Abstract
PURPOSE
Leptomeningeal metastases (LMs) exhibit a high incidence in patients with epidermal growth factor receptor (EGFR)–mutated non–small cell lung cancer (NSCLC) post-treatment with first- or second-generation EGFR tyrosine kinase inhibitors (TKIs). This investigation evaluates the efficacy, safety, and pharmacokinetics of 80 mg once daily osimertinib in patients with LMs resistant to prior first- or second-generation EGFR TKIs.
MATERIALS AND METHODS
In this phase II multicenter, open-label, single-arm study, 80 mg osimertinib was administered to patients with EGFR-mutated NSCLC who had developed LMs subsequent to treatment with prior EGFR TKIs. The primary end point was overall survival (OS), assessed alongside objective response rate by the blinded independent central review (BICR) and a pharmacokinetic analysis of plasma and cerebrospinal fluid (CSF) on the first day of cycles 3 and 6.
RESULTS
A total of 73 patients diagnosed with LM were treated with osimertinib, including 64 patients evaluable for the LM efficacy set—T790M negative (n = 62) and T790M positive (n = 2). The median OS in the full-analysis set was 15.6 months (95% CI, 11.5 to 20.2). The objective response rate for LM was 51.6%, including a 15.6% complete response, and the disease control rate was 81.3% by BICR in the LM efficacy evaluable set. The median LM progression-free survival by BICR was 11.2 months (95% CI, 7.7 to 15.3), the duration of response was 12.6 months (95% CI, 7.6 to 17.7), and OS was 15.0 months (95% CI, 11.3 to 18.7). Pharmacokinetic analysis showed that the CSF to free plasma osimertinib ratio was 22%. Most safety profiles were grade 1 and 2.
CONCLUSION
The study demonstrates significant intracranial efficacy and survival benefits of 80 mg once daily osimertinib in NSCLC patients with LMs. The data support considering daily 80 mg of osimertinib as a treatment option for EGFR-mutated NSCLC patients with LMs, irrespective of T790M mutation status.
80 mg osimertinib achieved a median OS of 15.0 months in T790M (-) EGFR-mutated NSCLC with LM, after 1st/2nd G TKI
INTRODUCTION
In patients with non–small cell lung cancer (NSCLC) who are undergoing treatment with epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs), leptomeningeal metastases (LMs) present a significant challenge, often leading to poor prognosis.1,2 Current LM treatment options are without a well-established therapy beyond conventional approaches, such as intrathecal chemotherapy or whole-brain radiotherapy (WBRT), both of which demonstrate limited clinical efficacy.2
CONTEXT
Key Objective
This study assesses the intracranial efficacy and overall survival (OS) benefits of 80 mg once daily osimertinib in patients with leptomeningeal metastases from epidermal growth factor receptor (EGFR)–mutated non–small cell lung cancer (NSCLC) resistant to first- or second-generation EGFR tyrosine kinase inhibitors. It specifically evaluates the response in T790M-negative patients without systemic progression.
Knowledge Generated
Treatment with 80 mg once daily osimertinib achieved a median OS of 15.0 months and an objective response rate of 51.6%, alongside a disease control rate of 81.3% in the efficacy-evaluable set as assessed by blinded independent central review, underscoring significant intracranial activity. Pharmacokinetic analysis revealed that the plasma-to-cerebrospinal fluid (CSF) ratio of 80 mg osimertinib mirrors that observed with the 160 mg dosage, supporting its effective CNS penetration.
Relevance (T.E. Stinchcombe)
This phase II study demonstrated the efficacy of osimertinib 80 mg daily in patients with EGFR mutant NSCLC with leptomeningeal disease. The study designed included BICR using the RANO-LM assessment criteria, patient reported outcomes, and the pharmacokinetic analyses of both plasma and CSF.*
*Relevance section written by JCO Associate Editor Thomas E. Stinchcombe, MD.
In cases where patients do not respond to standard doses of first- or second-generation EGFR TKIs and present as LMs, increased dosages have been tried to elevate the drug concentration within the cerebrospinal fluid (CSF). Pulsatile administration of erlotinib, for example, has shown a radiologic response in two thirds of patients who failed the standard dose, with some surviving up to a year.3 A third-generation EGFR TKI, osimertinib, has been formulated to target intracranial lesions more effectively, demonstrating superior blood-brain barrier penetration and increased CSF drug concentration relative to its predecessors.4,5 This enhanced brain distribution of osimertinib has been confirmed in human studies using positron emission tomography.6 Clinically, osimertinib has shown promise in controlling CNS progression, as seen in trials with lower rates of intracranial progression in both adjuvant and palliative settings.7,8
The efficacy of osimertinib for LM is particularly promising. At a doubled dose of 160 mg once daily, osimertinib has extended progression-free survival (PFS) and overall survival (OS) up to 8.6 and 13 months in patients, regardless of EGFR T790M mutation status.9,10 Using the standard dose, 80 mg once daily, osimertinib also showed comparable benefit to double dosage osimertinib in patients with LMs harboring the EGFR T790M mutation. From the retrospective analysis, we observed that patients with LMs without EGFR T790M mutation (18.8 months) showed similar OS to patients with EGFR T790M mutation (16.7 months) when treated with 80 mg osimertinib.11 In another retrospective analysis of studies across the AURA program (AURA extension, AURA2, AURA17 and AURA3),12-15 80 mg of osimertinib demonstrated by neuroradiological blinded independent central review (BICR), an LM objective response rate (ORR) of 55% with median PFS and OS reached up to 11.1 and 18.8 months in EGFR T790M mutant, respectively.16
Interestingly, even in the absence of this mutation, patients have exhibited similar survival benefits, suggesting a broader potential for osimertinib in LM treatment. The critical role of EGFR TKIs in improving outcomes for NSCLC patients with LMs, including those who are EGFR T790M mutation–negative, underscores the necessity for further research in this area. However, prospective studies involving a large number of patients with LM treated with 80 mg of osimertinib are still limited.
Encouraged by these data, our prospective study was initiated to assess the effectiveness and safety profile of 80 mg osimertinib in patients with EGFR-mutant NSCLC who developed LM after first- or second-generation EGFR TKI treatment. In addition, we generated comprehensive pharmacokinetic data to further elucidate the CSF penetration and systemic exposure of this dosage, aiming to optimize therapeutic strategies.
MATERIALS AND METHODS
Study Design and Treatment
This phase II, open-label, single-arm, multicenter study (BLOSSOM study) was conducted across six hospitals within the Republic of Korea. We enrolled patients who demonstrated progression of LM after treatment with first- or second-generation EGFR TKIs. These patients were treated with a daily dose of 80 mg osimertinib, which could be reduced to 40 mg based on observed toxicity. Treatment cycles were 28 days each, continuing until disease progression, onset of unacceptable toxicity, or patient withdrawal. Moreover, continuation of treatment beyond progression was permitted if the investigator anticipated additional clinical benefit. This study was originally planned to include 80 patients, including T790M-negative (n = 60) and T790M-positive (n = 20) patients. However, enrollment was terminated early after recruiting all preplanned T790M-negative patients. Investigators obtained informed consent from each participant or each participant's guardian. The protocol adhered to the ethical principles of the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice guidelines, with oversight by the institutional review board of Samsung Medical Center (IRB no. 2020-09-013) and the participating centers' ethics committees (ClinicalTrials.gov identifier: NCT04563871) This study was performed after approval by the Ministry of Food and Drug Safety of Korea and in accordance with an assurance filed with and approved by the Department of Clinical Trial Policy.
Participants
Patients with NSCLC harboring EGFR exon 19 deletions or L858R mutations and previously treated with first- or second-generation EGFR TKIs were eligible. Patients were evaluated for T790M mutation status after the failure of prior EGFR TKI treatment using either plasma or tissue molecular tests. Additionally, participants without the T790M mutation had to have stable extracranial disease. All participants were required to have at least one measurable LM lesion amenable to repeated magnetic resonance imaging (MRI) evaluations and an Eastern Cooperative Oncology Group performance status (ECOG PS) score of 0 to 2, maintaining this status for 2 weeks before enrollment with an expected minimum survival of 12 weeks. Previous radiotherapy, including WBRT or intrathecal chemotherapy, was permissible following a 2-week washout period. Patients with mild to minimal neurology symptoms who are tolerable for the treatment were allowed, and given the unique clinical nature of leptomeningeal metastasis, the use of corticosteroid is allowed. Detailed eligibility criteria are delineated in the Data Supplement Protocol (online only).
