doi: 10.1056/NEJM198805123181901. [DOI] [PubMed] [Google Scholar]
- Molina J. M., Cooper G. J., Leighton B., Olefsky J. M. Induction of insulin resistance in vivo by amylin and calcitonin gene-related peptide. Diabetes. 1990 Feb;39(2):260–265. doi: 10.2337/diab.39.2.260. [DOI] [PubMed] [Google Scholar]
- Nakazato M., Asai J., Kangawa K., Matsukura S., Matsuo H. Establishment of radioimmunoassay for human islet amyloid polypeptide and its tissue content and plasma concentration. Biochem Biophys Res Commun. 1989 Oct 16;164(1):394–399. doi: 10.1016/0006-291x(89)91732-4. [DOI] [PubMed] [Google Scholar]
- Ohlén A., Lindbom L., Staines W., Hökfelt T., Cuello A. C., Fischer J. A., Hedqvist P. Substance P and calcitonin gene-related peptide: immunohistochemical localisation and microvascular effects in rabbit skeletal muscle. Naunyn Schmiedebergs Arch Pharmacol. 1987 Jul;336(1):87–93. doi: 10.1007/BF00177756. [DOI] [PubMed] [Google Scholar]
- Popper P., Micevych P. E. Localization of calcitonin gene-related peptide and its receptors in a striated muscle. Brain Res. 1989 Sep 4;496(1-2):180–186. doi: 10.1016/0006-8993(89)91064-0. [DOI] [PubMed] [Google Scholar]
- RAFAELSEN O. J. GLYCOGEN CONTENT OF RAT DIAPHRAGM AFTER INTRAPERITONEAL INJECTION OF INSULIN AND OTHER HORMONES. Acta Physiol Scand. 1964 Aug;61:314–322. [PubMed] [Google Scholar]
- Reaven G. M. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988 Dec;37(12):1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- Richter E. A., Hansen B. F., Hansen S. A. Glucose-induced insulin resistance of skeletal-muscle glucose transport and uptake. Biochem J. 1988 Jun 15;252(3):733–737. doi: 10.1042/bj2520733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. N., Leighton B., Todd J. A., Cockburn D., Schofield P. N., Sutton R., Holt S., Boyd Y., Day A. J., Foot E. A. Molecular and functional characterization of amylin, a peptide associated with type 2 diabetes mellitus. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9662–9666. doi: 10.1073/pnas.86.24.9662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasson S., Cerasi E. Substrate regulation of the glucose transport system in rat skeletal muscle. Characterization and kinetic analysis in isolated soleus muscle and skeletal muscle cells in culture. J Biol Chem. 1986 Dec 25;261(36):16827–16833. [PubMed] [Google Scholar]
- Sasson S., Edelson D., Cerasi E. In vitro autoregulation of glucose utilization in rat soleus muscle. Diabetes. 1987 Sep;36(9):1041–1046. doi: 10.2337/diab.36.9.1041. [DOI] [PubMed] [Google Scholar]
- Takami K., Kawai Y., Uchida S., Tohyama M., Shiotani Y., Yoshida H., Emson P. C., Girgis S., Hillyard C. J., MacIntyre I. Effect of calcitonin gene-related peptide on contraction of striated muscle in the mouse. Neurosci Lett. 1985 Sep 30;60(2):227–230. doi: 10.1016/0304-3940(85)90248-4. [DOI] [PubMed] [Google Scholar]
- Takamori M., Yoshikawa H. Effect of calcitonin gene-related peptide on skeletal muscle via specific binding site and G protein. J Neurol Sci. 1989 Mar;90(1):99–109. doi: 10.1016/0022-510x(89)90049-x. [DOI] [PubMed] [Google Scholar]
- Walker P. S., Ramlal T., Donovan J. A., Doering T. P., Sandra A., Klip A., Pessin J. E. Insulin and glucose-dependent regulation of the glucose transport system in the rat L6 skeletal muscle cell line. J Biol Chem. 1989 Apr 15;264(11):6587–6595. [PubMed] [Google Scholar]
- Westermark P., Wernstedt C., Wilander E., Hayden D. W., O'Brien T. D., Johnson K. H. Amyloid fibrils in human insulinoma and islets of Langerhans of the diabetic cat are derived from a neuropeptide-like protein also present in normal islet cells. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3881–3885. doi: 10.1073/pnas.84.11.3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermark P., Wilander E. The influence of amyloid deposits on the islet volume in maturity onset diabetes mellitus. Diabetologia. 1978 Nov;15(5):417–421. doi: 10.1007/BF01219652. [DOI] [PubMed] [Google Scholar]
- Young A. A., Bogardus C., Wolfe-Lopez D., Mott D. M. Muscle glycogen synthesis and disposition of infused glucose in humans with reduced rates of insulin-mediated carbohydrate storage. Diabetes. 1988 Mar;37(3):303–308. doi: 10.2337/diab.37.3.303. [DOI] [PubMed] [Google Scholar]


