Abstract
PURPOSE
A head-to-head comparison of efficacy between a cyclin-dependent kinase 4/6 inhibitor plus endocrine therapy (ET) versus combination chemotherapy (CT) has never been reported in patients with clinically aggressive hormone receptor–positive, human epidermal growth factor receptor 2–negative (HR+/HER2–) advanced breast cancer (ABC).
METHODS
In this open-label, multicenter, randomized phase II trial, pre/perimenopausal women with clinically aggressive HR+/HER2– ABC were randomly assigned 1:1 to first-line ribociclib (600 mg once daily; 3 weeks on, 1 week off) plus letrozole/anastrozole and goserelin or investigator's choice of combination CT (docetaxel plus capecitabine, paclitaxel plus gemcitabine, or capecitabine plus vinorelbine). The primary end point was progression-free survival (PFS).
RESULTS
Among 222 patients randomly assigned to ribociclib plus ET (n = 112) or combination CT (n = 110), 150 (67.6%) had symptomatic visceral metastases, 41 (18.5%) had rapid disease progression per investigator's judgment, and 31 (14.0%) had symptomatic nonvisceral disease. Overall, 106 (47.7%) patients had investigator-assessed visceral crisis. The median follow-up time was 37.0 months. At data cutoff, 31.3% (ribociclib arm) and 15.5% (CT arm) of patients had completed study treatment and transitioned to post-trial access. The median PFS was 21.8 months (ribociclib plus ET; [95% CI, 17.4 to 26.7]) and 12.8 months (combination CT; [95% CI, 10.1 to 18.4); hazard ratio, 0.61 [95% CI, 0.43 to 0.87]; P = .003. The overall response rates and the median time to response in the ribociclib versus CT arms, respectively, were 66.1% and 61.8% and 4.9 months and 3.2 months (hazard ratio, 0.76 [95% CI, 0.55 to 1.06]). Lower rates of symptomatic adverse events were observed in the ribociclib versus CT arm.
CONCLUSION
First-line ribociclib plus ET showed a significant PFS benefit, similar response rates, and better tolerability over combination CT in patients with clinically aggressive HR+/HER2– ABC.
RIGHT Choice trial reports longer PFS with ribociclib + ET versus combination CT in clinically aggressive HR+/HER2–' ABC.
INTRODUCTION
Approximately one third of newly diagnosed breast cancer (BC) cases are in premenopausal women, in whom the disease is often aggressive.1-4 For hormone receptor–positive, human epidermal growth factor receptor 2–negative (HR+/HER2–) advanced breast cancer (ABC) with aggressive disease features, including symptomatic, rapidly progressing disease, or life-threatening visceral crisis requiring rapid disease control, combination chemotherapy (CT) remains a recommended first-line treatment.5,6 Several regimens (eg, docetaxel plus capecitabine, paclitaxel plus gemcitabine, or capecitabine plus vinorelbine) have demonstrated superior efficacy to that of single-agent CT but are associated with higher incidences of adverse events (AEs).7-13 Combination CT continues to be preferred in patients with critical disease features because of the need for a more rapid response and higher response rate in these patients.7-13 However, unlike the clear preference for CT for HR– ABC treatment, CT is generally less effective in HR+ ABC.14 Thus, an unmet medical need exists in the HR+/HER2– ABC patient population for therapy options that provide a rapid response and durable efficacy while sparing patients the toxicities associated with combination CT.
CONTEXT
Key Objective
Can a cyclin-dependent kinase 4/6 inhibitor (CDK4/6i) plus endocrine therapy (ET) be used instead of combination chemotherapy (CT) for treating patients with clinically aggressive hormone receptor–positive, human epidermal growth factor receptor 2–negative (HR+/HER2–) advanced breast cancer (ABC), in which combination CT is typically used to achieve a rapid response?
Knowledge Generated
This first ever prospective head-to-head comparison between a CDK4/6i (ribociclib) plus ET and combination CT showed improved progression-free survival, similar response rates, and lower symptomatic adverse event rates with ribociclib plus ET versus combination CT in patients with clinically aggressive HR+/HER2– ABC.
Relevance (K.D. Miller)
The “conventional wisdom” that patients with visceral disease need CT even if estrogen receptor–positive should be retired.*
*Relevance section written by JCO Senior Deputy Editor Kathy D. Miller, MD.
Cyclin-dependent kinase 4 and 6 inhibitors (CDK4/6i, eg, ribociclib, palbociclib, and abemaciclib) plus endocrine therapy (ET) have shown significant progression-free survival (PFS) benefit over ET alone and are now standard first-line treatment for patients with HR+/HER2– ABC.6,15-19 A significant PFS and overall survival (OS) benefit with a higher response rate was observed for first-line ribociclib plus ET over ET alone in the phase III MONALEESA-7 trial in premenopausal patients with HR+/HER2– ABC.18,20,21 However, although phase III CDK4/6i studies included patients with visceral disease, those with high burden of disease, extensive symptomatic visceral disease, or visceral crisis were excluded from these trials.15-19,22
To our knowledge, to date, no published randomized controlled trial (RCT) data have reported a comparison between a first-line CDK4/6i plus ET and combination CT in patients with clinically aggressive, high disease burden HR+/HER2– ABC. Here, we report the final analysis of the RIGHT Choice trial, the first prospective comparison of a first-line CDK4/6i (ribociclib) plus ET versus combination CT in premenopausal women with HR+/HER2– ABC with symptomatic visceral metastases, rapid disease progression or impending visceral compromise, or markedly symptomatic nonvisceral disease; these patients were defined as having clinically aggressive ABC.
METHODS
Study Design
This open-label phase II trial was conducted in 13 countries. Patients were randomly assigned (1:1) to oral ribociclib (600 mg once per day on a 3-week-on, 1-week-off schedule) plus ET (letrozole 2.5 mg or anastrozole 1 mg once daily orally; continuous daily schedule) with goserelin (3.6 mg subcutaneous implant administered once on day 1 of each 28-day cycle) or combination CT of investigator's choice among one of the three regimens (docetaxel plus capecitabine, paclitaxel plus gemcitabine, or capecitabine plus vinorelbine; Data Supplement, Table S1, online only). If one CT agent was discontinued because of AEs, patients could continue the other agent as monotherapy.
Random assignment was stratified by the presence of liver metastases (present or absent) and a disease-free interval (the time between complete tumor resection for primary BC lesion to disease recurrence) <2 years (yes or no; patients with de novo stage 4 disease were included in the disease-free interval ≥2 years group for the purpose of stratification only). The statistician was blinded to treatment until database lock. Patients received treatment until disease progression, unacceptable toxicity, death, or discontinuation for any other reason.
Participants
Eligible patients were pre/perimenopausal (hereby referred to as premenopausal) women age 18-59 years, with histologically or cytologically confirmed progesterone or estrogen (>10%) receptor–positive (ER+ or PR+), HER2– ABC (locoregionally recurrent or metastatic, not amenable to curative therapy) and an Eastern Cooperative Oncology Group performance status of 0-2. Measurable disease per RECIST version 1.1 was required.23 Patients were eligible if combination CT was clinically indicated per investigator's judgment for aggressive disease, namely symptomatic visceral metastases, rapid disease progression or impending visceral compromise, or markedly symptomatic nonvisceral disease. Patients who received (neo)adjuvant therapy for BC were eligible; adjuvant therapy with aromatase inhibitors was permitted if the subsequent treatment-free interval was >12 months.
Patients were ineligible if they received prior systemic anticancer therapy for ABC. Patients with liver metastases were ineligible if bilirubin levels were >1.5× the upper limit of normal (ULN) or if the AST or ALT levels were >5× the ULN.
End Points
The primary end point was locally assessed PFS (time from the date of random assignment to the date of the first documented progression or death due to any cause). Secondary end points were time to treatment failure (TTF), 3-month treatment failure rate (TFR), overall response rate (ORR), clinical benefit rate (CBR), time to response (TTR), OS, health-related quality of life, and safety (Data Supplement, Table S2). The 3-month TFR analysis was planned to assess the early efficacy of the treatments. The ORR, CBR, and TTR outcomes were without confirmation; confirmation imaging was not mandatory according to the study protocol as this was a phase II, nonregistrational study.23
Assessments
Tumor assessments were performed every 6 weeks (first 12 weeks), every 8 (next 32 weeks), and then every 12 weeks (Data Supplement, Table S3). AEs were characterized and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03. After discontinuation of study treatment, all patients were followed up for safety for 30 days (except in case of death, follow-up loss, or consent withdrawal). Exploratory end points were biomarker analyses and medical resource utilization. An exploratory PFS analysis of select subgroups is reported here; quality-of-life end points will be reported separately. Visceral crisis determination was according to investigator's judgment at start of the study, largely based on ABC 3 guidelines.5
The trial was conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki. The protocol and any amendments were approved by an independent ethics committee or institutional review board at each site and the health authority of participating countries. A steering committee comprising participating investigators, Novartis representatives, and one patient with BC who did not participate in this trial, supervised the study. All patients provided written informed consent. RIGHT Choice is registered with ClinicalTrials.gov (identifier: NCT03839823).