Data Management
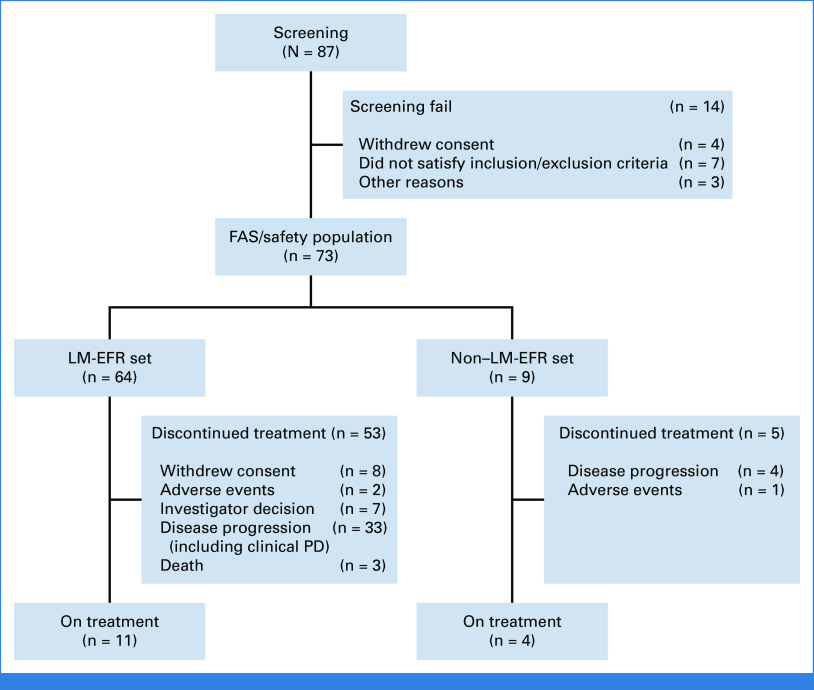
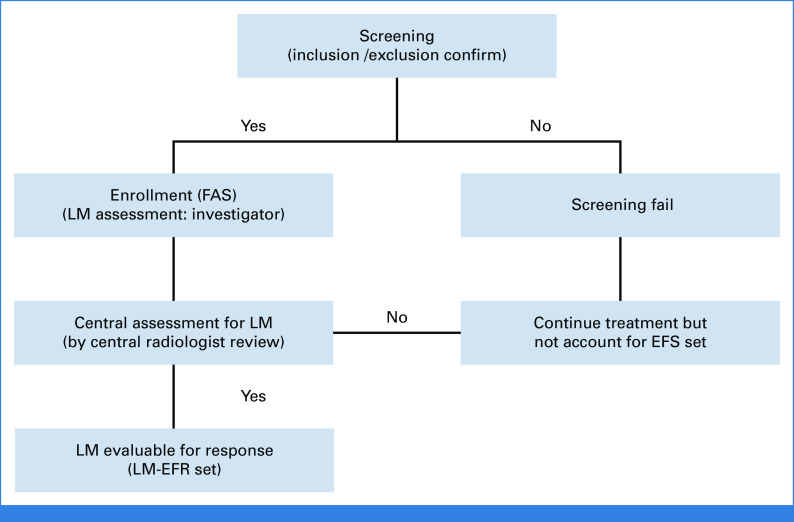
Patient enrollment was determined by the investigators based on LM visibility, with these individuals constituting the full-analysis set (FAS). Central review reassessed the same image for the sufficiency of the radiologic response of LM. Those with centrally reviewed LM, suitable for LM response assessment, were designated as evaluable for the response set (LM-EFR; Appendix Fig A1, online only). Management and assessments were consistently applied across all patients. Data were managed via an electronic case report form system (cubeCDMS v1.1, CRScube, Korea), compliant with FDA's 21 CFR Part 11. Regular data queries were conducted in line with the study's data management plan.
End Point and Assessment
The primary end point was OS among the LM-EFR cohort. Secondary end points included LM ORR, duration of response (DOR), disease control rate (DCR), and PFS, analyzed by neuroradiological BICR following response assessment in neuro-oncology leptomeningeal metastases (RANO-LM) assessment criteria.17 Investigator assessments used RECIST version 1.1 to evaluate ORR, DOR, and responses in LM, brain parenchyma, and extracranial lesions.18 Quality of life was evaluated using the European Organisation for Research and Treatment of Cancer (EORTC) 30-item Core Quality of Life questionnaire (QLQ-C30) and 20-item Quality of Life Questionnaire–Brain Neoplasm (QLQ-BN20) instruments every 8 weeks, equivalent to two cycles, up to 32 weeks, with safety profiles documented per Common Terminology Criteria for Adverse Events (CTCAE) 5.0. Neurologic examinations (Appendix Table A1) and patient-reported outcomes per National Cancer Institute's Patient Reported Outcome of the Common Terminology Criteria for Adverse Events (NCI-PRO-CTCAE) (Appendix Table A2) were recorded at each visit. Radiologic assessments, including brain MRI and chest and abdomen computed tomography scans, were performed every 8 weeks for the first 12 months and every 3 months thereafter. CSF cytology was assessed at the baseline, cycle three, and at the time of disease progression.
Pharmacokinetics
For pharmacokinetic analysis, we collected plasma (2 mL) and CSF (0.3 mL) samples pre-dose on the first day of cycle three and six, measuring the concentration of osimertinib and its metabolite, AZ5104. Sample collection between plasma and CSF was timed within 60 minutes for congruency. Labcorp (Burlington, NC) conducted the analyses, reporting free plasma and CSF concentrations.
Statistical Analysis
OS analysis was conducted approximately 14 months after the first dose for the last patient to report final OS outcomes. For the patient with EGFR T790M negative, we used H0 (μ = 5 months) and H1 (μ = 9 months) on the basis of previous retrospective analysis.11 Using a two-sided 5% significance level and 90% power and assuming exponential survival times, a sample size of 36 patients (accounting for 31 events) was required. To accommodate a potential 20% dropout rate, the target enrollment was set at 45 patients. Given the unmet clinical needs and anticipated benefits of osimertinib for T790M-negative patients, the recruitment aimed for up to 60 patients in this subgroup.
Scoring for QLQ-C30, QLQ-BN20, and NCI-PRO-CTCAE was based on the QLQ-C30 scoring manual, employing the following equation:
where I represents individual questions within a questionnaire, and n is the total number of questions in the target category questionnaire. Trends in QLQ-C30, QLQ-BN20, and NCI-PRO-CTCAE scores over time were evaluated through linear regression analysis. The composite QLQ-C30 score was derived from the mean values of physical functioning, global health status, and the inverse of symptom scores, scaled from 0 to 100. Consequently, elevated scores on the QLQ-C30 and reduced scores on the QLQ-BN20 and NCI-PRO-CTCAE signify improved outcomes.
RESULTS
Baseline Demographics
From November 2020 through November 2022, a total of 87 patients underwent screening, with 73 patients (FAS) being treated and analyzed for the OS and safety profile. Among them, 64 patients were qualified for the LM efficacy evaluation (LM-EFR) by central independent radiologist (Fig 1). Patients had either an EGFR deletion 19 (n = 29) or L858R mutation (n = 44) with a median age of 59 years. The cohort predominantly comprised females (65.7%), and ECOG PS of 0 (19.2%), 1 (61.6%), and 2 (19.2%) (Table 1). Prior treatments included gefitinib (31.5%), erlotinib (26.0%), and afatinib (42.5%). Of the study population, 97.3% were T790M-negative, and two T790M-positive patients (2.7%) were included in the LM-EFR. Prior management for LM included WBRT (13.7%), intrathecal methotrexate (12.3%), ommaya insertion (20.6%), and ventriculoperitoneal shunt (6.9%). Three required osimertinib administration via nasogastric tube because of dysphagia from cranial nerve dysfunction. Baseline CSF cytology was positive in 84.7% of patients available for CSF testing (n = 46). As of October 25, 2023, the median follow-up duration was 15.6 months (95% CI, 11.5 to 20.2), with 15 patients (20.5%) ongoing with treatment.
FIG 1.
Flow diagram of study population. EFR, evaluable for response; FAS, full analysis set; LM, leptomeningeal metastases; PD, progressive disease.
TABLE 1.
Baseline Demographics
| Characteristic | FAS Patients | LM-EFR Set Patients |
|---|---|---|
| No. of patients | 73 | 64 |
| Age, years, median (range) | 59 (36-82) | 59 (36-82) |
| Sex, No (%) | ||
| Male | 25 (34.3) | 24 (37.5) |
| Female | 48 (65.7) | 40 (62.5) |
| Race, No (%) | ||
| Asian | 73 (100.0) | 64 (100.0) |
| ECOG PS, No (%) | ||
| 0 | 14 (19.2) | 13 (20.3) |
| 1 | 45 (61.6) | 38 (59.4) |
| 2 | 14 (19.2) | 13 (20.3) |
| Smoking history, No (%) | ||
| Never | 56 (76.7) | 48 (75.0) |
| Current or former | 17 (23.3) | 16 (25.0) |
| Histologic type, No (%) | ||
| Adenocarcinoma | 67 (91.8) | 58 (90.6) |
| Poorly differentiated NSCLC | 6 (8.2) | 6 (9.4) |
| Initial EGFR mutation | ||
| Deletion 19 | 29 (39.7) | 27 (42.2) |
| L858R | 44 (60.3) | 37 (57.8) |
| EGFR T790M status, No (%) | ||
| Positive | 2 (2.7) | 2 (3.1) |
| Negative | 71 (97.3) | 62 (96.9) |
| Prior EGFR-TKI treatment, No (%) | ||
| Gefitinib | 23 (31.5) | 20 (31.3) |
| Erlotinib | 19 (26.0) | 15 (23.4) |
| Afatinib | 31 (42.5) | 29 (45.3) |
| Prior TKI treatment history, No (%) | ||
| First line | 73 (100.0) | 64 (100.0) |
| Previous CNS radiotherapy, No (%) | ||
| Whole-brain radiotherapy | 10 (13.7) | 8 (12.5) |
| Stereotactic radiotherapy | 30 (41.1) | 26 (40.6) |
| Intrathecal methotrexate treatment | 9 (12.3) | 8 (12.5) |
| Ommaya insertion | 15 (20.6) | 13 (20.3) |
| Ventriculoperitoneal shunt | 5 (6.9) | 5 (7.8) |
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group Performance Score; EGFR-TKI, epidermal growth factor receptor tyrosine kinase inhibitor; FAS, full analysis set; LM-EFR, leptomeningeal metastases evaluable for response; NSCLC, non–small cell lung cancer.
Clinical Efficacy and Survival Analysis
In the FAS subset (N = 73), according to RECIST by investigator assessment, the median intracranial PFS was 12.5 months (9.6 to 16.6) and OS was 15.6 months (11.5 to 20.2; Figs 2A and 2B).
FIG 2.
