Abstract
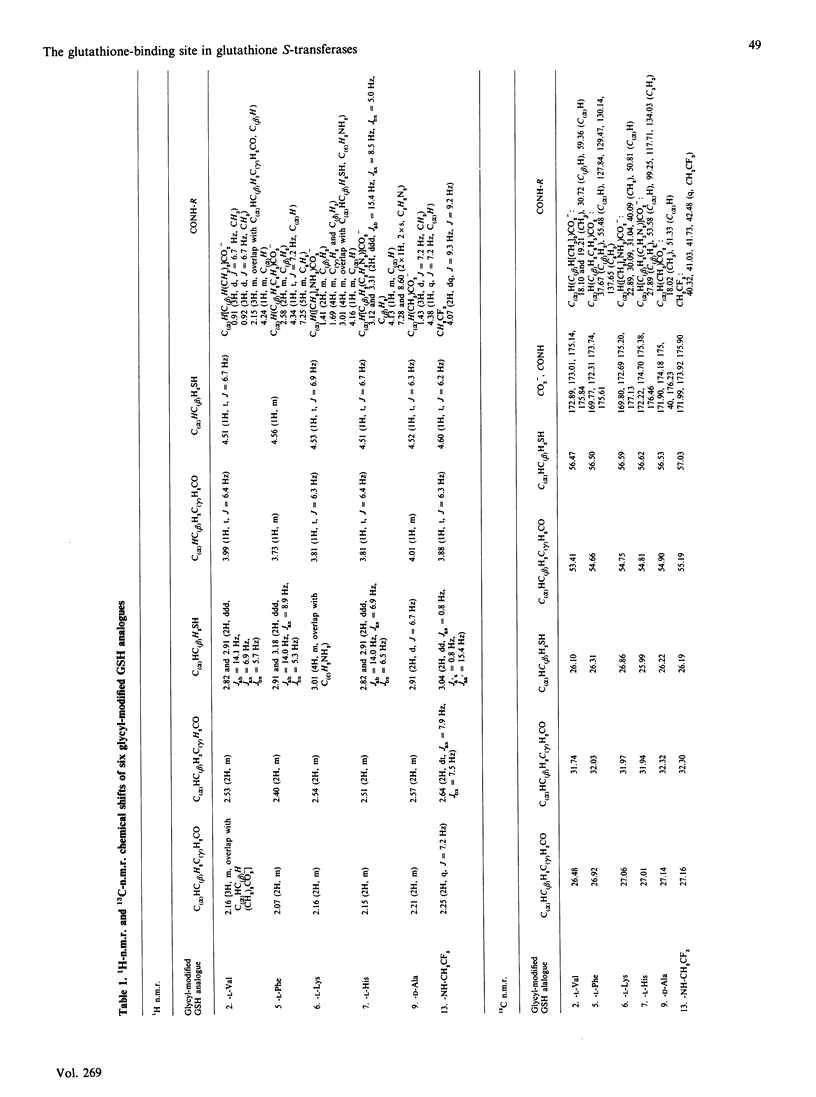
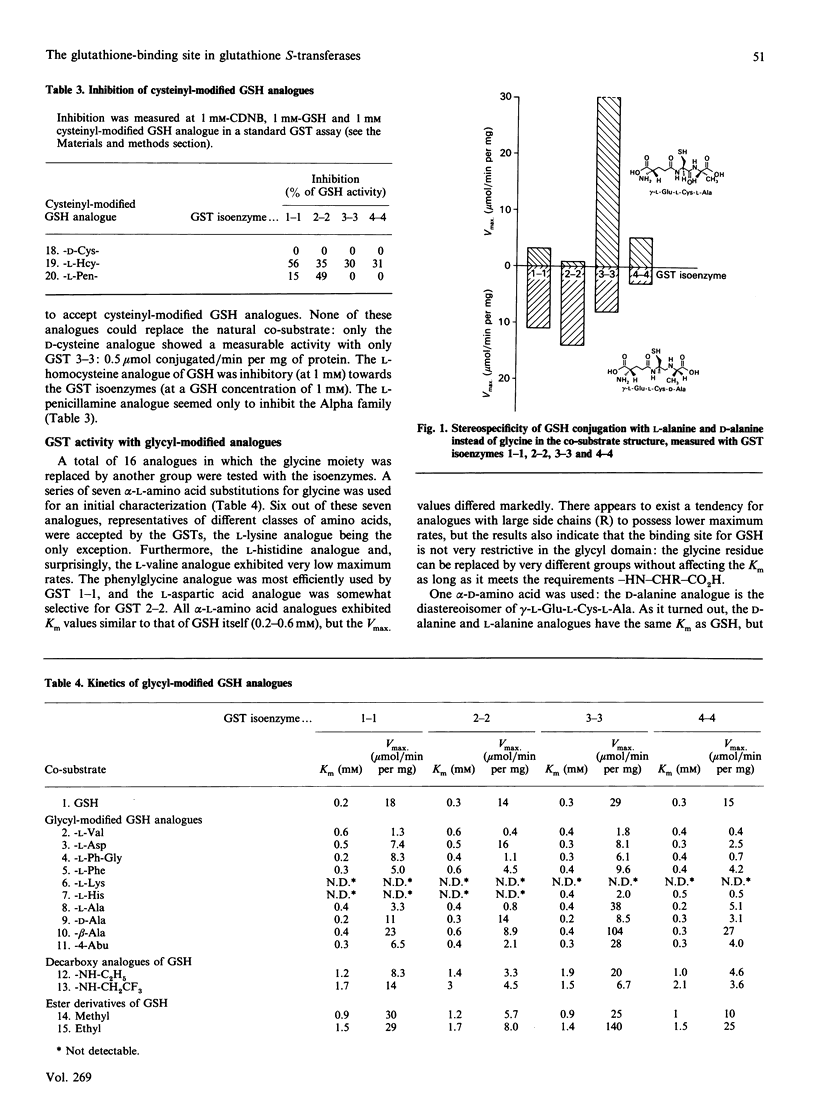
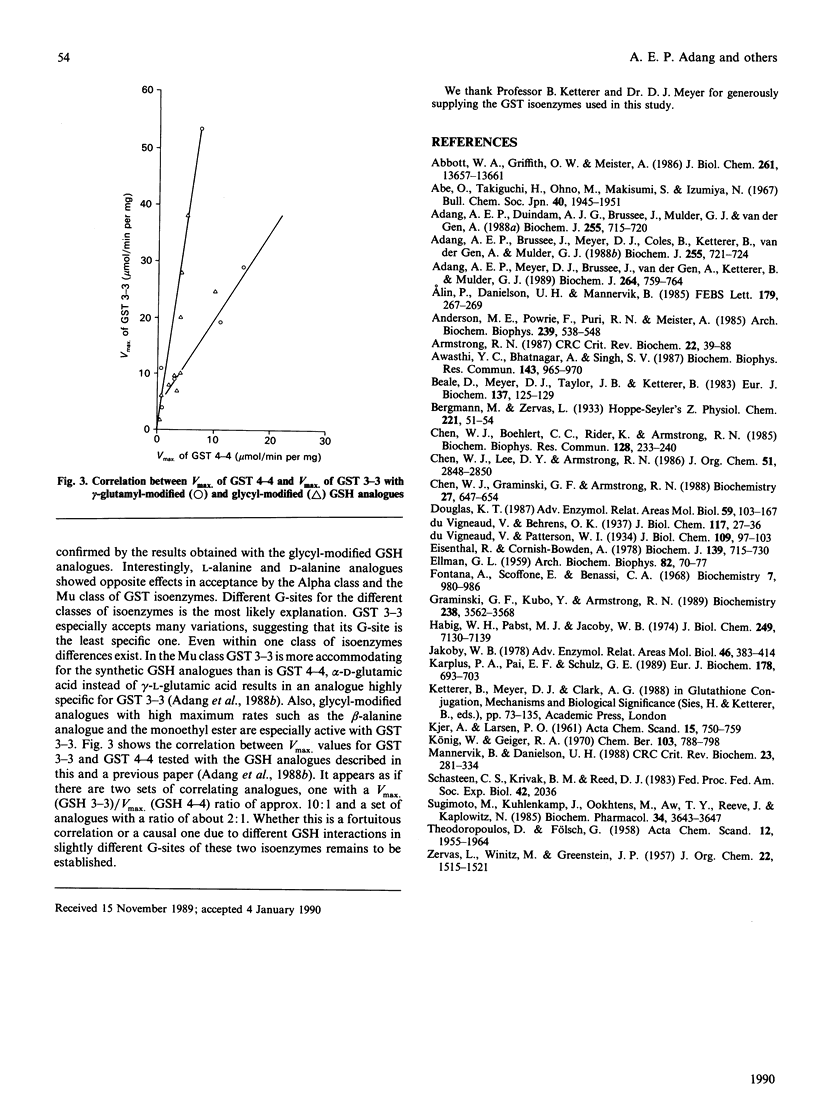
The GSH-binding site of glutathione S-transferase (GST) isoenzymes was studied by investigating their substrate-specificity for three series of GSH analogues; further, a model of the interactions of GSH with the G-site is proposed. Twelve glycyl-modified GSH analogues, four ester derivatives of GSH and three cysteinyl-modified GSH analogues were synthesized and tested with purified forms of rat liver GST (1-1, 2-2, 3-3 and 4-4). The glycyl analogues exhibited spontaneous chemical reaction rates with 1-chloro-2,4-dinitrobenzene comparable with the GSH rate. In contrast, the enzymic rates (Vmax.) differed greatly, from less than 1 up to 140 mumol/min per mg; apparently, a reaction mechanism is followed that is very sensitive to substitutions at the glycyl domain. No correlation exists between the chemical rates and Vmax. values for the analogues. Analogues of GSH in which L-cysteine was replaced by D-cysteine, L-homocysteine or L-penicillamine showed little or no capacity to replace GSH as co-substrate for the GSTs. GSH monomethyl and monoethyl esters showed Vmax. values greater than the Vmax. measured with GSH: the Vmax. for the monoethyl ester of GSH and GST 3-3 was 5-fold that for GSH. The data obtained in this and previous studies [Adang, Brussee, Meyer, Coles, Ketterer, van der Gen & Mulder (1988) Biochem. J. 255, 721-724; Adang, Meyer, Brussee, van der Gen, Ketterer & Mulder (1989) Biochem. J. 264, 759-764] allow a model of the interactions of GSH in the G-site in GSTs to be postulated. The gamma-glutamyl site is the main binding determinant: the alpha-carboxylate group is obligatory, whereas shifting of the amino group and shortening of the peptide backbone only decreased kcat./Km. Furthermore, the GSTs appear to be very critical with respect to a correct