Abstract
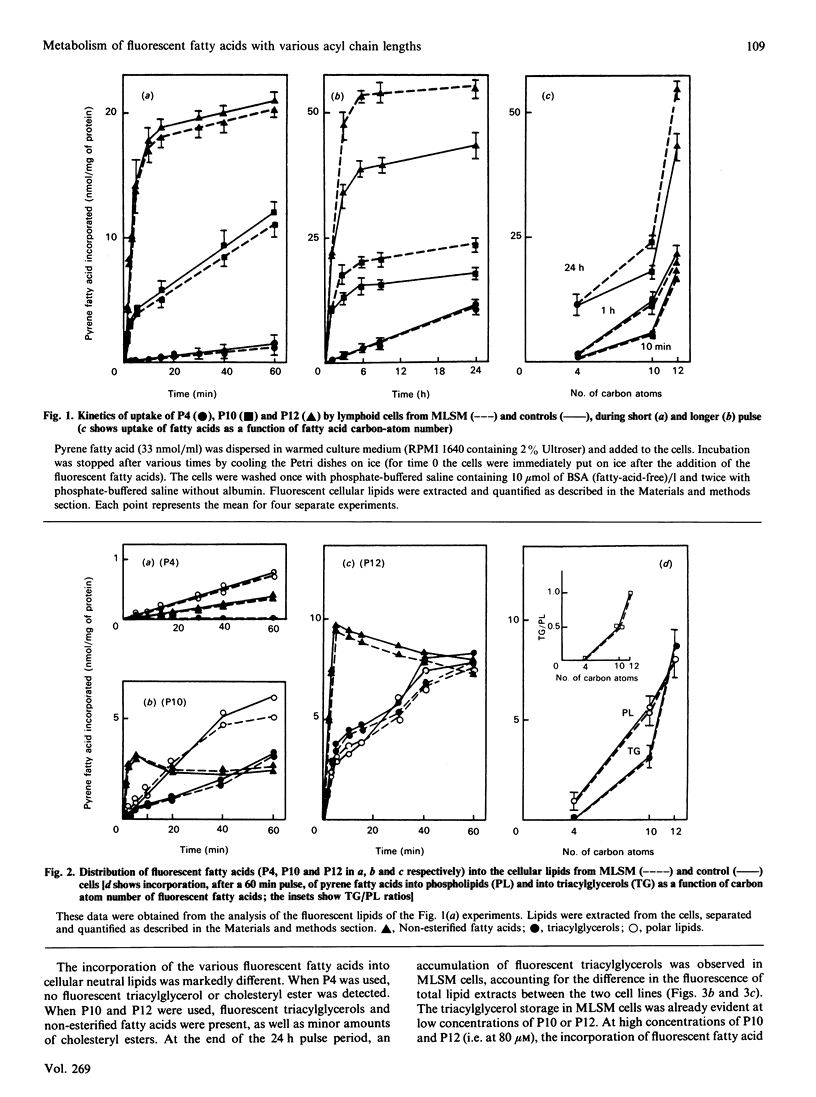
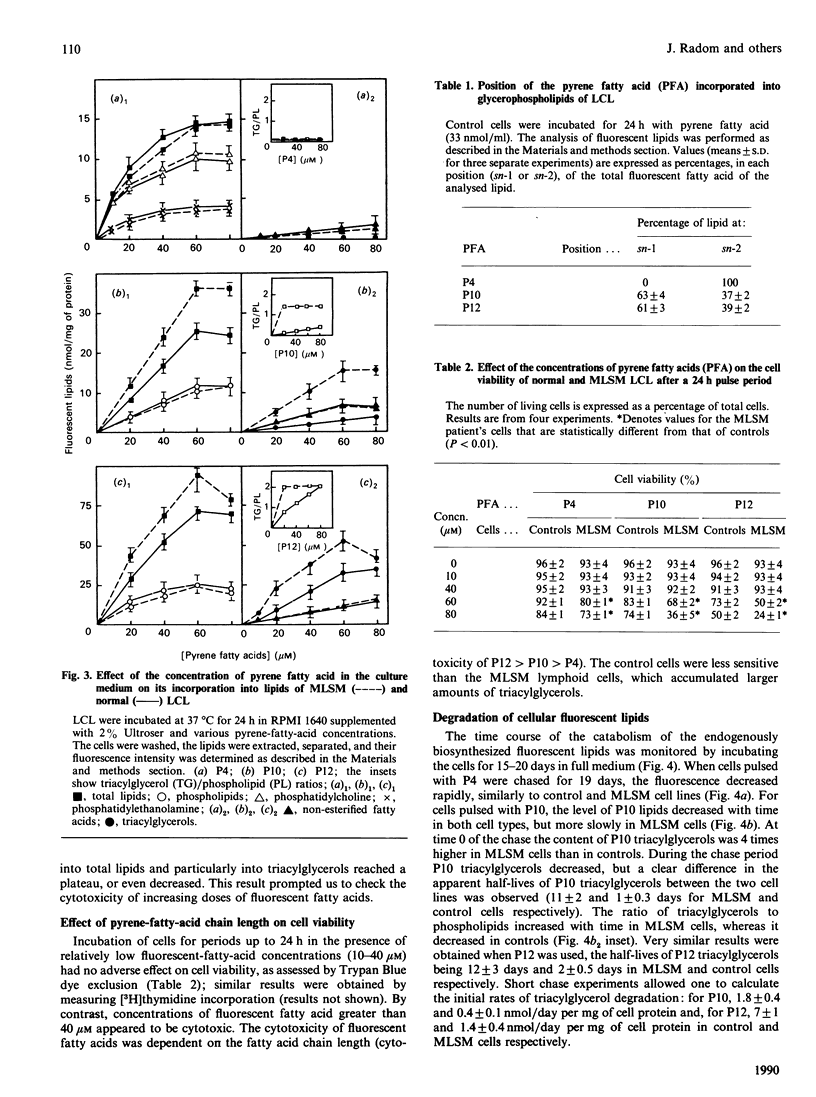
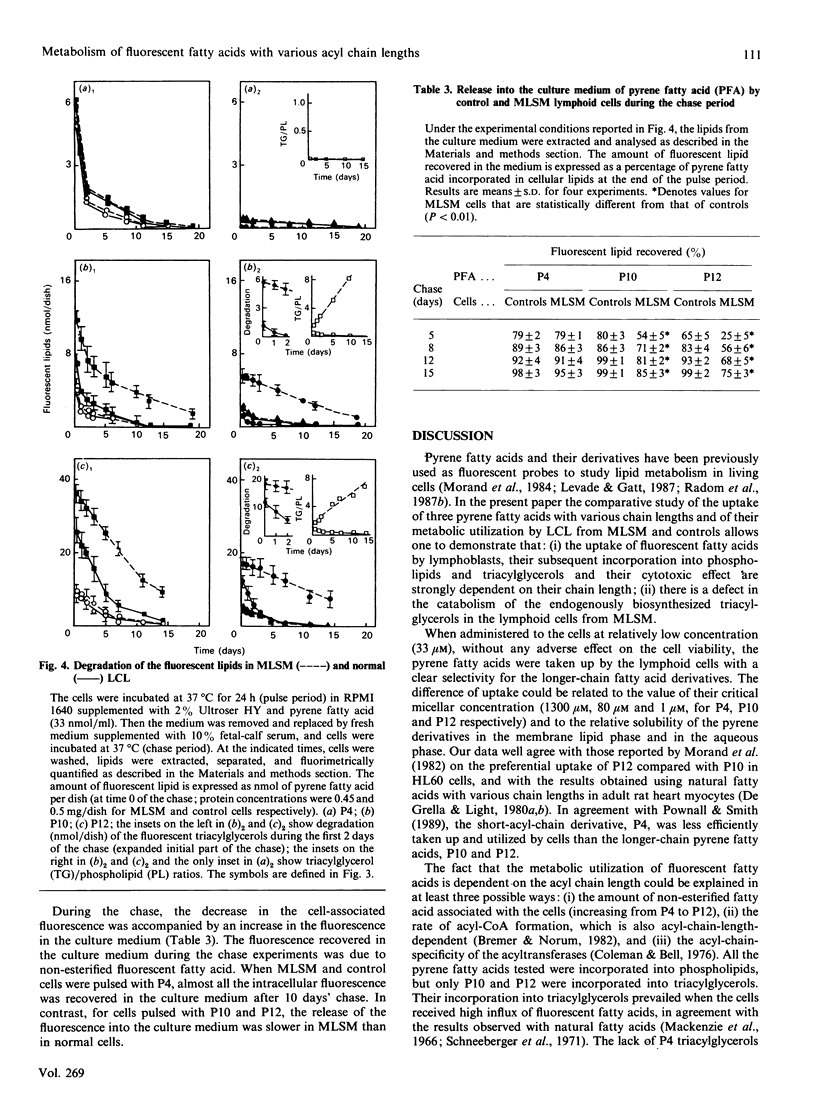
The uptake and intracellular metabolism of 4-(1-pyrene)butanoic acid (P4), 10-(1-pyrene)decanoic acid (P10) and 12-(1-pyrene)dodecanoic acid (P12) were investigated in cultured lymphoid cell lines from normal individuals and from a patient with multisystemic lipid storage myopathy (MLSM). The cellular uptake was shown to be dependent on the fatty-acid chain length, but no significant difference in the uptake of pyrene fatty acids was observed between MLSM and control lymphoid cells. After incubation for 1 h the distribution of fluorescent fatty acids taken up by the lymphoid cell lines also differed with the chain length, most of the fluorescence being associated with phospholipid and triacylglycerols. In contrast with P10 and P12, P4 was not incorporated into neutral lipids. When the cells were incubated for 24 h with the pyrene fatty acids, the amount of fluorescent lipids synthesized by the cells was proportional to the fatty acid concentration in the culture medium. After a 24 h incubation in the presence of P10 or P12, at any concentration, the fluorescent triacylglycerol content of MLSM cells was 2-5-fold higher than that of control cells. Concentrations of pyrene fatty acids higher than 40 microM seemed to be more toxic for mutant cells than for control cells. This cytotoxicity was dependent on the fluorescent-fatty-acid chain length (P12 greater than P10 greater than P4). Pulse-chase experiments permitted one to demonstrate the defect in the degradation of endogenously biosynthesized triacylglycerols in MLSM cells (residual activity was around 10-25% of controls on the basis of half-lives and initial rates of P10- or P12-labelled-triacylglycerol catabolism); MLSM lymphoid cells exhibited a mild phenotypic expression of the lipid storage (less severe than that observed in fibroblasts). P4 was not utilized in the synthesis of triacylglycerols, and thus did not accumulate in MLSM cells: this suggests that natural short-chain fatty acids might induce a lesser lipid storage in this disease.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angelini C., Govoni E., Bragaglia M. M., Vergani L. Carnitine deficiency: acute postpartum crisis. Ann Neurol. 1978 Dec;4(6):558–561. doi: 10.1002/ana.410040616. [DOI] [PubMed] [Google Scholar]