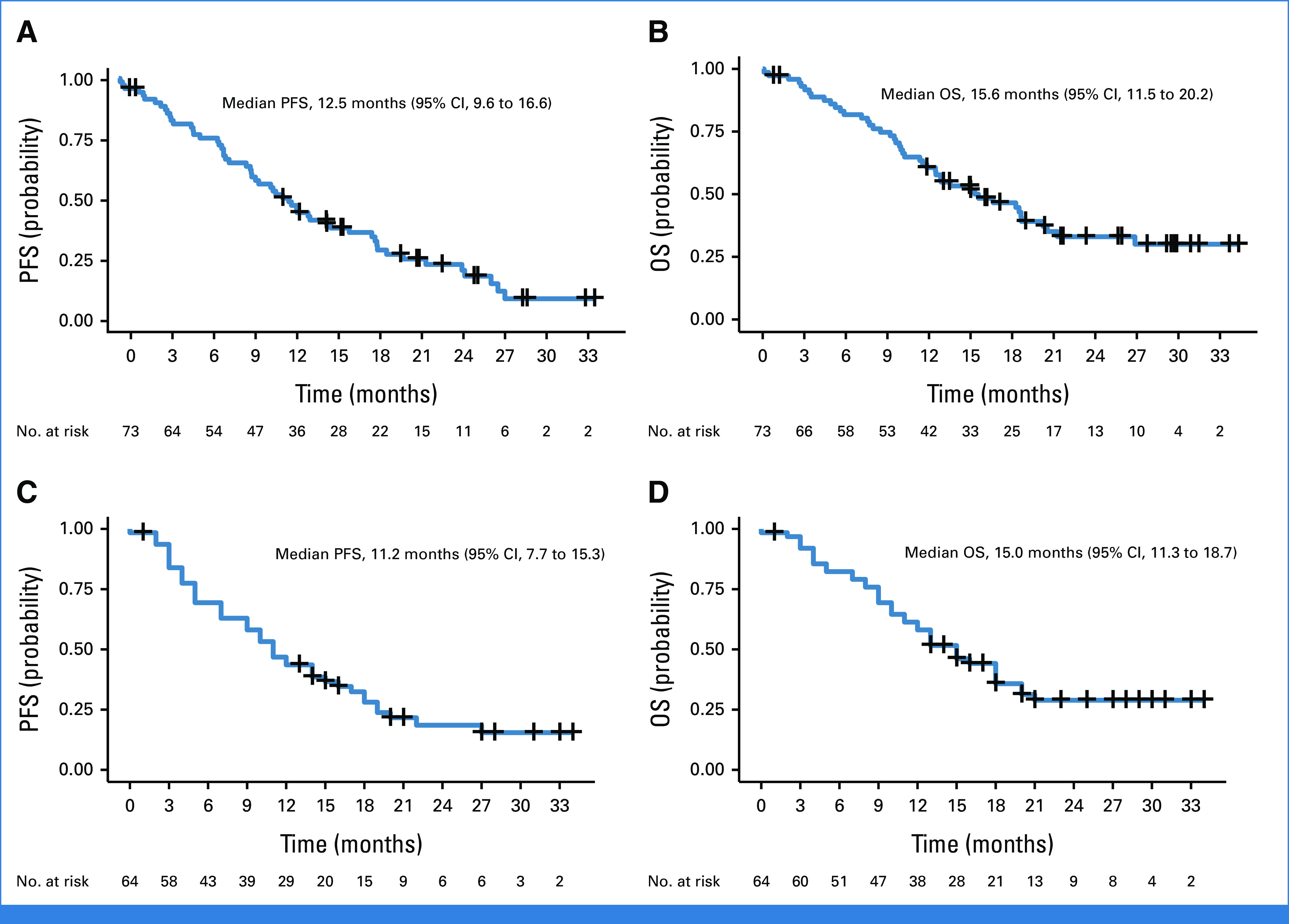
Survival analysis. (A) Intracranial PFS as per RECIST assessed by investigator and (B) OS from full analysis set. (C) Intracranial PFS as per RANO-LM criteria assessed by BICR and (D) OS from LM evaluable for response set. BICR, blinded independent central review; LM, leptomeningeal metastases; OS, overall survival; PFS, progression-free survival; RANO-LM, response assessment in neuro-oncology leptomeningeal metastases.
In the LM-EFR subset (n = 64), 48 patients (75%) exhibited disease progression or death at data cutoff. According to RECIST by investigator assessment, the median intracranial PFS was 12.5 months (9.3 to 16.6), and the DOR was 20.3 months (10.2 to NA). The clinical efficacy by BICR according to RANO-LM criteria showed the median intracranial PFS of 11.2 months (7.7-15.3) and the DOR of 12.6 months (7.6 to 17.7; Fig 2C). The median OS was 15.0 months (11.3 to 18.7), with OS rates at 6, 12, 18, and 24 months of 82%, 60%, 44%, and 29%, respectively (Fig 2D). For the T790M-negative group (n = 62), the median OS was 15.3 months (11.3 to 18.7). For the T790M-positive group (n = 2), the OS from two patients were 0.1 and 13.5 months.
From the LM-EFR set, the intracranial ORR assessed by the investigator, according to RECIST, was 6.3%, and the DCR was 85.9% (Table 2). The intracranial ORR assessed by BICR, according to RANO-LM criteria, was 51.6%, including 15.6% complete responses (CRs) and 35.9% partial response, and the DCR was 81.3%. In the subgroup analysis, the ORR was 52.9% in patients with prior WBRT or stereotactic radiotherapy and 62.5% in those receiving intrathecal methotrexate. The ORR for patients age ≥65 years or with ECOG PS of 2 was 52.6% and 38.5%, respectively.
TABLE 2.
Response to Treatment
| Measure | RANO-LM by BICRa | Intracranial Response by Investigatorb | Extracranial Response by Investigatorc | Overall Response by Investigatord |
|---|---|---|---|---|
| No. of patients | 64 | 64 | 52 | 64 |
| Best objective response, No. (%) | ||||
| Complete response | 10 (15.6) | 1 (1.6) | 1 (1.9) | 0 |
| Partial response | 23 (35.9) | 3 (4.7) | 8 (15.4) | 10 (15.6) |
| Stable disease | 19 (29.6) | 51 (79.7) | 37 (71.2) | 46 (71.9) |
| Progression | 5 (7.8) | 3 (4.7) | 0 | 3 (4.7) |
| Not evaluable | 7 (10.9) | 6 (9.4) | 6 (11.3) | 5 (7.8) |
| ORR (95% CI) | 51.6 (38.7 to 64.3) | 6.3 (1.7 to 15.2) | 17.3 (8.2 to 30.3) | 15.6 (7.8 to 26.9) |
| DCR (95% CI) | 81.3 (69.5 to 89.9) | 85.9 (75.0 to 93.4) | 88.5 (76.6 to 95.7) | 87.5 (76.9 to 94.5) |
| Median PFS, months (95% CI) | 11.2 (7.7 to 15.3) | 12.5 (9.3 to 16.6) | 13.5 (10.1 to 19.3) | 11.8 (9.2 to 15.0) |
| Median DOR, months (95% CI) | 12.6 (7.6 to 17.7) | 20.3 (10.2 to NA) | 17.6 (6.4 to NA) | 17.6 (6.4 to 20.3) |
Abbreviations: BICR, blinded independent central review; DCR, disease control rate; DOR, duration of response; ORR, objective response rate; PFS, progression-free survival; RANO-LM, response assessment in neuro-oncology leptomeningeal metastases.
Response of LMs only evaluated by BICR by RANO-LM criteria.
Response of LMs only evaluated by the individual investigator on the basis of RECIST version 1.1.
Extracranial response by the investigator on the basis of RECIST version 1.1, in those who available for extracranial lesion evaluation.
Overall response rate, including both intracranial and extracranial radiologic response, by the investigator on the basis of RECIST version 1.1.
The patients with the initial ECOG PS of 0 showed a longer median OS (26.9 months), compared with 9.8 months in those with an ECOG PS of 2 (P = .003; Appendix Figs A2A and A2B). Similarly, patients without neurologic symptoms at baseline exhibited a longer median OS of 21.3 months, compared with 11.5 months in patients with at least one neurologic symptom (P = .010; Appendix Figs A2C and A2D). The median OS was 9.6 months for patients who received the treatment after WBRT and 18.3 months for those who did not receive WBRT (P = .022). However, the median OS was similar between patients who received intrathecal methotrexate and those who did not (15.0 v 18.3 months, P = .513), respectively (Appendix Table A3). The CSF cytology positivity rate was 89.2% at cycle 3 in patients available for CSF sampling (n = 37).
Quality-of-Life Assessment and Neurologic Examination
The quality-of-life assessment was evaluated using the EORTC QLQ-C30, BN20, and NCI-PRO CTCAE, which showed compliance rates of 94.2%, 94.2%, and 79.0%, respectively, at cycle 5. The global health status score from EORTC QLQ-C30 showed an improvement in health status over time with osimertinib treatment but decreased at progression (Fig 3A), which was similar to functional scales and symptom scores (Appendix Figs A3A and A3B, Appendix Table A4). QLQ-BN20 assessments indicated a decline in mean scores after treatment but an increase at the point of disease progression (Appendix Fig A3C, Appendix Table A5). Patient-reported outcomes, as per NCI-PRO-CTCAE, including 12 questions related to symptoms, demonstrated a decrease in score over time, indicating improvement of symptoms (Fig 3B, Appendix Table A6).
FIG 3.