orientation of the thiol group of the GSH analogue. The glycyl site is the least restrictive domain in the G-site of GSTs: amino acid analogues all showed Km values between 0.2 and 0.6 mM (that for GSH is 0.2-0.3 mM), but large differences in Vmax. exist. The glycyl carboxylate group is not essential for substrate recognition, since decarboxy analogues and ester derivatives showed high activities. The possible mechanisms for an increased Vmax. in some analogues are briefly discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott W. A., Griffith O. W., Meister A. Gamma-glutamyl-glutathione. Natural occurrence and enzymology. J Biol Chem. 1986 Oct 15;261(29):13657–13661. [PubMed] [Google Scholar]
- Abe O., Takiguchi H., Ono M., Makisumi S., Izumiya N. Synthesis of cyclo-diglycyl-L-lysyl-diglycyl-L-lysyl and hydrolysis by trypsin. Bull Chem Soc Jpn. 1967 Aug;40(8):1945–1951. doi: 10.1246/bcsj.40.1945. [DOI] [PubMed] [Google Scholar]
- Adang A. E., Brussee J., Meyer D. J., Coles B., Ketterer B., van der Gen A., Mulder G. J. Substrate specificity of rat liver glutathione S-transferase isoenzymes for a series of glutathione analogues, modified at the gamma-glutamyl moiety. Biochem J. 1988 Oct 15;255(2):721–724. [PMC free article] [PubMed] [Google Scholar]
- Adang A. E., Duindam A. J., Brussee J., Mulder G. J., van der Gen A. Synthesis and nucleophilic reactivity of a series of glutathione analogues, modified at the gamma-glutamyl moiety. Biochem J. 1988 Oct 15;255(2):715–720. [PMC free article] [PubMed] [Google Scholar]
- Adang A. E., Meyer D. J., Brussee J., Van der Gen A., Ketterer B., Mulder G. J. Interaction of rat glutathione S-transferases 7-7 and 8-8 with gamma-glutamyl- or glycyl-modified glutathione analogues. Biochem J. 1989 Dec 15;264(3):759–764. doi: 10.1042/bj2640759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alin P., Danielson U. H., Mannervik B. 4-Hydroxyalk-2-enals are substrates for glutathione transferase. FEBS Lett. 1985 Jan 7;179(2):267–270. doi: 10.1016/0014-5793(85)80532-9. [DOI] [PubMed] [Google Scholar]