Statistical Analysis
All efficacy analyses were performed using the full analysis set, comprising all randomly assigned patients, per the intention-to-treat principle. Safety analyses were performed in patients who received ≥1 dose of any study treatment component (safety set).
For the primary efficacy analysis, PFS was compared between treatment arms using a log-rank test stratified according to randomly assigned stratification criteria. For the prespecified primary analysis, a determination that approximately 110 patients had disease progression or died was required to detect a hazard ratio of 0.67 with a power of 80% at a one-sided alpha level of 10%. The PFS was censored at the last adequate tumor assessment if no event was documented. Additionally, any event documented after two or more missing tumor assessments or initiation of a new antineoplastic therapy was censored at the adequate tumor assessment before the event. For TTF, discontinuation reasons that counted as events included AEs, death, loss to follow-up, pregnancy, progressive disease, physician or patient decision, or receipt of new antineoplastic therapy. Kaplan-Meier method was used to estimate time-to-event analyses. A stratified Cox proportional hazards model was used to estimate the hazard ratio and 95% CIs. This study was not powered to demonstrate a treatment difference in secondary end points. The PFS and secondary end points analysis presented here is from the final database lock (May 10, 2023).
RESULTS
Patients
From March 4, 2019, to November 16, 2021, a total of 222 patients were randomly assigned to receive ribociclib plus ET (n = 112) or combination CT (n = 110; CONSORT diagram [Fig 1]). Demographics and baseline characteristics were well balanced between the treatment arms (Table 1). Although 143 patients (64.4%) had de novo advanced or metastatic disease, 79 (35.6%) patients had relapsed from early disease. In total, 150 patients (67.6%) had symptomatic visceral metastases, 41 (18.5%) experienced rapid disease progression, and 31 (14.0%) had symptomatic nonvisceral metastases. Overall, 106 patients (47.7%) had investigator-assessed visceral crisis. In addition, most patients (n = 191; 86.0%) had ≥50% ER+ tumors. The majority of patients (n = 124, 55.9%) had ≥3 metastatic sites. Specifically, liver-only, lung-only, and liver or lung metastases were present in 107 (48.2%), 117 (52.7%), and 169 (76.1%) patients, respectively.
FIG 1.
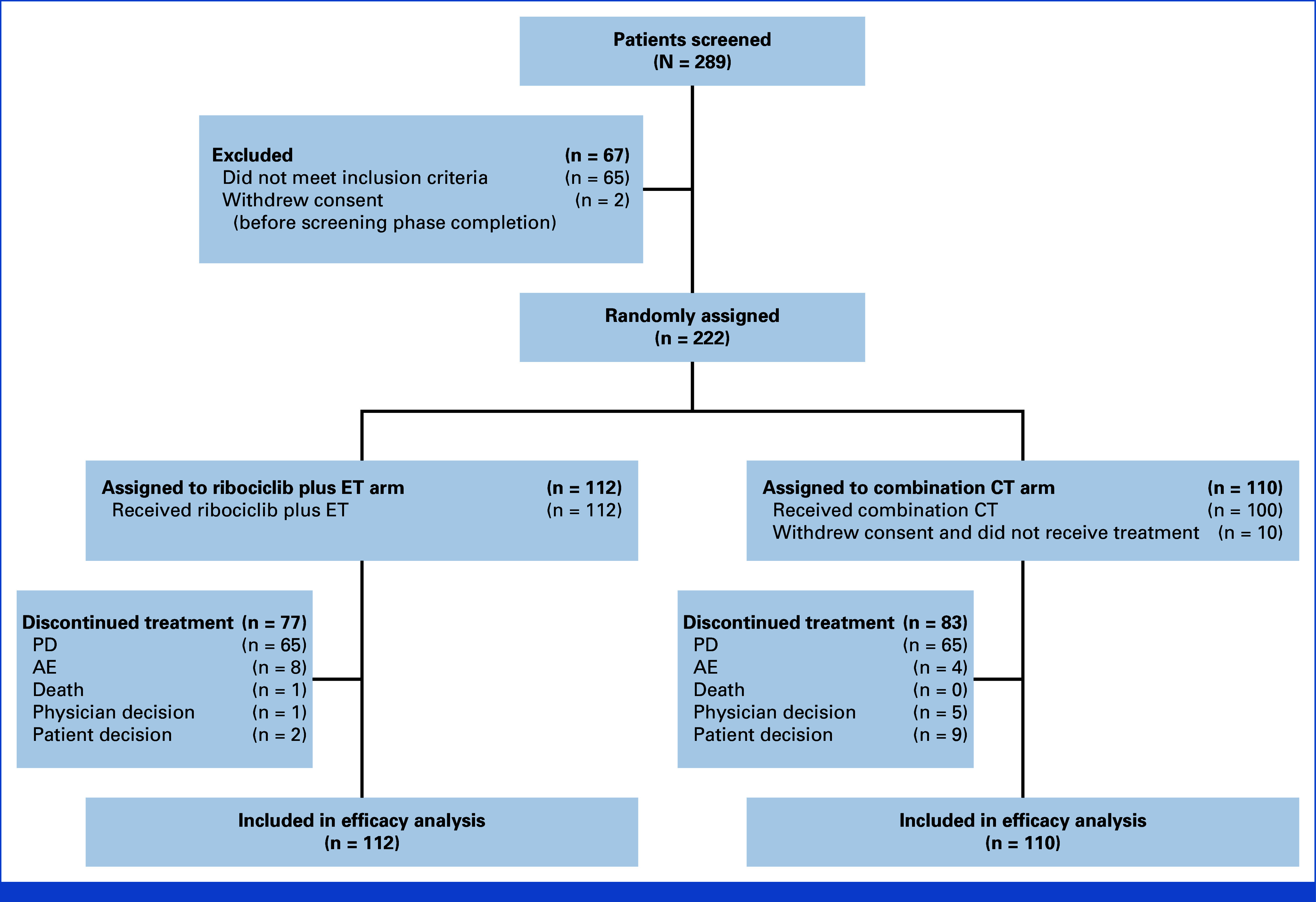
CONSORT diagram. AE, adverse event; CT, chemotherapy; ET, endocrine therapy; PD, progressive disease.
TABLE 1.
Patient Characteristics at Baseline
| Characteristics | Ribociclib + ET (n = 112) | Combination CT (n = 110) |
|---|---|---|
| Age, years, median (range) | 44.0 (26-58) | 43.0 (26-55) |
| Female sex, No. (%) | 112 (100.0) | 110 (100.0) |
| Race, No. (%) | ||
| Asian | 60 (53.6) | 58 (52.7) |
| White | 51 (45.5) | 52 (47.3) |
| Black or African American | 1 (0.9) | 0 |
| Histological tumor grade, No. (%) | ||
| I | 10 (8.9) | 16 (14.5) |
| II | 66 (58.9) | 61 (55.5) |
| III | 35 (31.3) | 29 (26.4) |
| Missing | 1 (0.9) | 4 (3.6) |
| ECOG performance status, No. (%) | ||
| 0 | 46 (41.1) | 42 (38.2) |
| 1 | 63 (56.3) | 62 (56.4) |
| 2 | 3 (2.7) | 6 (5.5) |
| Disease-free interval,a No. (%) | ||
| De novo disease | 70 (62.5) | 73 (66.4) |
| Relapsed from early breast cancer | 42 (37.5) | 37 (33.6) |
| ≤12 months | 6 (5.4) | 2 (1.8) |
| >12 and ≤24 months | 8 (7.1) | 7 (6.4) |
| >24 months | 28 (25.0) | 28 (25.5) |
| HER2 receptor negative, No. (%) | 112 (100.0) | 110 (100.0) |
| Estrogen receptor positive,b No. (%) | 112 (100.0) | 110 (100.0) |
| ≥50% | 95 (84.8) | 96 (87.3) |
| <50% | 8 (7.1) | 4 (3.6) |
| Progesterone receptor positive,c No. (%) | 99 (88.4) | 102 (92.7) |
| Disease history, No. (%) | ||
| Rapid progression | 23 (20.5) | 18 (16.4) |
| Symptomatic nonvisceral disease | 15 (13.4) | 16 (14.5) |
| Symptomatic visceral metastases | 74 (66.1) | 76 (69.1) |
| Visceral crisis status, No. (%) | ||
| Yes | 57 (50.9) | 49 (44.5) |
| Metastatic sites,d No. (%) | ||
| Bone | 60 (53.6) | 68 (61.8) |
| Bone only | 5 (4.5) | 4 (3.6) |
| CNS | 1 (0.9) | 3 (2.7) |
| Liver | 54 (48.2) | 53 (48.2) |
| Liver or lung | 87 (77.7) | 82 (74.5) |
| Lung | 62 (55.4) | 55 (50.0) |
| Lymph node | 74 (66.1) | 75 (68.2) |
| Other | 46 (41.1) | 38 (34.5) |
| Skin | 9 (8.0) | 2 (1.8) |
| Soft tissue | 3 (2.7) | 5 (4.5) |
| Metastatic sites, No. (%) | ||
| 1 | 19 (17.0) | 11 (10.0) |
| 2 | 29 (25.9) | 39 (35.5) |
| ≥3 | 64 (57.1) | 60 (54.5) |
Abbreviations: BC, breast cancer; CT, chemotherapy; ECOG, Eastern Cooperative Oncology Group; ET, endocrine therapy; HER2, human epidermal growth factor receptor 2.
