Abstract
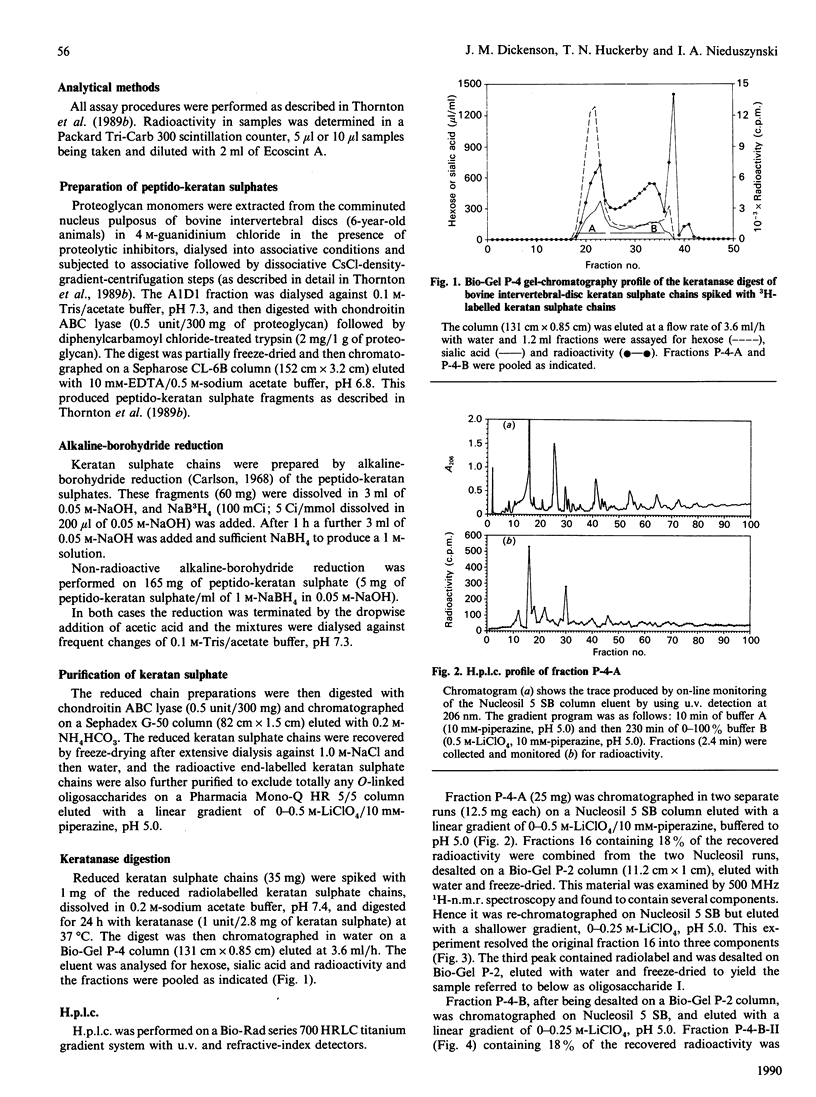
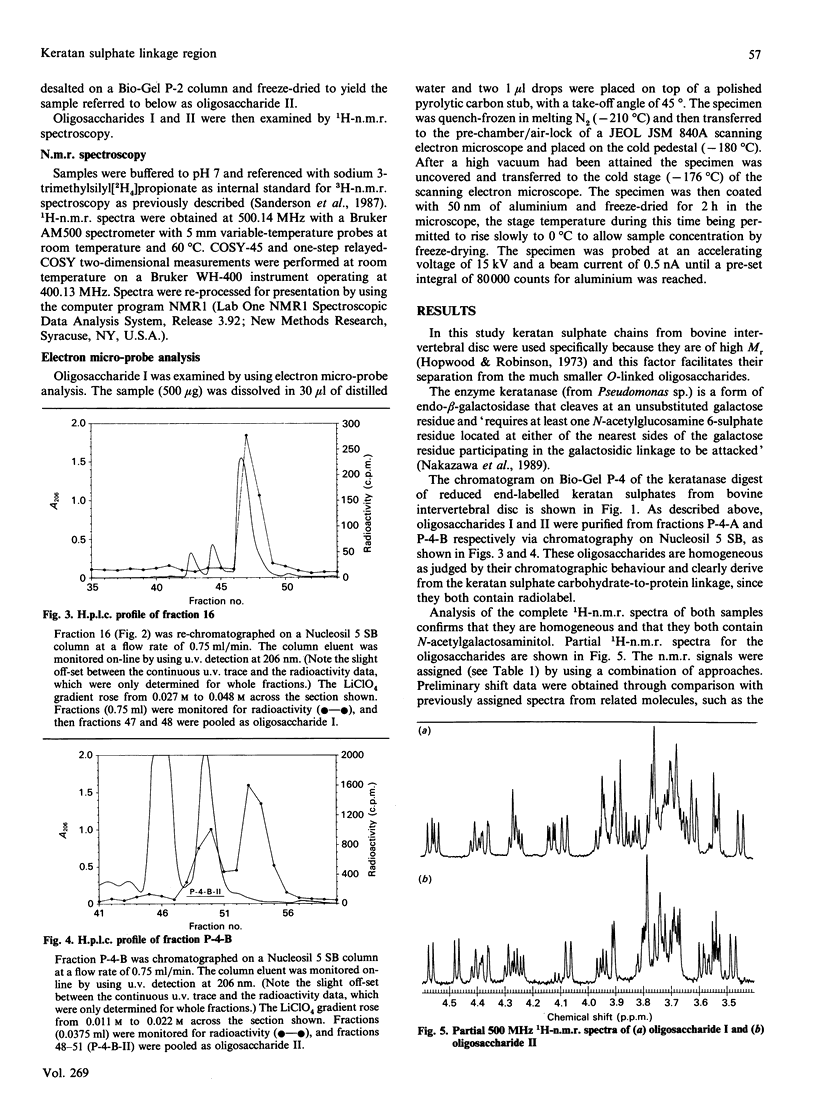
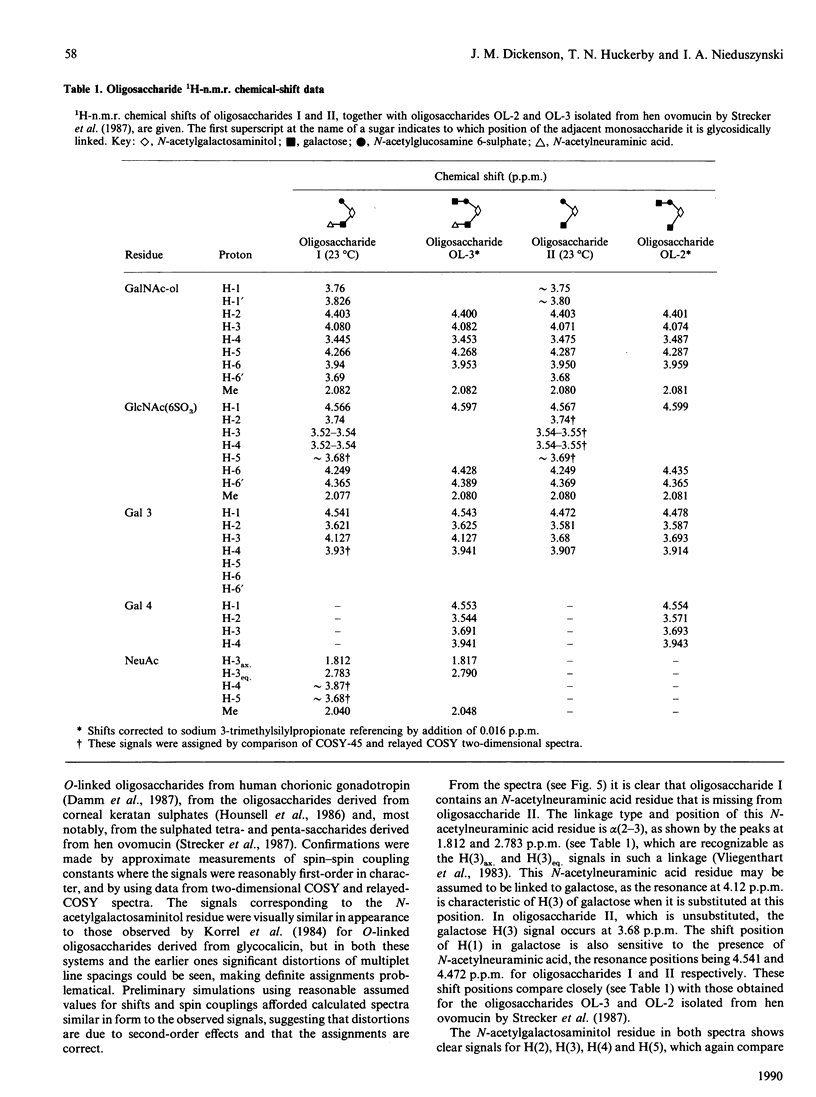
Peptido-keratan sulphate fragments were isolated from the nucleus pulposus of bovine intervertebral discs (6-year-old animals) after chondroitin ABC lyase digestion followed by digestion of A1D1 proteoglycans by diphenylcarbamoyl chloride-treated trypsin and gel-permeation chromatography on Sepharose CL-6B. Treatment of these peptido-keratan sulphate fragments with alkaline NaB3H4 yielded keratan sulphate chains with [3H]galactosaminitol end-labels, and these chains were further purified by gel-permeation chromatography on Sephadex G-50 and ion-exchange chromatography on a Pharmacia Mono-Q column in order to exclude any contamination with O-linked oligosaccharides. The chains were then treated with keratanase, and the digest was chromatographed on a Bio-Gel P-4 column followed by anion-exchange chromatography on a Nucleosil 5 SB column. Two oligosaccharides, each representing 18% of the recovered radiolabel, were examined by 500 MHz 1H-n.m.r. spectroscopy, and shown to have the following structures: [formula: see text] The structure of oligosaccharide (I) confirms the N-acetylneuraminylgalactose substitution at position 3 of N-acetylgalactosamine in the keratan sulphate-protein linkage region found by Hopwood & Robinson [(1974) Biochem. J. 141, 57-69] but additionally shows the presence of a 6-sulphated N-acetylglucosamine. Electron micro-probe analysis specifically confirmed the presence of sulphur in this sample. This sulphate ester group differentiates the keratan sulphate linkage region from similar structures derived from O-linked oligosaccharides [Lohmander, De Luca, Nilsson, Hascall, Caputo, Kimura & Heinegård (1980) J. Biol. Chem. 255, 6084-6091].