- Bremer J., Norum K. R. Metabolism of very long-chain monounsaturated fatty acids (22:1) and the adaptation to their presence in the diet. J Lipid Res. 1982 Feb;23(2):243–256. [PubMed] [Google Scholar]
- Chanarin I., Patel A., Slavin G., Wills E. J., Andrews T. M., Stewart G. Neutral-lipid storage disease: a new disorder of lipid metabolism. Br Med J. 1975 Mar 8;1(5957):553–555. doi: 10.1136/bmj.1.5957.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman R., Bell R. M. Triacylglycerol synthesis in isolated fat cells. Studies on the microsomal diacylglycerol acyltransferase activity using ethanol-dispersed diacylglycerols. J Biol Chem. 1976 Aug 10;251(15):4537–4543. [PubMed] [Google Scholar]
- DeGrella R. F., Light R. J. Uptake and metabolism of fatty acids by dispersed adult rat heart myocytes. I. Kinetics of homologous fatty acids. J Biol Chem. 1980 Oct 25;255(20):9731–9738. [PubMed] [Google Scholar]
- DeGrella R. F., Light R. J. Uptake and metabolism of fatty acids by dispersed adult rat heart myocytes. II. Inhibition by albumin and fatty acid homologues, and the effect of temperature and metabolic reagents. J Biol Chem. 1980 Oct 25;255(20):9739–9745. [PubMed] [Google Scholar]
- Di Donato S., Garavaglia B., Strisciuglio P., Borrone C., Andria G. Multisystem triglyceride storage disease is due to a specific defect in the degradation of endocellularly synthesized triglycerides. Neurology. 1988 Jul;38(7):1107–1110. doi: 10.1212/wnl.38.7.1107. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Fibach E., Gatt S. Clonal analysis of the response of human promyelocytic leukemia (HL-60) cells to photosensitization induced by a pyrene-containing fatty acid. Leuk Res. 1987;11(11):1019–1026. doi: 10.1016/0145-2126(87)90121-4. [DOI] [PubMed] [Google Scholar]
- Galla H. J., Hartmann W. Excimer-forming lipids in membrane research. Chem Phys Lipids. 1980 Oct;27(3):199–219. doi: 10.1016/0009-3084(80)90036-5. [DOI] [PubMed] [Google Scholar]