Defined as the duration between the date patient received complete tumor resection for primary BC lesion to the date of disease recurrence.
Among the nine patients in the ribociclib plus ET arm with missing estrogen receptor percentage, one patient had an Allred score of 5, two patients had an Allred score of 6, five patients had an Allred score of 8, and one patient did not have estrogen receptor percentage or Allred score.
Two patients in the ribociclib plus ET arm had an unknown progesterone receptor status.
The same patient may have multiple visceral metastatic sites.
In the CT arm, 10 patients did not receive any study treatment because of consent withdrawal (n = 9) and physician's decision to withdraw (n = 1); all patients in the ribociclib arm received study treatment. Among the 100 patients who received combination CT, 24 (24.0%) received docetaxel plus capecitabine, 34 (34.0%) received paclitaxel plus gemcitabine, and 42 (42.0%) received capecitabine plus vinorelbine. At the second and final database lock data cutoff, the median follow-up time (the time from random assignment to data cutoff date) was 37.01 months. Overall, 35 (31.3%) and 17 (15.5%) patients in the ribociclib and CT arms, respectively, completed study treatment and were transitioned to the post-trial access program. Treatment was discontinued in 77 (68.8%) and 83 (75.5%) patients in the ribociclib and CT arms, mostly because of disease progression (65 [58.0%] and 65 [59.1%] patients in the ribociclib and CT arms, respectively [Data Supplement, Table S4]). The median duration of treatment exposure was 17.6 months (IQR, 7.9-29.5) in the ribociclib arm and 10.9 months (IQR, 6.3-17.7) among the three combination CT regimens. The median relative dose intensity in the ribociclib arm was 97.35% (IQR, 73.02%-100%). In the ribociclib arm, 24.1% and 5.4% of patients required one or two ribociclib dose reductions, respectively; >2 ribociclib dose reductions were not allowed. In the CT arm, 13.0%, 16.0%, and 20.0% of patients required one, two, or three or more dose reductions, respectively.
Primary End Point
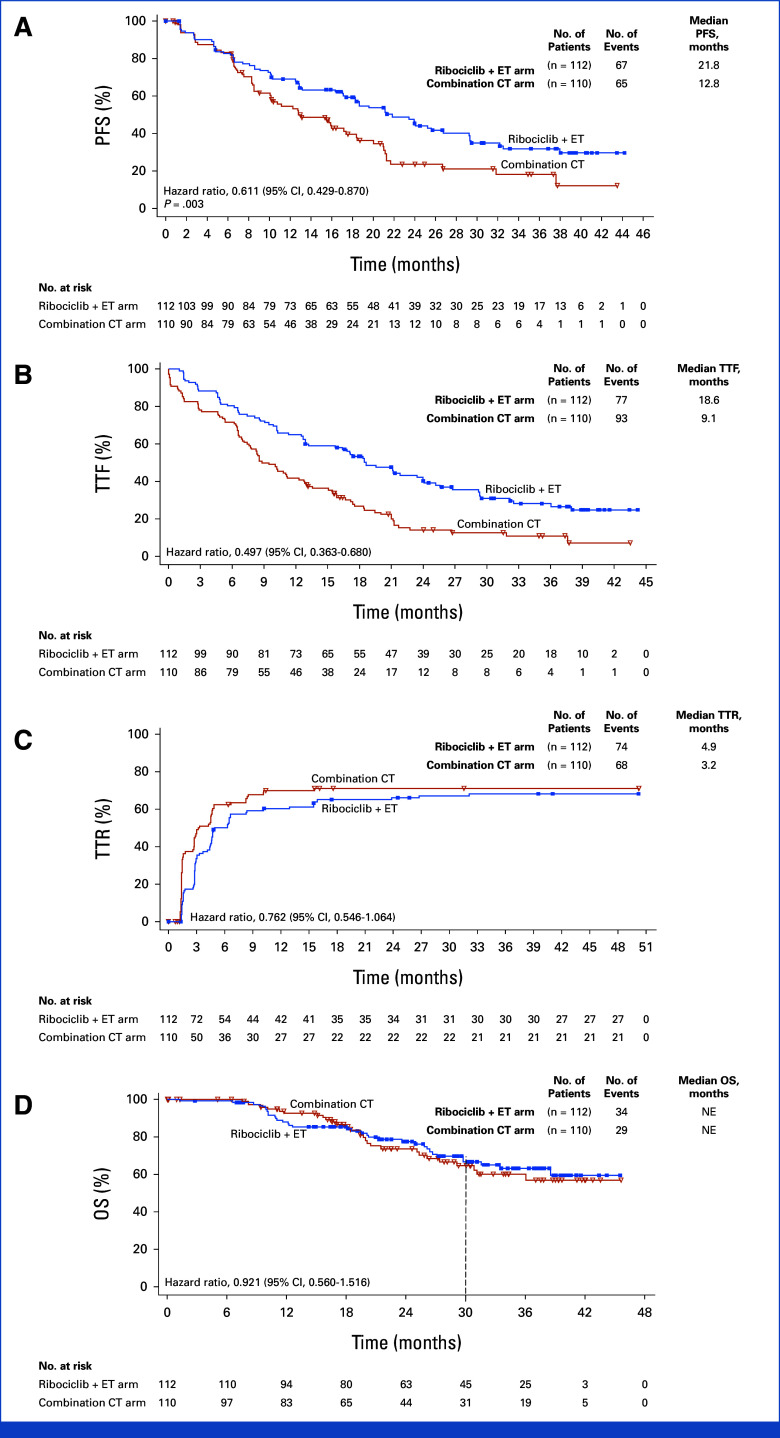
At data cutoff for final PFS analysis, at which 132 events had occurred, the median PFS was 21.8 months (95% CI, 17.4 to 26.7) with ribociclib plus ET versus 12.8 months (95% CI, 10.1 to 18.4) with combination CT (hazard ratio, 0.61 [95% CI, 0.43 to 0.87]; one-sided P = .003; Fig 2A). At 12 and 24 months, the PFS rates were 68.9% (95% CI, 59.3 to 76.7) and 46.5% (95% CI, 36.4 to 56.0) in the ribociclib versus 54.5% (95% CI, 43.7 to 64.0) and 23.6% (95% CI, 14.2 to 34.4) in the CT arm, respectively. The PFS benefit in the subgroups was generally consistent with the overall population; however, the degree of benefit was less in patients in visceral crisis and in those with recurrent disease (Fig 3).
FIG 2.
Kaplan-Meier analysis of (A) PFS, (B) TTF, (C) TTR, and (D) OS. CT, chemotherapy; ET, endocrine therapy; OS, overall survival; PFS, progression-free survival; TTF, time to treatment failure; TTR, time to response.
FIG 3.