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antonsson P., Heinegård D., Oldberg A. The keratan sulfate-enriched region of bovine cartilage proteoglycan consists of a consecutively repeated hexapeptide motif. J Biol Chem. 1989 Sep 25;264(27):16170–16173. [PubMed] [Google Scholar]
- Bray B. A., Lieberman R., Meyer K. Structure of human skeletal keratosulfate. The linkage region. J Biol Chem. 1967 Jul 25;242(14):3373–3380. [PubMed] [Google Scholar]
- Carlson D. M. Structures and immunochemical properties of oligosaccharides isolated from pig submaxillary mucins. J Biol Chem. 1968 Feb 10;243(3):616–626. [PubMed] [Google Scholar]
- Cockin G. H., Huckerby T. N., Nieduszynski I. A. High-field n.m.r. studies of keratan sulphates. 1H and 13C assignments of keratan sulphate from shark cartilage. Biochem J. 1986 Jun 15;236(3):921–924. doi: 10.1042/bj2360921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopwood J. J., Robinson H. C. The alkali-labile linkage between keratan sulphate and protein. Biochem J. 1974 Jul;141(1):57–69. doi: 10.1042/bj1410057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopwood J. J., Robinson H. C. The molecular-weight distribution of glycosaminoglycans. Biochem J. 1973 Dec;135(4):631–637. doi: 10.1042/bj1350631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounsell E. F., Feeney J., Scudder P., Tang P. W., Feizi T. 1H-NMR studies at 500 MHz of a neutral disaccharide and sulphated di-, tetra-, hexa- and larger oligosaccharides obtained by endo-beta-galactosidase treatment of keratan sulphate. Eur J Biochem. 1986 Jun 2;157(2):375–384. doi: 10.1111/j.1432-1033.1986.tb09679.x. [DOI] [PubMed] [Google Scholar]
- Korrel S. A., Clemetson K. J., Van Halbeek H., Kamerling J. P., Sixma J. J., Vliegenthart J. F. Structural studies on the O-linked carbohydrate chains of human platelet glycocalicin. Eur J Biochem. 1984 May 2;140(3):571–576. doi: 10.1111/j.1432-1033.1984.tb08140.x. [DOI] [PubMed] [Google Scholar]
- Lohmander L. S., De Luca S., Nilsson B., Hascall V. C., Caputo C. B., Kimura J. H., Heinegard D. Oligosaccharides on proteoglycans from the swarm rat chondrosarcoma. J Biol Chem. 1980 Jul 10;255(13):6084–6091. [PubMed] [Google Scholar]
- Sanderson P. N., Huckerby T. N., Nieduszynski I. A. Conformational equilibria of alpha-L-iduronate residues in disaccharides derived from heparin. Biochem J. 1987 Apr 1;243(1):175–181. doi: 10.1042/bj2430175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugahara K., Yamashina I., De Waard P., Van Halbeek H., Vliegenthart J. F. Structural studies on sulfated glycopeptides from the carbohydrate-protein linkage region of chondroitin 4-sulfate proteoglycans of swarm rat chondrosarcoma. Demonstration of the structure Gal(4-O-sulfate)beta 1-3Gal beta 1-4XYL beta 1-O-Ser. J Biol Chem. 1988 Jul 25;263(21):10168–10174. [PubMed] [Google Scholar]
- Thornton D. J., Morris H. G., Cockin G. H., Huckerby T. N., Nieduszynski I. A., Carlstedt I., Hardingham T. E., Ratcliffe A. Structural and immunological studies of keratan sulphates from mature bovine articular cartilage. Biochem J. 1989 May 15;260(1):277–282. doi: 10.1042/bj2600277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton D. J., Morris H. G., Cockin G. H., Huckerby T. N., Nieduszynski I. A. Structural studies of two populations of keratan sulphate chains from mature bovine articular cartilage. Glycoconj J. 1989;6(2):209–218. doi: 10.1007/BF01050649. [DOI] [PubMed] [Google Scholar]