- Gatt S., Nahas N., Fibach E. Continuous spectrofluorometric measurements of uptake by cultured cells of 12-(1-pyrene)-dodecanoic acid from its complex with albumin. Biochem J. 1988 Jul 15;253(2):377–380. doi: 10.1042/bj2530377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glade P. R., Beratis N. G. Long-term lymphoid cell lines in the study of human genetics. Prog Med Genet. 1976;1:1–48. [PubMed] [Google Scholar]
- Hosaka K., Schiele U., Numa S. Diacylglycerol acyltransferase from rat liver microsomes. Separation and acyl-donor specificity. Eur J Biochem. 1977 Jun 1;76(1):113–118. doi: 10.1111/j.1432-1033.1977.tb11576.x. [DOI] [PubMed] [Google Scholar]
- JORDANS G. H. The familial occurrence of fat containing vacuoles in the leukocytes diagnosed in two brothers suffering from dystrophia musculorum progressiva (ERB.). Acta Med Scand. 1953;145(6):419–423. doi: 10.1111/j.0954-6820.1953.tb07038.x. [DOI] [PubMed] [Google Scholar]
- Klar R., Levade T., Gatt S. Synthesis of pyrenesulfonylamido-sphingomyelin and its use as substrate for determining sphingomyelinase activity and diagnosing Niemann-Pick disease. Clin Chim Acta. 1988 Sep 15;176(3):259–267. doi: 10.1016/0009-8981(88)90185-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levade T., Gatt S. Uptake and intracellular degradation of fluorescent sphingomyelin by fibroblasts from normal individuals and a patient with Niemann-Pick disease. Biochim Biophys Acta. 1987 Apr 24;918(3):250–259. doi: 10.1016/0005-2760(87)90228-1. [DOI] [PubMed] [Google Scholar]
- Mackenzie C. G., Mackenzie J. B., Riss O. K., Philpott D. E. Regulation of cell lipid metabolism and accumulation. IV. The isolation and composition of cytoplasmic lipid-rich particles. Biochemistry. 1966 May;5(5):1454–1461. doi: 10.1021/bi00869a002. [DOI] [PubMed] [Google Scholar]
- Morand O., Fibach E., Dagan A., Gatt S. Transport of fluorescent derivatives of fatty acids into cultured human leukemic myeloid cells and their subsequent metabolic utilization. Biochim Biophys Acta. 1982 Jun 11;711(3):539–550. doi: 10.1016/0005-2760(82)90070-4. [DOI] [PubMed] [Google Scholar]
- Morand O., Fibach E., Livni N., Gatt S. Induction of lipid storage in cultured leukemic myeloid cells by pyrene-dodecanoic acid. Biochim Biophys Acta. 1984 Mar 27;793(1):95–104. doi: 10.1016/0005-2760(84)90057-2. [DOI] [PubMed] [Google Scholar]