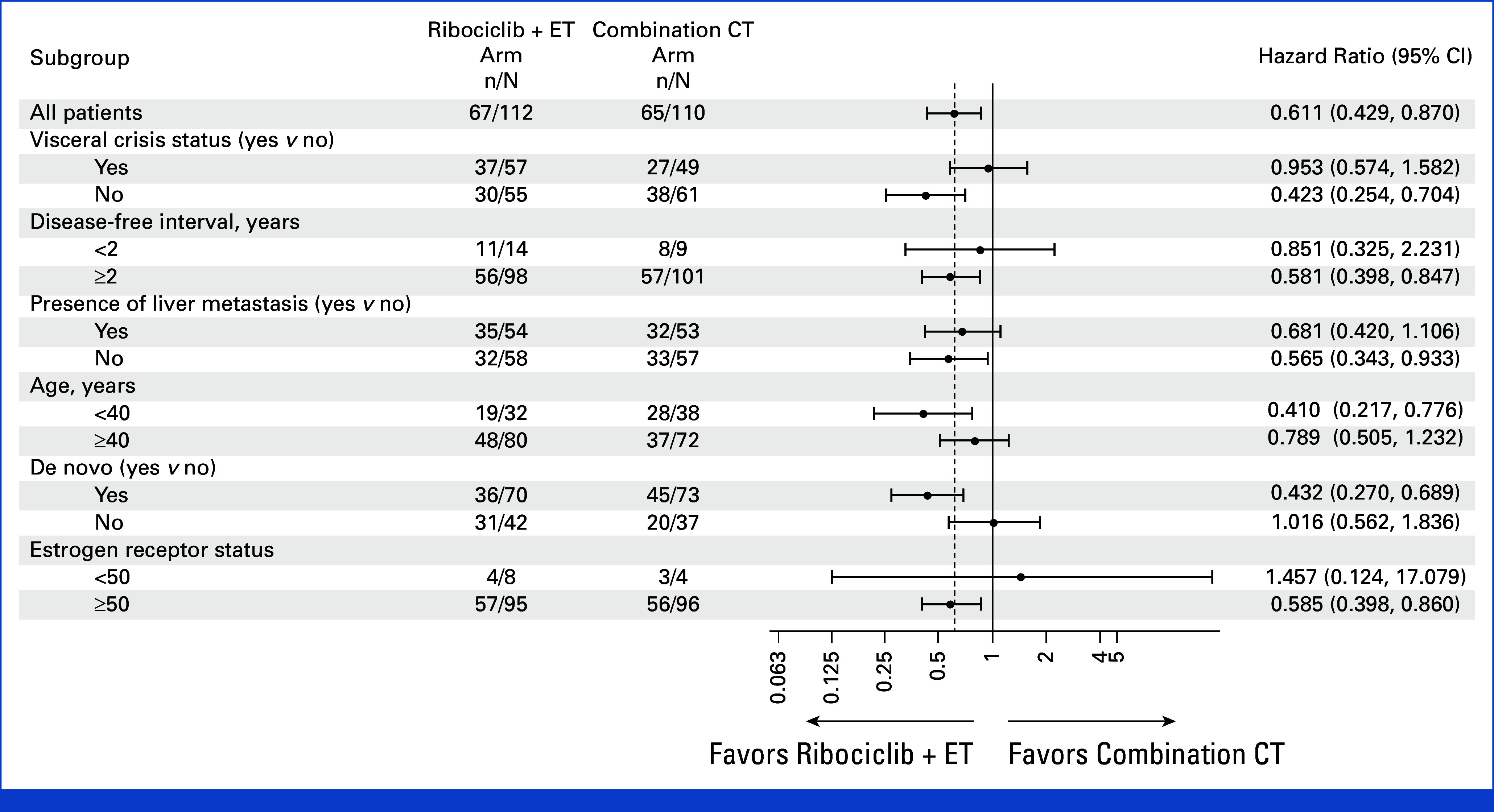
Subgroup analysis of PFS. The results from subgroups with small patient numbers (disease-free interval <2 years and low [<50] estrogen receptor–positive status) need to be interpreted with caution. CT, chemotherapy; ET, endocrine therapy; PFS, progression-free survival.
Secondary End Points
The median TTF was 18.6 months versus 9.1 months with ribociclib plus ET and combination CT (hazard ratio, 0.50 [95% CI, 0.36 to 0.68]; Fig 2B), respectively. The 3-month TFRs were 11.6% (95% CI, 6.3 to 19.0) with ribociclib plus ET versus 21.8% (95% CI, 14.5 to 30.7) with combination CT. Most 3-month treatment failure events were due to disease progression with similar rates in both arms (ribociclib plus ET, 9.8%; combination CT, 10.0%; Data Supplement, Table S5).
The median TTR was 4.9 months versus 3.2 months with ribociclib plus ET and combination CT (hazard ratio, 0.76 [95% CI, 0.55 to 1.06]; Fig 2C), respectively. Waterfall plots showed comparable tumor size changes from baseline to weeks 6 and 12 between the treatment arms (Data Supplement, Fig S1). The ORR was 66.1% in the ribociclib arm and 61.8% in the CT arm while the CBR was 81.3% in the ribociclib arm and 74.5% in the CT arm (Table 2). Sensitivity analyses in the safety set, which excluded the 10 patients in the CT arm who did not receive any study treatment, confirmed these findings (Data Supplement, Table S6, Fig S2).
TABLE 2.
ORR and CBR (full analysis set)
| Outcome Measures | Ribociclib + ET (n = 112)a | Combination CT (n = 110)a |
|---|---|---|
| Best overall response | ||
| Complete response | 7 (6.3) | 3 (2.7) |
| Partial response | 67 (59.8) | 65 (59.1) |
| Stable disease | 27 (24.1) | 20 (18.2) |
| Progressive disease | 9 (8.0) | 6 (5.5) |
| Unknown | 2 (1.8) | 16 (14.5) |
| ORR,b No. (%) 95% CI |
74 (66.1) 56.5 to 74.7 |
68 (61.8) 52.1 to 70.9 |
| CBR,c No. (%) 95% CI |
91 (81.3) 72.8 to 88.0 |
82 (74.5) 65.4 to 82.4 |
NOTE. Data are No. (%) or No. (%) (95% CI). The 95% CIs for the frequency distribution of each variable were computed using a normal approximation method.
Abbreviations: CBR, clinical benefit rate; CT, chemotherapy; ET, endocrine therapy; ORR, overall response rate.
Patients with measurable disease at baseline were included in these analyses.
Patients with complete or partial response without confirmation.
Patients with complete or partial response without confirmation (or stable disease lasting 24 weeks or more or noncomplete response without progressive disease lasting 24 weeks or more). Confirmation imaging was not mandatory according to the study protocol, as this was a phase II, nonregistrational study.23
The OS data were immature at database cutoff date, with 34 (30.4%) and 29 (26.4%) deaths in the ribociclib and CT arms, respectively. The median OS was not reached (NR) in the ribociclib arm (95% CI, 38.6 months to NR) or the CT arm (95% CI, 30.8 months to NR; hazard ratio, 0.92 [95% CI, 0.56 to 1.52]). The 12-, 18-, 24- and 30-month OS rates were 87.9%, 85.1%, 77.3%, and 66.6% and 92.5%, 86.5%, 73.7%, and 64.6% in the ribociclib and CT arm, respectively (Fig 2D).
Safety
The safety set included 112 and 100 patients in the ribociclib and CT arms, respectively. All patients experienced at least one all-grade AE (Table 3 and Data Supplement, Table S7). Higher rates of hematologic events including neutropenia and leukopenia were observed with ribociclib plus ET while higher rates of nonhematologic events including nausea, vomiting, diarrhea, and fatigue were observed with combination CT.
TABLE 3.
Adverse Events
| Events | Ribociclib + ET (n = 112), No. (%) | Combination CT (n = 100),a No. (%) | ||||
|---|---|---|---|---|---|---|
| All Grades | Grade 3 | Grade 4 | All Grades | Grade 3 | Grade 4 | |
| Any eventb | 112 (100.0) | 71 (63.4) | 18 (16.1) | 100 (100.0) | 62 (62.0) | 11 (11.0) |
| Hematologic events | ||||||
| Neutropenia | 94 (83.9) | 57 (50.9) | 10 (8.9) | 50 (50.0) | 29 (29.0) | 7 (7.0) |
| Leukopenia | 55 (49.1) | 28 (25.0) | 0 | 26 (26.0) | 7 (7.0) | 1 (1.0) |
| Anemia | 40 (35.7) | 6 (5.4) | 0 | 43 (43.0) | 11 (11.0) | 0 |
| Nonhematologic events | ||||||
| ALT increased | 23 (20.5) | 6 (5.4) | 0 | 30 (30.0) | 6 (6.0) | 0 |
| AST increased | 23 (20.5) | 8 (7.1) | 0 | 29 (29.0) | 5 (5.0) | 0 |
| Nausea | 14 (12.5) | 0 | 0 | 27 (27.0) | 1 (1.0) | 0 |
| Alopecia | 12 (10.7) | 0 | 0 | 20 (20.0) | 0 | 0 |
| Vomiting | 8 (7.1) | 1 (0.9) | 0 | 30 (30.0) | 0 | 0 |
| Diarrhea | 3 (2.7) | 0 | 0 | 26 (26.0) | 1 (1.0) | 0 |
| Fatigue | 9 (8.0) | 0 | 0 | 25 (25.0) | 2 (2.0) | 0 |
| Palmar-plantar erythrodysesthesia | 3 (2.7) | 0 | 0 | 32 (32.0) | 5 (5.0) | 0 |
Abbreviations: CT, chemotherapy; ET, endocrine therapy.