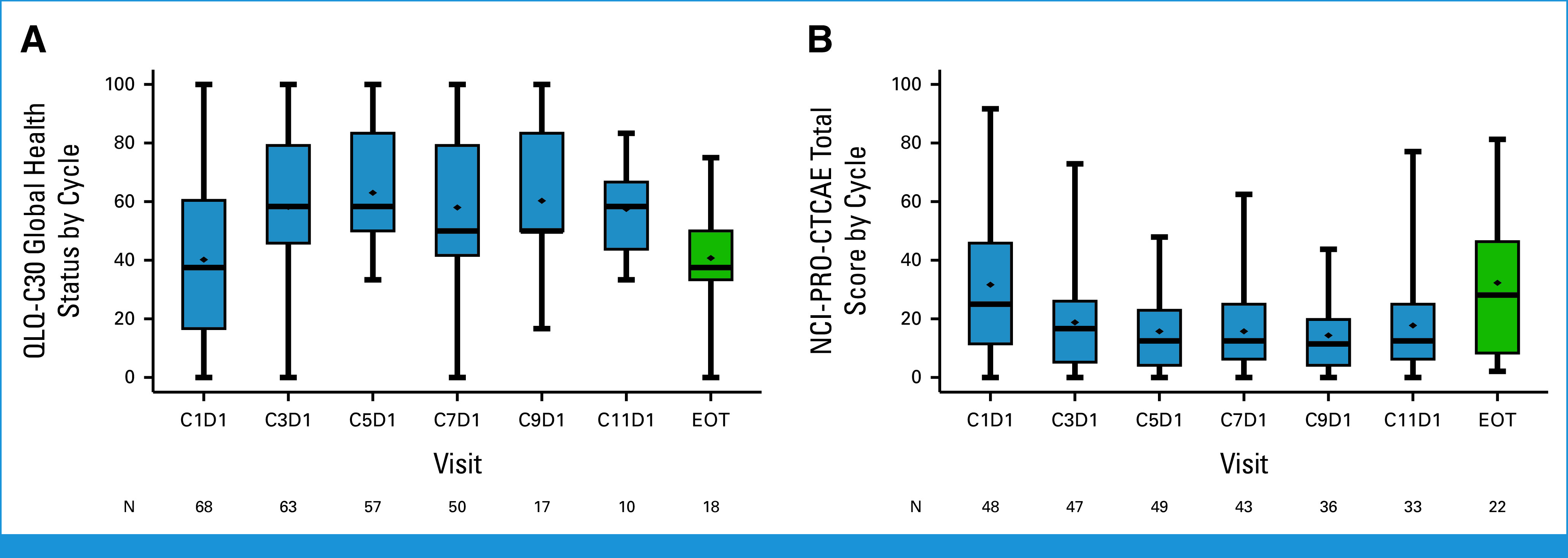
Quality-of-life assessment during the treatment. Changes in scores over the course of treatment compared with baseline: (A) Physical functioning score from QLQ-C30. (B) Symptom severity scores from NCI-PRO-CTCAE. EOT, end of treatment; NCI-PRO-CTCAE, National Cancer Institute's Patient Reported Outcome of the Common Terminology Criteria for Adverse Events; QLQ-C30, 30-item EORTC Core Quality of Life questionnaire.
Neurologic examinations covered 11 categories, scoring from 0 to 29 at each visit (Appendix Table A1). At baseline, normal neurologic function was reported in 57.8% of patients. Common abnormalities included gait disturbance (32.9%), decreased motor strength (31.5%), and visual changes (19.2%). The frequency of moderate, with a score ≥5, neurologic abnormalities remained stable during treatment but increased on disease progression (Appendix Fig A3D).
Pharmacokinetics
Pharmacokinetic analyses were conducted on 49 patients, with both plasma and CSF samples collected on the first day before cycles 3 and 6 (Appendix Table A7). The CSF concentrations of osimertinib on these days were found to be consistent. The CSF to free plasma ratio of osimertinib was approximately 22%, suggesting effective CNS penetration. The metabolite of osimertinib, AZD5104, displayed a plasma-to-CSF ratio of about 16%, and its concentration in the CSF was around 6%. The CSF-to-plasma ratio for AZD5104 was approximately 10%.
Safety Analysis
In terms of safety, treatment-emergent adverse events (TEAEs) were recorded for the FAS. 94.5% of patients experienced at least one TEAE associated with osimertinib, with 38.4% experiencing events of grade 3 or higher. Grade 5 adverse events occurred in four patients, and two experienced grade 4 events, none of which were directly attributable to the osimertinib treatment. Adverse events related to osimertinib were observed in 42 patients (57.5%), predominantly of grades 1 and 2 (Table 3). The most common osimertinib-related adverse events were pruritus (13.7%), followed by rash (12.3%), nail disorder (11.0%), and paronychia (9.6%). Four patients experienced grade 3 adverse events, which were manageable with appropriate clinical interventions. During the course of the treatment, four patients (5.5%) required a dosage reduction to 40 mg because of side effects, including grade 3 skin rash, weight loss, and depressive symptoms, as well as grade 2 vomiting. Treatment was discontinued in two patients (2.7%) because of a grade 3 acute cerebral infarction and grade 2 pneumonitis.
TABLE 3.
Treatment-Related Adverse Events
| Event | Total, No. (%) | Grade 1, No. (%) | Grade 2, No. (%) | Grade 3, No. (%) |
|---|---|---|---|---|
| Pruritus | 10 (13.7) | 5 (6.8) | 5 (6.8) | — |
| Rash | 9 (12.3) | 5 (6.8) | 3 (4.1) | 1 (1.4) |
| Nail disorder | 8 (11.0) | 3 (4.1) | 5 (6.8) | — |
| Paronychia | 7 (9.6) | 2 (2.7) | 5 (6.8) | — |
| Diarrhea | 5 (6.8) | 5 (6.8) | — | — |
| Nausea | 5 (6.8) | 4 (5.5) | 1 (1.4) | — |
| Mucositis | 4 (5.5) | 2 (2.7) | 2 (2.7) | — |
| Decreased appetite | 4 (5.5) | 3 (4.1) | 1 (1.4) | — |
| Headache | 3 (4.1) | — | 3 (4.1) | — |
| Dry skin | 3 (4.1) | 3 (4.1) | — | — |
| Insomnia | 2 (2.7) | — | 2 (2.7) | — |
| Stomatitis | 2 (2.7) | 2 (2.7) | — | — |
| Vomiting | 2 (2.7) | 1 (1.4) | 1 (1.4) | — |
| Alanine aminotransferase increased | 2 (2.7) | 2 (2.7) | — | — |
| Dizziness | 2 (2.7) | 1 (1.4) | 1 (1.4) | — |
| Muscle weakness | 1 (1.4) | — | 1 (1.4) | — |
| Dysphagia | 1 (1.4) | 1 (1.4) | — | — |
| Cardiac failure | 1 (1.4) | — | — | 1 (1.4) |
| Confusional state | 1 (1.4) | — | — | 1 (1.4) |
| Neutropenia | 1 (1.4) | — | — | 1 (1.4) |
DISCUSSION
In this study, we demonstrated the high efficacy of 80 mg once daily osimertinib in patients with LMs after treatment with first- or second-generation EGFR TKIs. The primary end point of OS was observed to be 15.6 months for the FAS and 15.0 months for the LM efficacy evaluable set, respectively. Notably, the ORR for LM reached 51.6%, which included a CR rate of 15.6%, while the median PFS was reported as 11.2 months based on RANO-LM evaluations by the BICR. These findings not only highlight a strong radiological response but also report significant improvements in quality of life and patient-reported outcomes. Furthermore, pharmacokinetic analysis showed that the CSF-to-free plasma ratio of osimertinib at an 80 mg dose was comparable with that of the 160 mg dose, supporting previous findings on its effectiveness.9
There remains a significant unmet medical need for patients with LM in EGFR-mutant NSCLC, largely attributable to the suboptimal pharmacologic levels achieved with first- or second-generation EGFR TKIs because of inadequate drug penetration. By contrast, third-generation EGFR TKI has demonstrated high CNS penetration and clinical efficacy in previous studies.7,8 In line with our results, a preclinical study demonstrates that osimertinib was associated with a higher CSF distribution (CSF Kpuu = 0.29) compared with other third-generation TKIs.6 Notably, although a double dose of daily osimertinib (160 mg) was investigated to achieve higher CSF drug concentrations for improved efficacy,9,10 our study found that daily 80 mg of osimertinib provided comparable OS, with marginal improvements over previous reports which might be ascribed to the enrollment of patients with stable extracranial disease. Of note, comprehensive pharmacokinetic data from our study indicates that daily 80 mg osimertinib achieved a CSF concentration almost on par with the daily 160 mg dosage, albeit with a marginally lower absolute CSF concentration. These results are consistent with our previous work, where preclinical pharmacokinetic/pharmacodynamic modeling indicated that more than 50% of patients are expected to have sufficient LM-free drug exposure to achieve maximal tumor growth inhibition at 80 mg, and the median LM dose response appeared to be saturated at ≥80 mg.16 Furthermore, the CSF-to-free plasma ratio maintained a linear correlation between the two dosages, reinforcing the potential for standard dosing to reach efficacious CSF therapeutic concentrations as reflected by similar, if not superior, OS metrics derived from previous data.9
The study's findings are particularly relevant as most patients were T790M negative, yet they exhibited comparable efficacy with that observed in patients with EGFR T790M-positive LMs. This was observed although some patients received the treatment immediately after prior therapies such as WBRT or intrathecal methotrexate. This suggests that osimertinib can overcome CNS failure, the so-called sanctuary lesion, in patients with LMs regardless of T790M mutation status. Additionally, the prevalence of the L858R mutation in 60% of our study patients suggests a potential association with a higher risk of CNS progression. The study also found that the LM ORR was consistent across patients with prior cranial radiotherapy (52.9%) or intrathecal chemotherapy (62.5%), indicating no significant impact of prior CNS local therapies on osimertinib's efficacy.