- Nahas N., Fibach E., Giloh H., Gatt S. Use of the fluorescence activated cell sorter for studying uptake of fluorescent fatty acids into cultured cells. Biochim Biophys Acta. 1987 Jan 13;917(1):86–91. doi: 10.1016/0005-2760(87)90287-6. [DOI] [PubMed] [Google Scholar]
- Nègre A., Maret A., Douste-Blazy L., Gatt S., Salvayre R. Relative fluorescence of normal and acid lipase-deficient cultured fibroblasts following administration of pyrene decanoic acid. Biochim Biophys Acta. 1988 Jun 15;960(3):401–409. doi: 10.1016/0005-2760(88)90048-3. [DOI] [PubMed] [Google Scholar]
- Nègre A., Salvayre R., Dousset N., Rogalle P., Dang Q. Q., Douste-Blazy L. Hydrolysis of fluorescent pyrenetriacylglycerols by lipases from human stomach and gastric juice. Biochim Biophys Acta. 1988 Nov 25;963(2):340–348. doi: 10.1016/0005-2760(88)90300-1. [DOI] [PubMed] [Google Scholar]
- Nègre A., Salvayre R., Maret A., Vieu C., Bes J. C., Borrone C., Durand P., Douste-Blazy L. Lymphoid cell lines as a model system for the study of Wolman's disease: enzymatic, metabolic and ultrastructural investigations. J Inherit Metab Dis. 1986;9(2):193–201. doi: 10.1007/BF01799458. [DOI] [PubMed] [Google Scholar]
- OKUYAMA H., NOJIMA S. STUDIES ON HYDROLYSIS OF CARDIOLIPIN BY SNAKE VENOM PHOSPHOLIPASE A. J Biochem. 1965 Apr;57:529–538. doi: 10.1093/oxfordjournals.jbchem.a128111. [DOI] [PubMed] [Google Scholar]
- Pope J. H., Horne M. K., Scott W. Transformation of foetal human keukocytes in vitro by filtrates of a human leukaemic cell line containing herpes-like virus. Int J Cancer. 1968 Nov 15;3(6):857–866. doi: 10.1002/ijc.2910030619. [DOI] [PubMed] [Google Scholar]
- Pownall H. J., Smith L. C. Pyrene-labeled lipids: versatile probes of membrane dynamics in vitro and in living cells. Chem Phys Lipids. 1989 Jun;50(3-4):191–211. doi: 10.1016/0009-3084(89)90050-9. [DOI] [PubMed] [Google Scholar]
- Radom J., Salvayre R., Maret A., Nègre A., Douste-Blazy L. Metabolism of 1-pyrenedecanoic acid and accumulation of neutral fluorescent lipids in cultured fibroblasts of multisystemic lipid storage myopathy. Biochim Biophys Acta. 1987 Jul 31;920(2):131–139. doi: 10.1016/0005-2760(87)90252-9. [DOI] [PubMed] [Google Scholar]
- Radom J., Salvayre R., Mussini J. M., De Lisle B., Negre A., Maret A., Billaudel S., Douste-Blazy L. Biochemical and ultrastructural features of human fibroblasts cultured from a new variant of type 3 lipid storage myopathy. Biol Cell. 1988;62(1):39–45. [PubMed] [Google Scholar]