- Anderson M. E., Powrie F., Puri R. N., Meister A. Glutathione monoethyl ester: preparation, uptake by tissues, and conversion to glutathione. Arch Biochem Biophys. 1985 Jun;239(2):538–548. doi: 10.1016/0003-9861(85)90723-4. [DOI] [PubMed] [Google Scholar]
- Armstrong R. N. Enzyme-catalyzed detoxication reactions: mechanisms and stereochemistry. CRC Crit Rev Biochem. 1987;22(1):39–88. doi: 10.3109/10409238709082547. [DOI] [PubMed] [Google Scholar]
- Awasthi Y. C., Bhatnagar A., Singh S. V. Evidence for the involvement of histidine at the active site of glutathione S-transferase psi from human liver. Biochem Biophys Res Commun. 1987 Mar 30;143(3):965–970. doi: 10.1016/0006-291x(87)90345-7. [DOI] [PubMed] [Google Scholar]
- Beale D., Meyer D. J., Taylor J. B., Ketterer B. Evidence that the Yb subunits of hepatic glutathione transferases represent two different but related families of polypeptides. Eur J Biochem. 1983 Dec 1;137(1-2):125–129. doi: 10.1111/j.1432-1033.1983.tb07805.x. [DOI] [PubMed] [Google Scholar]
- Chen W. J., Boehlert C. C., Rider K., Armstrong R. N. Synthesis and characterization of the oxygen and desthio analogues of glutathione as dead-end inhibitors of glutathione S-transferase. Biochem Biophys Res Commun. 1985 Apr 16;128(1):233–240. doi: 10.1016/0006-291x(85)91669-9. [DOI] [PubMed] [Google Scholar]
- Chen W. J., Graminski G. F., Armstrong R. N. Dissection of the catalytic mechanism of isozyme 4-4 of glutathione S-transferase with alternative substrates. Biochemistry. 1988 Jan 26;27(2):647–654. doi: 10.1021/bi00402a023. [DOI] [PubMed] [Google Scholar]