To date, the objective response assessment for patients with LM is not fully established. The RANO working group, with its LM expertise, has proposed a consensus for patient evaluation.17 In this study, the ORR reported by investigators (6.3%), according to RECIST, was notably lower than that by BICR (51.6%), according to RANO-LM. The CR incidence also varied, being 15.6% for BICR versus 1.6% for investigators, though the DCR was comparably high across both evaluations (81.3% by BICR v 85.9% by investigators). This variance largely stems from the stringent RANO-LM criteria used by BICR, contrasting with the RECIST criteria for investigators. Nonetheless, the median intracranial PFS was similar between the two assessments, underscoring the need for further validation of RANO-LM in large-scale prospective studies.
This study also employed a comprehensive quality-of-life assessment, revealing significant improvements across various domains, particularly in QLQ-C30 and QLQ-BN20 scores. Patient-reported outcomes, including a 12-question symptom survey from NCI-PRO-CTCAE, indicated notable benefits after osimertinib treatment. Moreover, these quality-of-life enhancements corresponded with positive neurologic and radiologic findings, highlighting osimertinib's substantial therapeutic value in enhancing daily activities for patients with LMs. Osimertinib was well tolerated, with most side effects being mild (grade 1 or 2 skin rash and pruritus) and affecting only about 13% of patients, which is lower compared with a 160 mg dose of osimertinib, where more than 50% of patients experienced rash or diarrhea. Only 5% of patients required a dose reduction because of side effects.
A notable limitation of our study is its focus on patients initially treated with first- or second-generation EGFR TKIs, a practice that contrasts with the emerging standard that includes third-generation EGFR TKIs as frontline therapy.19 With the evolution of first-line treatments to include combinations of third-generation EGFR TKIs with chemotherapy or bispecific antibodies,20,21 the effectiveness of third-generation EGFR TKI in managing LM post-combination therapy warrants further exploration. In addition, the T790M mutation status was confirmed using either blood or tissue samples, which have limitations in accurately capturing the presence of the T790M mutation in the CSF. To address this issue, we conducted an exploratory analysis using targeted sequencing (Liquid SCAN, GENINUS, Seoul, Korea) on CSF samples from 35 patients. The results revealed that all but one patient were T790M negative; the exception was a patient who was also confirmed to be T790M positive in a tissue sample. Despite this, to our knowledge, this is the pioneering prospective study evaluating the efficacy of a standard dose of osimertinib in treating EGFR-mutated NSCLC with LMs, a notably challenging condition.
In conclusion, the standard dose of osimertinib has shown promise in enhancing disease control and OS for patients with LM who do not have systemic progression, particularly those who are T790M negative and have progressed on prior first- or second-generation EGFR TKI therapies. These findings support the consideration of 80 mg once daily osimertinib as a viable treatment option for these patients.
ACKNOWLEDGMENT
This study was operated by the Samsung Medical Center Academic CRO (Seoha Kim), Central laboratory at Samsung Medical Center (Mi Soon Kim, Kyoung Young Lee, You-Kyung Lee, Jiae Koh, Bo Mi Ku).
APPENDIX
FIG A1.
Distribution of patients based on the presence of leptomeningeal metastases. EFS, event-free survival; FAS, full analysis set; LM-EFR, leptomeningeal metastases evaluable for response.
FIG A2.
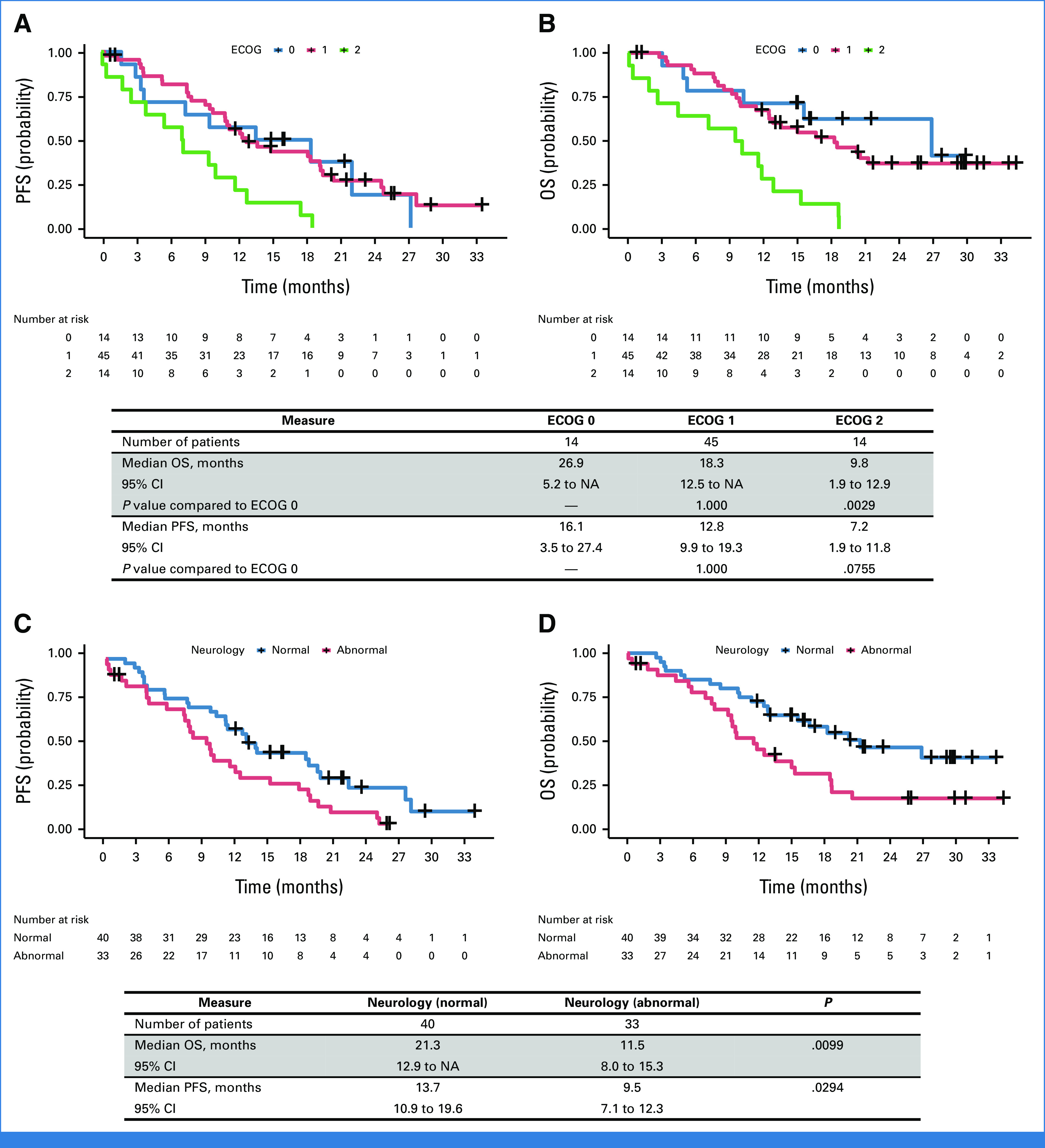
Survival analysis on the basis of the subgroup from the full analysis set. The KM curve on the basis of initial ECOG performance status: (A) PFS and (B) OS. Survival analysis on the basis of the presence of neurology symptoms at the baseline: (C) PFS and (D) OS. ECOG, Eastern Cooperative Oncology Group; KM, Kaplan-Meier; NA, not available; OS, overall survival; PFS, progression-free survival.
FIG A3.
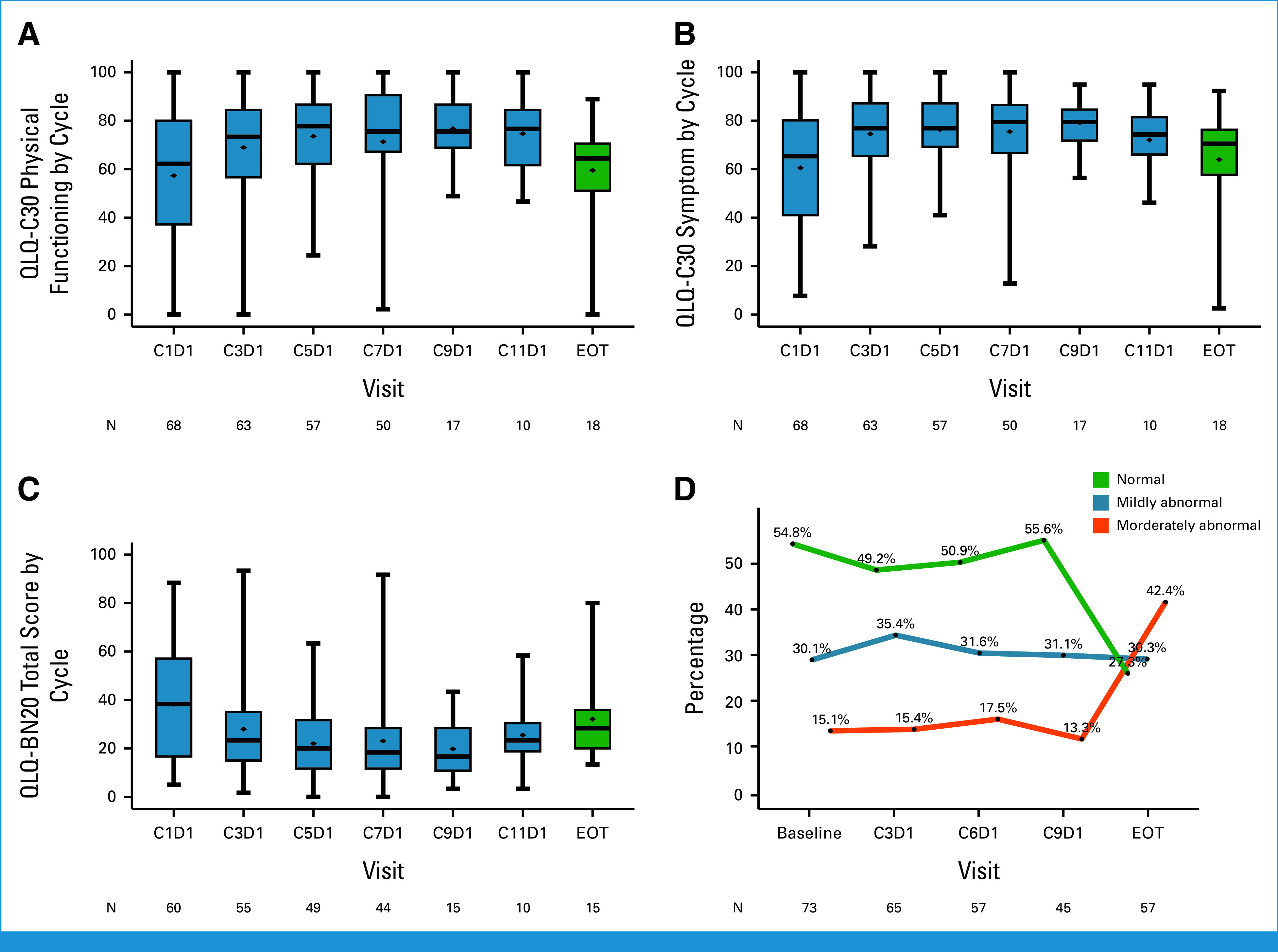
Detailed quality-of-life assessment and neurology examination. Changes in scores over the treatment course compared to the baseline: (A) Physical functioning score. (B) Symptom score from QLQ-C30. (C) Scores from QLQ-BN20. (D) Changes in neurologic examination results: normal (score 0), mildly abnormal (scores 1-4), moderately abnormal (score 5 or higher). EOT, end of treatment; QLQ-BN20, 20-item EORTC Quality of Life Questionnaire–Brain Neoplasm questionnaire; QLQ-C30, 30-item EORTC Core Quality of Life questionnaire.