- Radom J., Salvayre R., Negre A., Douste-Blazy L. Metabolism of pyrenedecanoic acid in Epstein-Barr virus-transformed lymphoid cell lines from normal subjects and from a patient with multisystemic lipid storage myopathy. Biochim Biophys Acta. 1989 Sep 25;1005(2):130–136. doi: 10.1016/0005-2760(89)90178-1. [DOI] [PubMed] [Google Scholar]
- Radom J., Salvayre R., Negre A., Maret A., Douste-Blazy L. Metabolism of neutral lipids in cultured fibroblasts from multisystemic (or type 3) lipid storage myopathy. Eur J Biochem. 1987 May 4;164(3):703–708. doi: 10.1111/j.1432-1033.1987.tb11183.x. [DOI] [PubMed] [Google Scholar]
- Salvayre R., Maret A., Negre A., Lenoir G., Vuillaume M., Icart J., Didier J., Douste-Blazy L. Molecular forms of beta-N-acetylhexosaminidase in Epstein-Barr virus-transformed lymphoid cell lines from normal subjects and patients with Tay-Sachs disease. Eur J Biochem. 1983 Jul 1;133(3):627–633. doi: 10.1111/j.1432-1033.1983.tb07509.x. [DOI] [PubMed] [Google Scholar]
- Salvayre R., Negre A., Maret A., Lenoir G., Douste-Blazy L. Separation and properties of molecular forms of alpha-galactosidase and alpha-N-acetylgalactosaminidase from blood lymphocytes and lymphoid cell lines transformed by Epstein-Barr virus. Biochim Biophys Acta. 1981 Jun 15;659(2):445–456. doi: 10.1016/0005-2744(81)90070-x. [DOI] [PubMed] [Google Scholar]
- Salvayre R., Nègre A., Radom J., Douste-Blazy L. Fluorometric assay for pancreatic lipase. Clin Chem. 1986 Aug;32(8):1532–1536. [PubMed] [Google Scholar]
- Samuel D., Paris S., Ailhaud G. Uptake and metabolism of fatty acids and analogues by cultured cardiac cells from chick embryo. Eur J Biochem. 1976 May 1;64(2):583–595. doi: 10.1111/j.1432-1033.1976.tb10338.x. [DOI] [PubMed] [Google Scholar]
- Schneeberger E. E., Lynch R. D., Geyer R. P. Formation and disappearance of triglyceride droplets in strain L fibroblasts. An electron microscopic study. Exp Cell Res. 1971 Nov;69(1):193–206. doi: 10.1016/0014-4827(71)90325-9. [DOI] [PubMed] [Google Scholar]
- Sun A. S., Aggarwal B. B., Packer L. Enzyme levels of normal human cells: aging in culture. Arch Biochem Biophys. 1975 Sep;170(1):1–11. doi: 10.1016/0003-9861(75)90092-2. [DOI] [PubMed] [Google Scholar]