The 10 patients in the combination CT arm who were randomly assigned to CT but did not receive any treatment were not included in the safety set.
Listed are events that were reported in at least 20% of the patients in either arm irrespective of causality.
Overall, 79.5% and 73.0% of patients in the ribociclib and CT arms, respectively, experienced an all-cause grade 3 or 4 AEs. The most common grade 3 or 4 AEs were neutropenia (59.8% and 36.0%) and leukopenia (25.0% and 8.0%) in the ribociclib and CT arm, respectively. The most common grade 3 or 4 biochemical abnormality was an increased ALT level (ribociclib arm, 6.3%; CT arm, 12.0%; Data Supplement, Table S8). Two patients (1.8%) in the ribociclib arm experienced grade ≥3 QTc prolongation without evidence of arrythmia. Grade ≥3 febrile neutropenia was reported in three patients (3.0%) in the CT arm only. All-grade and grade 3/4 infections occurred in 39.3% and 5.4% versus 44.0% and 12.0% of patients in the ribociclib and CT arms, respectively. The colony-stimulating factors were used in 4.5% of patients in the ribociclib arm (not recommended per protocol for patients receiving ribociclib with neutropenia without infection) versus 25.0% in the CT arm. Overall, treatment-related AEs led to discontinuation of any study component in 6.3% versus 27.0% of patients in the ribociclib and CT arms, respectively. In the ribociclib arm, patients discontinued because of increased AST (four patients) or bilirubin (two patients); in the CT arm, patients discontinued because of neutropenia (six patients), palmar-plantar erythrodysesthesia (five patients), peripheral sensory neuropathy (three patients), or pulmonary embolism (two patients). Treatment-related serious AEs were reported in two (1.8%) and eight (8.0%) patients in the ribociclib and CT arms, respectively.
Five deaths (4.5%) occurred in the ribociclib arm during the 30 days after the end of study treatment; these deaths were attributed to BC progression. These five patients had a ribociclib treatment duration of 1.0 month, 8.6 months, 9.9 months, 18.2 months, and 23.4 months. No on-treatment deaths occurred in the combination CT arm. The patient in the ribociclib arm who died during the first 6 months of treatment experienced a serious AE of sepsis, which was not considered treatment related according to the principal investigator's judgment, with death on study day 38 attributed to ABC.
DISCUSSION
This final analysis of the RIGHT Choice trial showed a clinically meaningful, statistically significant PFS benefit with first-line ribociclib plus ET over combination CT in premenopausal women with clinically aggressive HR+/HER2– ABC in which combination CT is typically is used to achieve a rapid tumor response. This PFS benefit was observed in most subgroups. In this trial, PFS with combination CT was longer than the historical data in advanced disease.7-13 Ribociclib plus ET showed a longer TTF and a similar ORR as combination CT, matching historical combination CT tumor response rates.7-9 Although the median TTR slightly favored combination CT over ribociclib plus ET by 1.7 months in this premenopausal patient population (Fig 2C), the similar ORR along with the similar changes in tumor size from baseline to weeks 6 and 12 with ribociclib plus ET and combination CT (Data Supplement, Fig S1), indicated comparable activity at those time points. The OS data, although immature at final database lock, showed a similar survival trend for both arms, suggesting there is likely no meaningful difference in survival benefit with combination CT versus ribociclib plus ET.
AEs with ribociclib plus ET were in line with the known safety profile, with no new safety signals observed.17-19 AEs with combination CT were also consistent with previously published data, with higher rates of symptomatic AEs including nausea, vomiting, fatigue, and diarrhea, compared with ribociclib plus ET.7-9,24 Additionally, treatment-related AEs that led to discontinuation of any study component were seen in a higher percentage of patients receiving combination CT versus those receiving ribociclib plus ET, thus supporting a favorable tolerability of ribociclib plus ET. As determining the choice of treatment includes taking into account the relative toxicity of each treatment, these efficacy and safety data collectively show that ribociclib plus ET may be a better alternative to combination CT in this patient population.
In RIGHT Choice, 47.7% of patients were determined to have visceral crisis by investigators' assessment (principally based on ABC 3 guidelines available at the time of study design), reflecting the considerable disease burden of the trial patients.5 The visceral crisis definition remains imprecise, and determination largely depends on clinical judgment; thus, some subjectivity was involved when characterizing patients in this regard. The ABC 5 guidelines, published in 2020, further clarified the visceral crisis definition by adding laboratory evaluation of liver function on the basis of elevated bilirubin levels.6 However, patients with liver metastases and bilirubin levels >1.5 times the ULN were ineligible for this trial, as such patients require immediate individualized treatment, which clearly impedes their inclusion in a RCT. Exploratory subgroup analysis of patients with investigator-assessed visceral crisis in this trial showed similar PFS and TTR durations in the two arms; however, the symptomatic AE rates were lower in those in the ribociclib versus the CT arm.25
A few specificities of this trial must be considered. The sample size was smaller in this phase II proof-of-concept study, as performing large-scale phase III studies for this specific patient population was not possible. As treatment blinding could not be implemented in the open-label design of this trial, investigators and patients were aware of treatment assignment information that may have led to detection and performance bias. Ten patients in the CT arm did not receive any study treatment; however, this fact likely did not affect the efficacy results for the intention-to-treat population, as confirmed by sensitivity analyses in the safety set that excluded these patients (Data Supplement, Fig S2, Data Supplement, Table S6). The CT regimens used here are commonly used CT regimens in the ABC clinical setting. Not all combination CT regimens used in the ABC setting in clinics could be included in the comparator arm. Anthracycline-based combination CT regimens, which have been shown to have efficacy as first-line treatments in patients with ABC, were not included because of potential of increased cardiotoxicities associated with them; notably, 32 (14.4%) patients had received anthracycline in (neo)adjuvant setting and relapsed.26-29 In addition, most patients had >50% ER+ tumors and PR+ tumors; therefore, these findings may not apply to patients with low ER+ or PR– tumors. The 50% ER cutoff to split patients with lower versus higher endocrine sensitivity was used based on significant differences in ET benefit between these tumor ER expression levels.30 Finally, the majority of patients in this trial have de novo ABC disease and thus the validity of these findings in patients with recurrent disease warrants further investigation.
The results of the RIGHT Choice trial are aligned with those from the MONALEESA-7 trial, which showed PFS benefit (median PFS: 23.8 months) with first-line ribociclib plus ET in premenopausal patients with HR+/HER2– ABC.18 However, MONALEESA-7 excluded patients with extensive symptomatic disease or visceral crisis and included patients with prior CT in the advanced setting.18 The Young-PEARL and PEARL trials are the only published examples comparing a CDK4/6i plus ET with single-agent CT in patients with HR+/HER2– ABC.31,32 In Young-PEARL, which excluded patients with symptomatic serious visceral metastases, second-line palbociclib plus exemestane demonstrated longer PFS over capecitabine by 5.7 months in premenopausal patients.32 In PEARL, second-line palbociclib plus ET did not meet the superiority threshold versus single-agent CT in postmenopausal women with less aggressive disease.31 Conversely, RIGHT Choice investigated first-line treatment of patients with a significant disease burden using combination CT as the comparator.
In conclusion, we report the final analysis of the phase II RIGHT Choice trial of first-line ribociclib plus ET versus combination CT in premenopausal women with clinically aggressive HR+/HER2– ABC, including investigator-assessed visceral crisis. The data show PFS superiority with ribociclib plus ET over combination CT, with similar response rates, lower symptomatic AE rates, and fewer discontinuations because of treatment-related AEs. Thus, ribociclib plus ET could be considered a first-line treatment option in this patient population.
ACKNOWLEDGMENT
We thank the patients enrolled in these studies and their families, as well as the study investigators. We also thank Dr Malwinder Singh Sandhu, the site personnel, and the team that supported this trial. The authors would also like to thank Christine Chateauneuf, Michael Goldbrunner, Cassandra Slader, Laura Torres Perez, Huilin Hu, Soyoun Park, Shraddha Serathia, and Ajit Patil for their support. Medical editorial assistance with earlier versions of this manuscript was provided by Shashank Tandon, PhD, and Aaron Runkle, PhD, of Nucleus Global. Ribociclib was discovered by the Novartis Institutes for BioMedical Research in collaboration with Astex Pharmaceuticals.