TABLE A1.
Neurologic Examination Results From Baseline
| Neurologic Examination | n = 73, No. (%) |
|---|---|
| Gait | |
| 0—Normal | 49 (67.1) |
| 1—Abnormal but walks without assistance | 14 (19.2) |
| 2—Abnormal and requires assistance (companion, cane, walker, etc) | 8 (11.0) |
| 3—Unable to walk | 2 (2.7) |
| Strength | |
| 0—Normal | 50 (68.5) |
| 1—Movement present but decreased against resistance | 19 (26.0) |
| 2—Movement present but none against resistance | 4 (5.5) |
| 3—No movement | 0 |
| Sensation | |
| 0—Norma | 66 (90.4) |
| 1—Decreased but aware of sensory modality | 7 (9.6) |
| 2—Unaware of sensory modality | 0 |
| Vision | |
| 0—Normal | 59 (80.8) |
| 1—Partial monocular visual loss | 12 (16.4) |
| 2—Complete monocular visual loss | 0 |
| 3—Bilateral visual loss | 2 (2.7) |
| Eye movements | |
| 0—Normal | 71 (97.3) |
| 1—Abnormality noted in one direction of gaze | 2 (2.7) |
| 2—Abnormality noted in more than one gaze direction, but not all | 0 |
| 3—Unable to move the eye in any gaze direction | 0 |
| Facial strength | |
| 0—Normal | 67 (91.8) |
| 1—Mild facial weakness (nasolabial fold flattening, asymmetric smile, decreased forehead contraction, or partial eye closure) | 6 (8.2) |
| 2—Severe facial weakness (severe nasolabial fold flattening, asymmetric smile with limited or no movement of face, incomplete eye closure, or labial incompetence | 0 |
| 3—Bilateral facial weakness | 0 |
| Hearing | |
| 0—Normal | 65 (89.0) |
| 1—Impaired but residual serviceable hearing | 7 (9.6) |
| 2—Absent unilateral hearing | 0 |
| 3—Bilateral hearing loss | 1 (1.4) |
| Swallowing | |
| 0—Normal | 70 (95.9) |
| 1—Impaired but not requiring change in diet formulation, not aspirating by bedside testing | 2 (2.7) |
| 2- Unable to swallow without risk of aspiration by bedside testing | 1 (1.4) |
| Level of consciousness | |
| 0—Normal | 71 (97.3) |
| 1—Drowsy (easily arousable and responsive) | 1 (1.4) |
| 2—Somnolent (difficult to arouse and poorly responsive) | 1 (1.4) |
| 3—Coma (unarousable and unresponsive) | 0 |
| Behavior | |
| 0—Normal | 65 (89.04) |
| 1—Mild/moderate alteration | 7 (9.59) |
| 2—Severe alteration | 1 (1.37) |
| Other | |
| 0—Normal | 73 (100) |
| 1—Occasional or mild | 0 (0.00) |
| 2—Persistent, moderate to severe | 0 (0.00) |
| Neurologic examination total score | |
| Mean | 1.67 |
| SD | 2.65 |
| Median | 0 |
| Min, Max | 0, 10 |
Abbreviation: SD, standard deviation.
TABLE A2.
Questions Selected for Patient-Reported Outcome From NCI-PRO-CTCAE
| Headache | ||||
| In the last 7 days, how OFTEN did you have a HEADACHE? | ||||
| ○ Never | ○ Rarely | ○ Occasionally | ○ Frequently | ○ Almost constantly |
| In the last 7 days, what was the SEVERITY of your HEADACHE at its WORST? | ||||
| ○ None | ○ Mild | ○ Moderate | ○ Severe | ○ Very severe |
| In the last 7 days, how much did your HEADACHE INTERFERE with your usual or daily activities? | ||||
| ○ Not at all | ○ A little bit | ○ Somewhat | ○ Quite a bit | ○ Very much |
| Difficulty swallowing | ||||
| In the last 7 days, what was the SEVERITY of your DIFFICULTY SWALLOWING at its WORST? | ||||
| ○ None | ○ Mild | ○ Moderate | ○ Severe | ○ Very severe |
| Dizziness | ||||
| In the last 7 days, what was the SEVERITY of your DIZZINESS at its WORST? | ||||
| ○ None | ○ Mild | ○ Moderate | ○ Severe | ○ Very severe |
| In the last 7 days, how much did DIZZINESS INTERFERE with your usual or daily activities? | ||||
| ○ Quite a bit | ○ Quite a bit | ○ Quite a bit | ○ Quite a bit | ○ Quite a bit |
| Nausea | ||||
| In the last 7 days, how OFTEN did you have NAUSEA? | ||||
| ○ Never | ○ Rarely | ○ Occasionally | ○ Frequently | ○ Almost constantly |
| In the last 7 days, what was the SEVERITY of your NAUSEA at its WORST? | ||||
| ○ None | ○ Mild | ○ Moderate | ○ Severe | ○ Very severe |
| Vomiting | ||||
| In the last 7 days, how OFTEN did you have VOMITING? | ||||
| ○ Never | ○ Rarely | ○ Occasionally | ○ Frequently | ○ Almost constantly |
| In the last 7 days, what was the SEVERITY of your VOMITING at its WORST? | ||||
| ○ None | ○ Mild | ○ Moderate | ○ Severe | ○ Very severe |
| Memory | ||||
| In the last 7 days, what was the SEVERITY of your PROBLEMS WITH MEMORY at their WORST? | ||||
| ○ None | ○ Mild | ○ Moderate | ○ Severe | ○ Very severe |
| In the last 7 days, how much did PROBLEMS WITH MEMORY INTERFERE with your usual or daily activities? | ||||
| ○ Not at all | ○ A little bit | ○ Somewhat | ○ Quite a bit | ○ Very much |
Abbreviation: NCI-PRO-CTCAE, National Cancer Institute's Patient Reported Outcome of the Common Terminology Criteria for Adverse Events.
TABLE A3.
OS on the Basis of Previous Exposurbrain Radiotherapy and Intrathecal Methotrexate Treatment
| Measure | Did Not Receive WBRT | Received WBRT | P |
|---|---|---|---|
| No. of patients | 63 | 10 | |
| Median OS, months | 18.3 | 9.6 | .0222 |
| 95% CI | 12.5 to 21.3 | 4.9 to 15.3 | |
| Measure | Did Not Receive IT-MTX | Received IT-MTX | P |
| No. of patients | 64 | 9 | |
| Median OS, months | 18.3 | 15.0 | .5129 |
| 95% CI | 10.2 to 21.3 | 5.6 to 16.6 |
Abbreviations: IT-MTX, intrathecal-methotrexate; OS, overall survival; WBRT, whole-brain radiotherapy
TABLE A4.