- Cornish-Bowden A., Eisenthal R. Statistical considerations in the estimation of enzyme kinetic parameters by the direct linear plot andother methods. Biochem J. 1974 Jun;139(3):721–730. doi: 10.1042/bj1390721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas K. T. Mechanism of action of glutathione-dependent enzymes. Adv Enzymol Relat Areas Mol Biol. 1987;59:103–167. doi: 10.1002/9780470123058.ch3. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Fontana A., Scoffone E., Benassi C. A. Sulfenyl halides as modifying reagents for polypeptides and proteins. II. Modification of cysteinyl residues. Biochemistry. 1968 Mar;7(3):980–986. doi: 10.1021/bi00843a015. [DOI] [PubMed] [Google Scholar]
- Graminski G. F., Kubo Y., Armstrong R. N. Spectroscopic and kinetic evidence for the thiolate anion of glutathione at the active site of glutathione S-transferase. Biochemistry. 1989 Apr 18;28(8):3562–3568. doi: 10.1021/bi00434a062. [DOI] [PubMed] [Google Scholar]
- Habig W. H., Pabst M. J., Jakoby W. B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974 Nov 25;249(22):7130–7139. [PubMed] [Google Scholar]
- Jakoby W. B. The glutathione S-transferases: a group of multifunctional detoxification proteins. Adv Enzymol Relat Areas Mol Biol. 1978;46:383–414. doi: 10.1002/9780470122914.ch6. [DOI] [PubMed] [Google Scholar]
- Karplus P. A., Pai E. F., Schulz G. E. A crystallographic study of the glutathione binding site of glutathione reductase at 0.3-nm resolution. Eur J Biochem. 1989 Jan 2;178(3):693–703. doi: 10.1111/j.1432-1033.1989.tb14500.x. [DOI] [PubMed] [Google Scholar]
- König W., Geiger R. Eine neue Methode zur Synthese von Peptiden: Aktivierung der Carboxylgruppe mit Dicyclohexycarbodiimid unter Zusatz von 1-Hydroxy-benzotriazolen. Chem Ber. 1970;103(3):788–798. doi: 10.1002/cber.19701030319. [DOI] [PubMed] [Google Scholar]
- Sugimoto M., Kuhlenkamp J., Ookhtens M., Aw T. Y., Reeve J., Jr, Kaplowitz N. Gamma-glutamylcysteine: a substrate for glutathione S-transferases. Biochem Pharmacol. 1985 Oct 15;34(20):3643–3647. doi: 10.1016/0006-2952(85)90224-2. [DOI] [PubMed] [Google Scholar]