List of Principal Investigators and Recruitment Sites are available at Appendix Table A1 (online only).
APPENDIX
TABLE A1.
List of Principal Investigators and Recruitment Sites
| Investigator | Country |
|---|---|
| Adher Alsayed | Saudi Arabia |
| Ahmed El Bastawisy | Egypt |
| Ajay Gogia | India |
| Chaiyut Charoentum | Thailand |
| Chanchal Goswami | India |
| Chien-Ting Liu | Taiwan |
| Chun Sen Lim | Malaysia |
| Elena Artamonova | Russian Federation |
| Erhan Gokmen | Turkey |
| Esat Namal | Turkey |
| Eznal Izwadi Mohd Mahidin | Malaysia |
| Fadi Farhat | Lebanon |
| Flora Chong Li Tze | Malaysia |
| Gül Başaran | Turkey |
| Hakan Harputluoglu | Turkey |
| Hamdy Abdel Azim | Egypt |
| Hikmat Abdelrazeq | Jordan |
| Hesham ElGhazaly | Egypt |
| K. Govind Babu | India |
| Konstantin Penkov | Russian Federation |
| Le Thanh Duc | Vietnam |
| Ling-Ming Tseng | Taiwan |
| Liudmila Osmanova | Russian Federation |
| Ludmila Zhukova | Russian Federation |
| Meher Lakshmi Konatum | India |
| Mehmet Ali Nahit Şendur | Turkey |
| Ming-Shen Dai | Taiwan |
| Mona Ayoubi | Lebanon |
| Nagi El-Saghir | Lebanon |
| Naiyarat Prasongsook | Thailand |
| Napa Parinyanitikul | Thailand |
| Nikita Volkov | Russian Federation |
| Nuri Karadurmus | Turkey |
| Patrapim Sunpaweravong | Thailand |
| Peter Cher Siang Ang | Singapore |
| Pei Jye Voon | Malaysia |
| Rabab Gaafar | Egypt |
| Rasha Abdel Motagaly | Egypt |
| Richard Khanyile | South Africa |
| Sema Sezgin Goksu | Turkey |
| Shin-Cheh Chen | Taiwan |
| Sudeep Gupta | India |
| Su Mien Lynette Ngo | Singapore |
| Swee Hsia Choong | Malaysia |
| Terence Aik Huang Tan | Singapore |
| Umut Demirci | Turkey |
| Wei-Pang Chung | Taiwan |
| Wen-Son Hsieh | Singapore |
| Yen-Shen Lu | Taiwan |
| Yesim Eralp | Turkey |
| Yoon Sim Yap | Singapore |
| Yuan-Ching Chang | Taiwan |
| Yueh Ni Lim | Malaysia |
Yen-Shen Lu
Honoraria: Pfizer, Roche, MSD, Novartis, Lilly, Eisai, Daiichi Sankyo/UCB Japan, AstraZeneca, EuroPharma
Consulting or Advisory Role: Pfizer, Roche, Novartis, Lilly
Research Funding: Novartis (Inst), MSD (Inst), AstraZeneca (Inst)
Travel, Accommodations, Expenses: Novartis
Hamdy Azim
Honoraria: AstraZeneca, Bristol Myers Squibb, Lilly, MSD, Novartis, Pfizer, Roche, GlaxoSmithKline, Sanofi/Aventis
Consulting or Advisory Role: AstraZeneca, Bristol Myers Squibb, Lilly, MSD, Novartis, Pfizer, Roche, GlaxoSmithKline, Sanofi/Aventis
Speakers' Bureau: AstraZeneca, Bristol Myers Squibb, Lilly, MSD, Novartis, Pfizer, Roche
Research Funding: Roche, Novartis
Yesim Eralp
Honoraria: Gilead Sciences, Amgen, BMS Turkey, Amgen, AstraZeneca
Consulting or Advisory Role: Novartis, MSD Oncology, Gilead Sciences, MSD Oncology, Lilly, Novartis, MSD Oncology, MSD Oncology, GlaxoSmithKline, MSD Oncology, Novartis
Research Funding: Novartis
Other Relationship: Novartis
Yoon Sim Yap
Honoraria: Novartis, Pfizer, AstraZeneca, MSD, Gilead Sciences, DKSH
Consulting or Advisory Role: Novartis, Lilly, Pfizer, AstraZeneca, MSD
Research Funding: MSD Oncology (Inst)
Travel, Accommodations, Expenses: DKSH, AstraZeneca, MSD, Pfizer, Daiichi Sankyo
Seock-Ah Im
Consulting or Advisory Role: AstraZeneca, Novartis, Roche/Genentech, Eisai, Pfizer, Amgen, Hanmi, Lilly, MSD, Daiichi Sankyo
Research Funding: AstraZeneca (Inst), Pfizer (Inst), Roche/Genentech (Inst), Daewoong Pharmaceutical (Inst), Eisai (Inst), Boryung Pharmaceuticals (Inst)
Other Relationship: Roche
Erhan Gokmen
Stock and Other Ownership Interests: Immunogen, MacroGenics, G1 Therapeutics, Fibrogen
Honoraria: Lilly, Roche, Pfizer, Novartis, Janssen Oncology, Astellas Pharma, Amgen, AstraZeneca, Bristol Myers Squibb/Celgene, Gilead Sciences, Sandoz-Novartis, Abdi Ibrahim, MSD Oncology, Genekor, Abbvie
Consulting or Advisory Role: Roche, Novartis, Pfizer, Lilly, MSD Oncology, Gilead Sciences, AstraZeneca
Speakers' Bureau: Pfizer, Roche, Novartis, Lilly, Bristol Myers Squibb/Celgene, Genekor, AstraZeneca
Travel, Accommodations, Expenses: Pfizer, Roche, MSD Oncology, Novartis, BMS
Ahmed El Bastawisy
Honoraria: Pfizer, Novartis, AstraZeneca, MSD Oncology, Eva Pharma (Inst), Amgen
Consulting or Advisory Role: MSD Oncology, Novartis, Pfizer
Speakers' Bureau: AstraZeneca, Pfizer, Amgen, Novartis, MSD Oncology, Eva Pharma
Research Funding: Roche, Novartis, AstraZeneca, MSD Oncology
Travel, Accommodations, Expenses: Pfizer, Eva Pharma, Pierre Fabre, MSD Oncology
Yueh Ni Lim
Consulting or Advisory Role: AstraZeneca, Pfizer
Speakers' Bureau: Novartis, Eisai, Roche
Research Funding: Arcus Biosciences, Yuhan, AstraZeneca, Novartis, Pfizer, Roche, MSD Oncology
Travel, Accommodations, Expenses: Roche, Roche, Amgen, MSD Oncology, AstraZeneca, Ipsen, Novartis, Arcus Biosciences, Varian Medical Systems, MSD Oncology, Roche, Astellas Pharma
Wei-Pang Chung
Honoraria: Amgen, AstraZeneca, Daiichi Sankyo/AstraZeneca, Lilly, Gilead Sciences, MSD Oncology, Novartis, Pfizer, Roche, Viatris
Consulting or Advisory Role: AstraZeneca, Daiichi Sankyo/AstraZeneca, Lilly, Gilead Sciences, Novartis, Pfizer, Roche
Travel, Accommodations, Expenses: Pfizer, Novartis, AstraZeneca
Konstantin Penkov
Consulting or Advisory Role: Nektar
Research Funding: AstraZeneca/MedImmune (Inst), MSD (Inst), Nektar (Inst), Pfizer (Inst), Regeneron (Inst), Roche (Inst)
James Bowles
Employment: Novartis
Stock and Other Ownership Interests: AstraZeneca, Novartis, BeiGene, Pfizer, BioNTech SE
Travel, Accommodations, Expenses: Novartis
Teresa Delgar Alfaro
Employment: Novartis
Jiwen Wu
Employment: Novartis, Geron
Stock and Other Ownership Interests: Novartis, Geron
Melissa Gao
Employment: Novartis
Stock and Other Ownership Interests: Novartis
Khemaies Slimane
Employment: Novartis
Stock and Other Ownership Interests: Novartis
Honoraria: Novartis
Travel, Accommodations, Expenses: Novartis
Nagi S. El Saghir
Honoraria: Roche, Novartis, Pfizer, MSD Oncology, Lilly, Pierre Fabre
Travel, Accommodations, Expenses: Novartis, Roche
No other potential conflicts of interest were reported.
SUPPORT
Supported by Novartis Pharma AG.
