To the Editor:
Monoclonal gammopathy of thrombotic significance (MGTS) is a recently described highly prothrombotic neoplastic condition characterized by monoclonal anti-platelet factor 4 (PF4) antibodies1. Here, we describe the first report on eradicating an MGTS antibody using plasma cell-directed therapy.
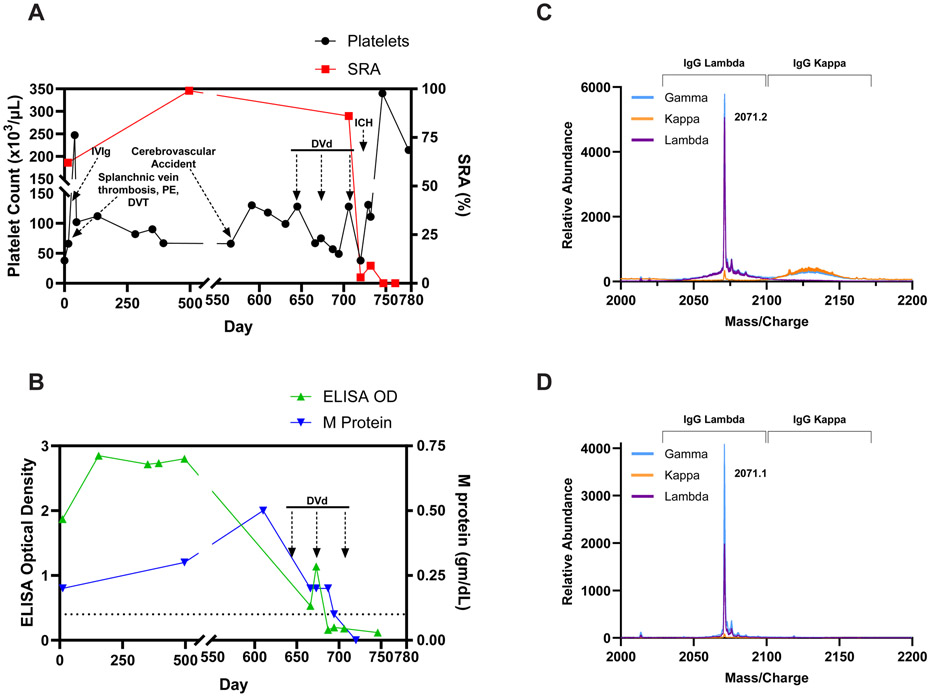
A 67-year-old male on aspirin treatment for coronary artery disease presented with thrombocytopenia (38 x 103/uL) and mesenteric ischemia secondary to extensive splanchnic vein thrombosis. Emergent small bowel resection was performed. Heparin treatment worsened splanchnic vein thrombosis, requiring additional resection. Heparin-induced thrombocytopenia (HIT) testing was positive (Figure 1A & B), and he was switched to argatroban. He developed bilateral deep vein thrombosis and pulmonary embolism, leading to cardiac arrest, requiring resuscitation. Testing also showed a monoclonal gammopathy of undetermined significance (MGUS, an IgG lambda at 0.2g/dL). He was switched to therapeutic daily fondaparinux before discharge. Positive HIT testing, MGUS, and thrombocytopenia were persistent (Figure 1A & B), and bone marrow biopsy showed 5% lambda-restricted plasma cells. Despite compliance with aspirin and fondaparinux, he presented with a cerebrovascular accident due to acute right middle cerebral and internal carotid artery occlusion, requiring thrombectomy and stent placement at which point clopidogrel was added to his regimen.
Figure 1.
(A) Key clinical events, platelet counts and serotonin release assay (SRA) results over a two year period are shown. (B) HIT ELISA results and M-protien/MGUS level in relation to DVd treatment is presented. Chemiluminescent HIT immunoassay (HemosIL AcuStar HIT-IgG (PF4/H, Werfen) was negative on five different patient samples, reminiscent of vaccine-induced immune thrombotic thrombocytopenia (VITT) antibodies (data not shown)3. Abbreviations used: IVIg- Intravenous Immunoglobulin G; DVT- deep venous thrombosis; PE- pulmonary embolism; DVd- Daratumumab-bortezomib-dexamethasone; ICH- Intracranial hemorrhage; M protein- MGUS (monoclonal antibody) protein level. Arrows under “DVd” represent first day of each treatment cycle. (C) Displayed are liquid chromatography-electrospray ionization quadrupole time-of-flight mass spectrometry (LC-ESI-QTOF MS) light chain (LC) +11 (m/z) distributions of serum proteins and (D) Anti-PF4 antibodies isolated from the patient’s serum as described in the Supplementary Appendix.
Study of antibody light chains1,2 from the MGUS (Figure 1C) and immuno-enriched anti-PF4 antibody (Figure 1D) demonstrated monoclonality in both cases, and the identical molecular masses of the MGUS and anti-PF4 antibody suggested they were synonymous. This was further supported by the ability of the immuno-enriched anti-PF4 antibody to bind PF4-polyanion complexes and activate PF4-treated platelets (Supplemental Figures 1A & 1B). Given recurrent breakthrough life-threatening thrombosis, plasma-cell-directed therapy with daratumumab, bortezomib, and dexamethasone (DVd) was initiated. Both MGUS and HIT serology improved concordantly, with HIT testing becoming negative and MGUS protein becoming undetectable after three treatment cycles (Figure 1A & B). After completion of his third cycle, he sustained a fall leading to significant intracranial hemorrhage likely due to concomitant anticoagulation/antiplatelet therapy. Likely due to eradication of the MGTS antibody he was able to receive platelet transfusions and andexanet alfa without recurrent thrombosis. He was thereafter de-escalated to aspirin and prophylactic fondaparinux without recurrent thrombosis. Complete platelet recovery was attained as a result of DVd therapy with the most recent platelet count of 214 which was remote (46 days) from platelet transfusion.
In summary, MGTS is a highly prothrombotic disorder for which anticoagulation/anti-platelet therapy may be inadequate and which could benefit from plasma cell-directed therapies.
Supplementary Material
Acknowledgments
This work was supported, in part, by National Institutes of Health grant HL158932 (AP). Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
Footnotes
Publisher's Disclaimer: This is an Author Accepted Manuscript, which is the version after external peer review and before publication in the Journal. The publisher’s version of record, which includes all New England Journal of Medicine editing and enhancements, is available at https://www.neim.orq/doi/full/10.1056/NEJMc2406453.
Contributor Information
Giselle Ghazal Salmasi, Stanford University Medical Center, Palo Alto, CA
David L. Murray, Mayo Clinic College of Medicine and Science, Rochester, MN
Anand Padmanabhan, Mayo Clinic College of Medicine and Science, Rochester, MN
References
- 1.Kanack AJ, Schaefer JK, Sridharan M, et al. Monoclonal gammopathy of thrombotic/thrombocytopenic significance. Blood 2023;141:1772–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kanack AJ, Bayas A, George G, et al. Monoclonal and oligoclonal anti-platelet factor 4 antibodies mediate VITT. Blood 2022;140:73–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vayne C, Rollin J, Gruel Y, et al. PF4 Immunoassays in Vaccine-Induced Thrombotic Thrombocytopenia. N Engl J Med 2021;385:376–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.