EORTC QLQ-C30 Score Changes Compared With the Baseline
| Category | Visit | Total (N = 60) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | Mean | SD | Q1 | Median | Q3 | Minimum | Maximum | ||
| Total score | Cycle 3 – cycle 1 | 53 | –8.58 | 16.27 | –22.00 | –2.00 | 2.00 | –45.00 | 23.00 |
| Cycle 5 – cycle 1 | 47 | –10.04 | 18.13 | –21.00 | –4.00 | 3.00 | –54.00 | 23.00 | |
| Cycle 7 – cycle 1 | 42 | –9.02 | 18.08 | –21.00 | –2.00 | 3.00 | –48.00 | 17.00 | |
| Cycle 9 – cycle 1 | 14 | –18.64 | 17.93 | –32.00 | –18.50 | –1.00 | –46.00 | 4.00 | |
| Cycle 11 – cycle 1 | 9 | –14.56 | 15.13 | –26.00 | –14.00 | 1.00 | –34.00 | 5.00 | |
| Cycle 13 – cycle 1 | 5 | –6.20 | 13.81 | –13.00 | –11.00 | 6.00 | –23.00 | 10.00 | |
| Cycle 15 – cycle 1 | 2 | –3.50 | 13.44 | –13.00 | –3.50 | 6.00 | –13.00 | 6.00 | |
| EOT – cycle 1 | 15 | 7.33 | 23.00 | –6.00 | 8.00 | 23.00 | –32.00 | 55.00 | |
| Physical functioning | Cycle 3 – cycle 1 | 53 | 11.47 | 20.47 | –2.22 | 4.44 | 24.44 | –26.67 | 62.22 |
| Cycle 5 – cycle 1 | 47 | 14.94 | 24.43 | –2.22 | 8.89 | 28.89 | –28.89 | 73.33 | |
| Cycle 7 – cycle 1 | 42 | 11.69 | 25.17 | –6.67 | 3.33 | 24.44 | –20.00 | 68.89 | |
| Cycle 9 – cycle 1 | 14 | 22.22 | 25.83 | 0.00 | 13.33 | 51.11 | –17.78 | 62.22 | |
| Cycle 11 – cycle 1 | 9 | 17.04 | 20.25 | 6.67 | 13.33 | 31.11 | –11.11 | 46.67 | |
| Cycle 13 – cycle 1 | 5 | 2.22 | 16.56 | –8.89 | 2.22 | 11.11 | –17.78 | 24.44 | |
| Cycle 15 – cycle 1 | 2 | –3.33 | 17.28 | –15.56 | –3.33 | 8.89 | –15.56 | 8.89 | |
| EOT – cycle 1 | 15 | –11.56 | 29.07 | –33.33 | –13.33 | 4.44 | –75.56 | 37.78 | |
| Global health status | Cycle 3 – cycle 1 | 53 | 17.45 | 29.16 | 0.00 | 16.67 | 41.67 | –33.33 | 75.00 |
| Cycle 5 – cycle 1 | 47 | 19.68 | 28.68 | 0.00 | 16.67 | 33.33 | –33.33 | 83.33 | |
| Cycle 7 – cycle 1 | 42 | 13.29 | 29.68 | 0.00 | 4.17 | 33.33 | –41.67 | 91.67 | |
| Cycle 9 – cycle 1 | 14 | 24.40 | 32.27 | 0.00 | 16.67 | 41.67 | –16.67 | 91.67 | |
| Cycle 11 – cycle 1 | 9 | 20.37 | 25.38 | 0.00 | 25.00 | 33.33 | –25.00 | 58.33 | |
| Cycle 13 – cycle 1 | 5 | 20.00 | 25.41 | 0.00 | 8.33 | 33.33 | 0.00 | 58.33 | |
| Cycle 15 – cycle 1 | 2 | –4.17 | 5.89 | –8.33 | –4.17 | 0.00 | –8.33 | 0.00 | |
| EOT – cycle 1 | 15 | –17.22 | 25.29 | –33.33 | –16.67 | 8.33 | –66.67 | 16.67 | |
| Symptom | Cycle 3 – cycle 1 | 53 | –13.98 | 25.72 | –30.77 | –7.69 | 2.56 | –79.49 | 38.46 |
| Cycle 5 – cycle 1 | 47 | –14.57 | 27.68 | –35.90 | –5.13 | 2.56 | –79.49 | 33.33 | |
| Cycle 7 – cycle 1 | 42 | –13.74 | 26.69 | –33.33 | –5.13 | 5.13 | –76.92 | 25.64 | |
| Cycle 9 – cycle 1 | 14 | –29.67 | 27.28 | –58.97 | –23.08 | –7.69 | –74.36 | 5.13 | |
| Cycle 11 – cycle 1 | 9 | –23.93 | 23.47 | –43.59 | –28.21 | –2.56 | –51.28 | 7.69 | |
| Cycle 13 – cycle 1 | 5 | –19.49 | 22.98 | –38.46 | –25.64 | –5.13 | –41.03 | 12.82 | |
| Cycle 15 – cycle 1 | 2 | –11.54 | 16.32 | –23.08 | –11.54 | 0.00 | –23.08 | 0.00 | |
| EOT – cycle 1 | 15 | 10.77 | 30.80 | –12.82 | 7.69 | 28.21 | –43.59 | 74.36 | |
Abbreviations: EORTC QLQ-C30, European Organisation for Research and Treatment of Cancer 30-item Quality of Life questionnaire; EOT, end of treatment; Q1, 1st quartile; Q3, 3rd quartile; SD, standard deviation.
TABLE A5.
EORTC QLQ-BN20 Score Changes Compared With the Baseline
| Category | Visit | Total (N = 60) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | Mean | SD | Q1 | Median | Q3 | Minimum | Maximum | ||
| Total score | Cycle 3 – cycle 1 | 53 | –5.66 | 11.64 | –13 | –2 | 1 | –32 | 26 |
| Cycle 5 – cycle 1 | 47 | –7.89 | 12.43 | –17 | –8 | 1 | –39 | 15 | |
| Cycle 7 – cycle 1 | 42 | –6.45 | 11.81 | –14 | –3 | 1 | –40 | 11 | |
| Cycle 9 – cycle 1 | 14 | –12.57 | 12.20 | –20 | –13 | –5 | –40 | 11 | |
| Cycle 11 – cycle 1 | 9 | –10.33 | 13.72 | –12 | –11 | –8 | –38 | 12 | |
| Cycle 13 – cycle 1 | 5 | –8.80 | 7.92 | –14 | –11 | –7 | –16 | 4 | |
| Cycle 15 – cycle 1 | 2 | –9.00 | 5.66 | –13 | –9 | –5 | –13 | –5 | |
| EOT – cycle 1 | 15 | 2.80 | 13.45 | –2 | 1 | 10 | –25 | 38 | |
Abbreviations: EORTC QLQ-BN20, 20-item EORTC Quality of Life Questionnaire–Brain Neoplasm questionnaire; EOT, end of treatment; Q1, 1st quartile; Q3, 3rd quartile; SD, standard deviation.
TABLE A6.
NCI-PRO-CTCAE Score Changes Compared With the Baseline
| Category | Visit | Total (N = 48) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | Mean | SD | Q1 | Median | Q3 | Minimum | Maximum | ||
| Total score | Cycle 3 – cycle 1 | 42 | –5.07 | 13.14 | –8 | –3 | 2 | –43 | 23 |
| Cycle 5 – cycle 1 | 35 | –5.14 | 13.76 | –10 | –1 | 2 | –43 | 15 | |
| Cycle 7 – cycle 1 | 32 | –5.44 | 13.81 | –11 | –2 | 5 | –43 | 13 | |
| Cycle 9 – cycle 1 | 26 | –7.08 | 14.77 | –21 | –2 | 3 | –43 | 13 | |
| Cycle 11 – cycle 1 | 24 | –4.96 | 15.93 | –12 | –0.5 | 5.5 | –40 | 21 | |
| Cycle 13 – cycle 1 | 19 | –4.21 | 16.09 | –10 | 0 | 9 | –43 | 18 | |
| Cycle 15 – cycle 1 | 15 | –6.80 | 17.54 | –24 | 0 | 7 | –43 | 16 | |
| EOT – cycle 1 | 17 | 4.00 | 10.58 | –3 | 5 | 14 | –18 | 21 | |
Abbreviations: EOT, end of treatment: NCI-PRO-CTCAE, National Cancer Institute's Patient Reported Outcome of the Common Terminology Criteria for Adverse Events; Q1, 1st quartile; Q3, 3rd quartile; SD, standard deviation.
TABLE A7.
Pharmacokinetics Result of Osimertinib and AZD5104
| Visit | Category | Statistic | Osimertinib, nM | AZ5104, nM | ||||
|---|---|---|---|---|---|---|---|---|
| T790M– | T790M+ | All | T790M– | T790M+ | All | |||
| C3D1 | Free plasma concentration, nM | No. Geometric mean (CV%) Range |
48 19.07 (84.46) 1.00-74.20 |
1 29.20 |
49 19.24 (83.70) 1.00-74.20 |
48 3.03 (81.91) 0.22-20.22 |
1 42.30 |
49 3.04 (80.84) 0.22-20.22 |
| CSF concentration, nM | No. Geometric mean (CV%) Range |
36 4.55 (105.37) 0.46-47.70 |
1 0.41 |
37 4.58 (103.49) 0.46-47.70 |
36 0.30 (97.81) 0.08-5.98 |
1 0.41 |
37 0.30 (96.19) 0.08-5.98 |
|
| Free plasma metabolite-to-parent ratio | No. Ratio (CV%) Range |
NA | NA | NA | 48 0.16 (64.87) 0.09-0.55 |
1 0.11 |
49 0.16 (34.84) 0.09-0.55 |
|
| CSF metabolite-to-parent ratio | No. Ratio (CV%) Range |
NA | NA | NA | 36 0.07 (52.85) 0.037-0.290 |
1 0.07 |
37 0.066 (52.02) 0.037-0.290 |
|
| CSF-to-free plasma ratio | No. Ratio (CV%) Range |
36 22.25 (63.34) 3.80-64.29 |
1 20.44 |
37 22.20 (62.47) 3.80-64.29 |
36 10.21 (53.05) 3.29-29.57 |
1 12.21 |
37 10.25 (52.32) 3.29-29.57 |
|
| C6D1 | Free plasma concentration, nM | No. Geometric mean (CV%) Range |
41 21.87 (59.73) 3.91-69.96 |
NA | 41 21.87 (59.73) 3.91-69.96 |
41 3.34 (71.11) 0.64-19.51 |
NA | 41 3.34 (71.11) 0.64-19.51 |
| CSF concentration, nM | No. Geometric mean (CV%) Range |
30 4.81 (88.16) 0.84-21.70 |
1 2.74 |
31 4.72 (87.25) 0.84-21.70 |
30 0.29 (72.75) 0.11-1.35 |
1 0.22 |
31 0.29 (71.58) 0.11-1.35 |
|
| Free plasma metabolite-to-parent ratio | No. Ratio (CV%) Range |
NA | NA | NA | 41 0.15 (24.51) 0.09-0.30 |
0 | 41 0.15 (24.51) 0.09-0.30 |
|
| CSF metabolite-to-parent ratio | No. Ratio (CV%) Range |
NA | NA | NA | 30 0.06 (48.09) 0.03-0.19 |
1 0.078 |
31 0.06 (47.46) 0.03-0.19 |
|
| CSF-to-free plasma ratio | No. Ratio (CV%) Range |
30 22.12 (80.14) 4.73-82.29 |
NA | 30 22.12 (80.14) 4.73-82.29 |
30 9.33 (59.57) 3.78-29.41 |
NA | 30 9.33 (59.57) 3.78-29.41 |
|
Abbreviations: CSF, cerebrospinal fluid; CV, Coefficient of Variation; NA, not available.