CLINICAL TRIAL INFORMATION
Contributor Information
Collaborators: Adher Alsayed, Ahmed El Bastawisy, Ajay Gogia, Chaiyut Charoentum, Chanchal Goswami, Chien-Ting Liu, Chun Sen Lim, Elena Artamonova, Erhan Gokmen, Esat Namal, Eznal Izwadi Mohd Mahidin, Fadi Farhat, Flora Chong Li Tze, Gül Başaran, Hakan Harputluoglu, Hamdy Abdel Azim, Hikmat Abdelrazeq, Hesham ElGhazaly, K. Govind Babu, Konstantin Penkov, Le Thanh Duc, Ling-Ming Tseng, Liudmila Osmanova, Ludmila Zhukova, Meher Lakshmi Konatum, Mehmet Ali Nahit Şendur, Ming-Shen Dai, Mona Ayoubi, Nagi El-Saghir, Naiyarat Prasongsook, Napa Parinyanitikul, Nikita Volkov, Nuri Karadurmus, Patrapim Sunpaweravong, Peter Cher Siang Ang, Pei Jye Voon, Rabab Gaafar, Rasha Abdel Motagaly, Richard Khanyile, Sema Sezgin Goksu, Shin-Cheh Chen, Sudeep Gupta, Su Mien Lynette Ngo, Swee Hsia Choong, Terence Aik Huang Tan, Umut Demirci, Wei-Pang Chung, Wen-Son Hsieh, Yen-Shen Lu, Yesim Eralp, Yoon Sim Yap, Yuan-Ching Chang, and Yueh Ni Lim
DATA SHARING STATEMENT
A data sharing statement provided by the authors is available with this article at DOI https://doi.org/10.1200/JCO.24.00144.
AUTHOR CONTRIBUTIONS
Conception and design: Yen-Shen Lu, Hamdy Azim, Yesim Eralp, Yoon Sim Yap, Seock-Ah Im, Teresa Delgar Alfaro, Melissa Gao, Khemaies Slimane, Nagi S. El Saghir
Administrative support: Ahmed El Bastawisy, Konstantin Penkov, James Bowles, Teresa Delgar Alfaro, Melissa Gao
Provision of study materials or patients: Yen-Shen Lu, Hamdy Azim, Yoon Sim Yap, Seock-Ah Im, Julie Rihani, Ahmed El Bastawisy, Nuri Karadurmus, Yueh Ni Lim, Chun Sen Lim, Le Thanh Duc, Wei-Pang Chung, K. Govind Babu, Konstantin Penkov, Teresa Delgar Alfaro, Melissa Gao, Nagi S. El Saghir
Collection and assembly of data: Yen-Shen Lu, Eznal Izwadi Bin Mohd Mahidin, Yesim Eralp, Yoon Sim Yap, Seock-Ah Im, Erhan Gokmen, Ahmed El Bastawisy, Nuri Karadurmus, Yueh Ni Lim, Chun Sen Lim, Le Thanh Duc, Wei-Pang Chung, K. Govind Babu, Konstantin Penkov, James Bowles, Teresa Delgar Alfaro, Melissa Gao, Nagi S. El Saghir
Data analysis and interpretation: Yen-Shen Lu, Eznal Izwadi Bin Mohd Mahidin, Hamdy Azim, Yesim Eralp, Yoon Sim Yap, Seock-Ah Im, Julie Rihani, Erhan Gokmen, Ahmed El Bastawisy, Wei-Pang Chung, K. Govind Babu, James Bowles, Teresa Delgar Alfaro, Jiwen Wu, Melissa Gao, Nagi S. El Saghir
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Final Results of RIGHT Choice: Ribociclib Plus Endocrine Therapy Versus Combination Chemotherapy in Premenopausal Women With Clinically Aggressive Hormone Receptor–Positive/Human Epidermal Growth Factor Receptor 2–Negative Advanced Breast Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Yen-Shen Lu
Honoraria: Pfizer, Roche, MSD, Novartis, Lilly, Eisai, Daiichi Sankyo/UCB Japan, AstraZeneca, EuroPharma
Consulting or Advisory Role: Pfizer, Roche, Novartis, Lilly
Research Funding: Novartis (Inst), MSD (Inst), AstraZeneca (Inst)
Travel, Accommodations, Expenses: Novartis
Hamdy Azim
Honoraria: AstraZeneca, Bristol Myers Squibb, Lilly, MSD, Novartis, Pfizer, Roche, GlaxoSmithKline, Sanofi/Aventis
Consulting or Advisory Role: AstraZeneca, Bristol Myers Squibb, Lilly, MSD, Novartis, Pfizer, Roche, GlaxoSmithKline, Sanofi/Aventis
Speakers' Bureau: AstraZeneca, Bristol Myers Squibb, Lilly, MSD, Novartis, Pfizer, Roche
Research Funding: Roche, Novartis
Yesim Eralp
Honoraria: Gilead Sciences, Amgen, BMS Turkey, Amgen, AstraZeneca
Consulting or Advisory Role: Novartis, MSD Oncology, Gilead Sciences, MSD Oncology, Lilly, Novartis, MSD Oncology, MSD Oncology, GlaxoSmithKline, MSD Oncology, Novartis
Research Funding: Novartis
Other Relationship: Novartis
Yoon Sim Yap
Honoraria: Novartis, Pfizer, AstraZeneca, MSD, Gilead Sciences, DKSH
Consulting or Advisory Role: Novartis, Lilly, Pfizer, AstraZeneca, MSD
Research Funding: MSD Oncology (Inst)
Travel, Accommodations, Expenses: DKSH, AstraZeneca, MSD, Pfizer, Daiichi Sankyo
Seock-Ah Im
Consulting or Advisory Role: AstraZeneca, Novartis, Roche/Genentech, Eisai, Pfizer, Amgen, Hanmi, Lilly, MSD, Daiichi Sankyo
Research Funding: AstraZeneca (Inst), Pfizer (Inst), Roche/Genentech (Inst), Daewoong Pharmaceutical (Inst), Eisai (Inst), Boryung Pharmaceuticals (Inst)
Other Relationship: Roche
Erhan Gokmen
Stock and Other Ownership Interests: Immunogen, MacroGenics, G1 Therapeutics, Fibrogen
Honoraria: Lilly, Roche, Pfizer, Novartis, Janssen Oncology, Astellas Pharma, Amgen, AstraZeneca, Bristol Myers Squibb/Celgene, Gilead Sciences, Sandoz-Novartis, Abdi Ibrahim, MSD Oncology, Genekor, Abbvie
Consulting or Advisory Role: Roche, Novartis, Pfizer, Lilly, MSD Oncology, Gilead Sciences, AstraZeneca
Speakers' Bureau: Pfizer, Roche, Novartis, Lilly, Bristol Myers Squibb/Celgene, Genekor, AstraZeneca
Travel, Accommodations, Expenses: Pfizer, Roche, MSD Oncology, Novartis, BMS
Ahmed El Bastawisy
Honoraria: Pfizer, Novartis, AstraZeneca, MSD Oncology, Eva Pharma (Inst), Amgen
Consulting or Advisory Role: MSD Oncology, Novartis, Pfizer
Speakers' Bureau: AstraZeneca, Pfizer, Amgen, Novartis, MSD Oncology, Eva Pharma
Research Funding: Roche, Novartis, AstraZeneca, MSD Oncology
Travel, Accommodations, Expenses: Pfizer, Eva Pharma, Pierre Fabre, MSD Oncology
Yueh Ni Lim
Consulting or Advisory Role: AstraZeneca, Pfizer
Speakers' Bureau: Novartis, Eisai, Roche
Research Funding: Arcus Biosciences, Yuhan, AstraZeneca, Novartis, Pfizer, Roche, MSD Oncology
Travel, Accommodations, Expenses: Roche, Roche, Amgen, MSD Oncology, AstraZeneca, Ipsen, Novartis, Arcus Biosciences, Varian Medical Systems, MSD Oncology, Roche, Astellas Pharma
Wei-Pang Chung
Honoraria: Amgen, AstraZeneca, Daiichi Sankyo/AstraZeneca, Lilly, Gilead Sciences, MSD Oncology, Novartis, Pfizer, Roche, Viatris
Consulting or Advisory Role: AstraZeneca, Daiichi Sankyo/AstraZeneca, Lilly, Gilead Sciences, Novartis, Pfizer, Roche
Travel, Accommodations, Expenses: Pfizer, Novartis, AstraZeneca
Konstantin Penkov
Consulting or Advisory Role: Nektar
Research Funding: AstraZeneca/MedImmune (Inst), MSD (Inst), Nektar (Inst), Pfizer (Inst), Regeneron (Inst), Roche (Inst)
James Bowles
Employment: Novartis
Stock and Other Ownership Interests: AstraZeneca, Novartis, BeiGene, Pfizer, BioNTech SE
Travel, Accommodations, Expenses: Novartis
Teresa Delgar Alfaro
Employment: Novartis
Jiwen Wu
Employment: Novartis, Geron
Stock and Other Ownership Interests: Novartis, Geron
Melissa Gao
Employment: Novartis
Stock and Other Ownership Interests: Novartis
Khemaies Slimane
Employment: Novartis
Stock and Other Ownership Interests: Novartis
Honoraria: Novartis
Travel, Accommodations, Expenses: Novartis
Nagi S. El Saghir
Honoraria: Roche, Novartis, Pfizer, MSD Oncology, Lilly, Pierre Fabre
Travel, Accommodations, Expenses: Novartis, Roche
No other potential conflicts of interest were reported.
REFERENCES
- 1.El Saghir NS, Khalil MK, Eid T, et al. : Trends in epidemiology and management of breast cancer in developing Arab countries: A literature and registry analysis. Int J Surg 5:225-233, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Youlden DR, Cramb SM, Yip CH, et al. : Incidence and mortality of female breast cancer in the Asia-Pacific region. Cancer Biol Med 11:101-115, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bardia A, Hurvitz S: Targeted therapy for premenopausal women with HR+, HER2− advanced breast cancer: Focus on special considerations and latest advances. Clin Cancer Res 24:5206-5218, 2018 [DOI] [PubMed] [Google Scholar]
- 4.Heer E, Harper A, Escandor N, et al. : Global burden and trends in premenopausal and postmenopausal breast cancer: A population-based study. Lancet Glob Health 8:e1027-e1037, 2020 [DOI] [PubMed] [Google Scholar]
- 5.Cardoso F, Costa A, Senkus E, et al. : 3rd ESO-ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 3). Ann Oncol 28:16-33, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cardoso F, Paluch-Shimon S, Senkus E, et al. : 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann Oncol 31:1623-1649, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Shaughnessy J: Extending survival with chemotherapy in metastatic breast cancer. Oncologist 10:20-29, 2005. (suppl 3) [DOI] [PubMed] [Google Scholar]
- 8.Albain KS, Nag SM, Calderillo-Ruiz G, et al. : Gemcitabine plus paclitaxel versus paclitaxel monotherapy in patients with metastatic breast cancer and prior anthracycline treatment. J Clin Oncol 26:3950-3957, 2008 [DOI] [PubMed] [Google Scholar]
- 9.O'Shaughnessy J, Miles D, Vukelja S, et al. : Superior survival with capecitabine plus docetaxel combination therapy in anthracycline-pretreated patients with advanced breast cancer: Phase III trial results. J Clin Oncol 20:2812-2823, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Carrick S, Parker S, Thornton CE, et al. : Single agent versus combination chemotherapy for metastatic breast cancer. Cochrane Database Syst Rev 2009:Cd003372, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gennari A, André F, Barrios CH, et al. : ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann Oncol 32:1475-1495, 2021 [DOI] [PubMed] [Google Scholar]
- 12.Vernieri C, Prisciandaro M, Nichetti F, et al. : Oral capecitabine-vinorelbine is associated with longer overall survival when compared to single-agent capecitabine in patients with hormone receptor-positive advanced breast cancer. Cancers (Basel) 12:617, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glück S, Russell C, O'Shaughnessy J, et al. : Treatment effect of capecitabine and docetaxel or docetaxel alone by oestrogen receptor status in patients with metastatic breast cancer: Results of an exploratory analysis. Breast 22:1087-1093, 2013 [DOI] [PubMed] [Google Scholar]
- 14.Barrios CH, Sampaio C, Vinholes J, et al. : What is the role of chemotherapy in estrogen receptor-positive, advanced breast cancer?. Ann Oncol 20:1157-1162, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Finn RS, Martin M, Rugo HS, et al. : Palbociclib and letrozole in advanced breast cancer. N Engl J Med 375:1925-1936, 2016 [DOI] [PubMed] [Google Scholar]
- 16.Goetz MP, Toi M, Campone M, et al. : MONARCH 3: Abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol 35:3638-3646, 2017 [DOI] [PubMed] [Google Scholar]
- 17.Hortobagyi GN, Stemmer SM, Burris HA, et al. : Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med 375:1738-1748, 2016 [DOI] [PubMed] [Google Scholar]
- 18.Tripathy D, Im SA, Colleoni M, et al. : Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): A randomised phase 3 trial. Lancet Oncol 19:904-915, 2018 [DOI] [PubMed] [Google Scholar]
- 19.Slamon DJ, Neven P, Chia S, et al. : Phase III randomized study of ribociclib and fulvestrant in hormone receptor–positive, human epidermal growth factor receptor 2–negative advanced breast cancer: MONALEESA-3. J Clin Oncol 36:2465-2472, 2018 [DOI] [PubMed] [Google Scholar]
- 20.Im S-A, Lu Y-S, Bardia A, et al. : Overall survival with ribociclib plus endocrine therapy in breast cancer. N Engl J Med 381:307-316, 2019 [DOI] [PubMed] [Google Scholar]
- 21.Lu YS, Im SA, Colleoni M, et al. : Updated overall survival of ribociclib plus endocrine therapy versus endocrine therapy alone in pre- and perimenopausal patients with HR+/HER2- advanced breast cancer in MONALEESA-7: A phase III randomized clinical trial. Clin Cancer Res 28:851-859, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sledge GW Jr., Toi M, Neven P, et al. : The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor-positive, ERBB2-negative breast cancer that progressed on endocrine therapy-MONARCH 2: A randomized clinical trial. JAMA Oncol 6:116-124, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eisenhauer EA, Therasse P, Bogaerts J, et al. : New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 45:228-247, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Cardoso F, Rihani J, Aubel D, et al. : 178P Assessment of side effects (SEs) impacting quality of life (QoL) in patients (pts) undergoing treatment (tx) for advanced breast cancer (ABC) in clinical practice: A real-world (RW) multi-country survey. Ann Oncol 33:S208-S209, 2022 [Google Scholar]
- 25.Azim H, Saghir N, Yap Y, et al. : First-line ribociclib (RIB) + endocrine therapy (ET) vs combination chemotherapy (combo CT) in aggressive HR+/HER2− advanced breast cancer (ABC): A subgroup analysis of patients (pts) with or without visceral crisis from the phase II RIGHT Choice study. Ann Oncol 34:S350-S351, 2023. (abstr 402P) [Google Scholar]
- 26.Sledge GW, Neuberg D, Bernardo P, et al. : Phase III trial of doxorubicin, paclitaxel, and the combination of doxorubicin and paclitaxel as front-line chemotherapy for metastatic breast cancer: An intergroup trial (E1193). J Clin Oncol 15:588-592, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Piccart-Gebhart M, Burzykowski T, Buyse M, et al. : Taxanes alone or in combination with anthracyclines as first-line therapy of patients with metastatic breast cancer. J Clin Oncol 26:1980-1986, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Gehl J, Boesgaard M, Paaske T, et al. : Combined doxorubicin and paclitaxel in advanced breast cancer: Effective and cardiotoxic. Ann Oncol 7:687-693, 1996 [DOI] [PubMed] [Google Scholar]
- 29.Cai F, Luis MAF, Lin X, et al. : Anthracycline-induced cardiotoxicity in the chemotherapy treatment of breast cancer: Preventive strategies and treatment. Mol Clin Oncol 11:15-23, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Makhlouf S, Quinn C, Toss M, et al. : Quantitative expression of oestrogen receptor in breast cancer: Clinical and molecular significance. Eur J Cancer 197:113473, 2024 [DOI] [PubMed] [Google Scholar]
- 31.Martin M, Zielinski C, Ruiz-Borrego M, et al. : Palbociclib in combination with endocrine therapy versus capecitabine in hormonal receptor-positive, human epidermal growth factor 2-negative, aromatase inhibitor-resistant metastatic breast cancer: A phase III randomised controlled trial-PEARL. Ann Oncol 32:488-499, 2021 [DOI] [PubMed] [Google Scholar]
- 32.Park YH, Kim TY, Kim GM, et al. : Palbociclib plus exemestane with gonadotropin-releasing hormone agonist versus capecitabine in premenopausal women with hormone receptor-positive, HER2-negative metastatic breast cancer (KCSG-BR15-10): A multicentre, open-label, randomised, phase 2 trial. Lancet Oncol 20:1750-1759, 2019 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
A data sharing statement provided by the authors is available with this article at DOI https://doi.org/10.1200/JCO.24.00144.