Richard Baldry
Employment: AstraZeneca
Stock or Other Ownership: AstraZeneca
Hyun Ae Jung
Consulting or Advisory Role: Yuhan, Guardant Health, AIMEDBIO
Research Funding: Yuhan
Jong-Mu Sun
Research Funding: Yuhan (Inst), Bristol Myers Squibb (Inst), pfizer (Inst)
Se-Hoon Lee
Honoraria: AstraZeneca/MedImmune, Roche, Lilly, Amgen, Yuhan, Merck Sharp & Dohme
Consulting or Advisory Role: AstraZeneca, Roche, Pfizer, Lilly, BMS/Ono, Takeda, Janssen, IMBdx, Abion, BeiGene, Daiichi Sankyo, ImmuneOncia, Merck (German), Merck Sharp & Dohme, Novartis
Speakers' Bureau: Abion
Research Funding: AstraZeneca (Inst), Lunit (Inst), Merck Sharp & Dohme (Inst)
Jin Seok Ahn
Honoraria: Pfizer, Roche, BC World Pharmaceutical, Yuhan, Novartis, Amgen, Boehringer Ingelheim, Menarini, Kyowa Kirin, AstraZeneca, Bayer, Lilly, Takeda, Boryung, Samyang, Nokwon Medical
Consulting or Advisory Role: Pharmbio Korea, Guardant Health, Yuhan, ImmuneOncia, Therapex, Daiichi Sankyo Korea, Roche
Youngjoo Lee
Consulting or Advisory Role: Roche, MSD, Yuhan
Dong-Wan Kim
Research Funding: Alpha Biopharma (Inst), AstraZeneca/MedImmune (Inst), Hanmi (Inst), Janssen (Inst), Merus (Inst), Mirati Therapeutics (Inst), MSD (Inst), Novartis (Inst), Ono Pharmaceutical (Inst), Pfizer (Inst), Roche/Genentech (Inst), Takeda (Inst), TP Therapeutics (Inst), Xcovery (Inst), Yuhan (Inst), Boehringer Ingelheim (Inst), Amgen (Inst), Daiichi Sankyo (Inst), Chong Kun Dang Pharmaceutical (Inst), BridgeBio Pharma (Inst), GlaxoSmithKline (Inst), Merck (Inst), inno.N (Inst), IMBdx (Inst), IMBdx (Inst)
Ki Hyeong Lee
Consulting or Advisory Role: Bristol Myers Squibb, MSD, AstraZeneca, Pfizer, Lilly
Research Funding: Merck
Myung-Ju Ahn
Honoraria: AstraZeneca, Lilly, MSD, Takeda, Amgen, Merck Serono, Yuhan, Daiichi Sankyo/Astra Zeneca
Consulting or Advisory Role: AstraZeneca, Lilly, MSD, Takeda, Alpha pharmaceutical, Amgen, Merck Serono, Pfizer, Yuhan, Arcus Ventures, Daiichi Sankyo/Astra Zeneca, Daiichi Sankyo/AstraZeneca
Research Funding: Yuhan
No other potential conflicts of interest were reported.
See accompanying Editorial, p. 2727
DISCLAIMER
The source of funding had no role in the study design, data collection, data analysis, data interpretation, or report writing.
PRIOR PRESENTATION
Presented at the 2024 ASCO annual meeting, Chicago, IL, June 3, 2024.
SUPPORT
Supported by AstraZeneca by providing osimertinib and funds for this study.
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
A data sharing statement provided by the authors is available with this article at DOI https://doi.org/10.1200/JCO.24.00708.
AUTHOR CONTRIBUTIONS
Conception and design: Sehhoon Park, Jung Won Choi, Myung-Ju Ahn
Financial support: Myung-Ju Ahn
Provision of study materials or patients: Jong-Mu Sun, Jin Seok Ahn, Yu Jung Kim, Dong-Wan Kim, Sang-We Kim, Ki Hyeong Lee, Won Jae Lee, Kyuha Chong, Jung-Il Lee, Myung-Ju Ahn
Collection and assembly of data: Sehhoon Park, Hyun Ae Jung, Jong-Mu Sun, Jin Seok Ahn, Yu Jung Kim, Youngjoo Lee, Sang-We Kim, Ki Hyeong Lee, Won Jae Lee, Kyuha Chong, Jung-Il Lee, Myung-Ju Ahn
Data analysis and interpretation: Sehhoon Park, Richard Baldry, Hyun Ae Jung, Se-Hoon Lee, Jin Seok Ahn, Yu Jung Kim, Dong-Wan Kim, Sang-We Kim, Ki Hyeong Lee, So-Hyeon Gwon, Nak-Hoon Son, Myung-Ju Ahn
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Phase II Efficacy and Safety of 80 mg Osimertinib in Patients With Leptomeningeal Metastases Associated With Epidermal Growth Factor Receptor Mutation–Positive Non–Small Cell Lung Cancer (BLOSSOM)
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Richard Baldry
Employment: AstraZeneca
Stock or Other Ownership: AstraZeneca
Hyun Ae Jung
Consulting or Advisory Role: Yuhan, Guardant Health, AIMEDBIO
Research Funding: Yuhan
Jong-Mu Sun
Research Funding: Yuhan (Inst), Bristol Myers Squibb (Inst), pfizer (Inst)
Se-Hoon Lee
Honoraria: AstraZeneca/MedImmune, Roche, Lilly, Amgen, Yuhan, Merck Sharp & Dohme
Consulting or Advisory Role: AstraZeneca, Roche, Pfizer, Lilly, BMS/Ono, Takeda, Janssen, IMBdx, Abion, BeiGene, Daiichi Sankyo, ImmuneOncia, Merck (German), Merck Sharp & Dohme, Novartis
Speakers' Bureau: Abion
Research Funding: AstraZeneca (Inst), Lunit (Inst), Merck Sharp & Dohme (Inst)
Jin Seok Ahn
Honoraria: Pfizer, Roche, BC World Pharmaceutical, Yuhan, Novartis, Amgen, Boehringer Ingelheim, Menarini, Kyowa Kirin, AstraZeneca, Bayer, Lilly, Takeda, Boryung, Samyang, Nokwon Medical
Consulting or Advisory Role: Pharmbio Korea, Guardant Health, Yuhan, ImmuneOncia, Therapex, Daiichi Sankyo Korea, Roche
Youngjoo Lee
Consulting or Advisory Role: Roche, MSD, Yuhan
Dong-Wan Kim
Research Funding: Alpha Biopharma (Inst), AstraZeneca/MedImmune (Inst), Hanmi (Inst), Janssen (Inst), Merus (Inst), Mirati Therapeutics (Inst), MSD (Inst), Novartis (Inst), Ono Pharmaceutical (Inst), Pfizer (Inst), Roche/Genentech (Inst), Takeda (Inst), TP Therapeutics (Inst), Xcovery (Inst), Yuhan (Inst), Boehringer Ingelheim (Inst), Amgen (Inst), Daiichi Sankyo (Inst), Chong Kun Dang Pharmaceutical (Inst), BridgeBio Pharma (Inst), GlaxoSmithKline (Inst), Merck (Inst), inno.N (Inst), IMBdx (Inst), IMBdx (Inst)
Ki Hyeong Lee
Consulting or Advisory Role: Bristol Myers Squibb, MSD, AstraZeneca, Pfizer, Lilly
Research Funding: Merck
Myung-Ju Ahn
Honoraria: AstraZeneca, Lilly, MSD, Takeda, Amgen, Merck Serono, Yuhan, Daiichi Sankyo/Astra Zeneca
Consulting or Advisory Role: AstraZeneca, Lilly, MSD, Takeda, Alpha pharmaceutical, Amgen, Merck Serono, Pfizer, Yuhan, Arcus Ventures, Daiichi Sankyo/Astra Zeneca, Daiichi Sankyo/AstraZeneca
Research Funding: Yuhan
No other potential conflicts of interest were reported.
REFERENCES
- 1.Wu YL, Zhao Q, Deng L, et al. : Leptomeningeal metastasis after effective first-generation EGFR TKI treatment of advanced non-small cell lung cancer. Lung Cancer 127:1-5, 2019 [DOI] [PubMed] [Google Scholar]
- 2.Li YS, Jiang BY, Yang JJ, et al. : Leptomeningeal metastases in patients with NSCLC with EGFR mutations. J Thorac Oncol 11:1962-1969, 2016 [DOI] [PubMed] [Google Scholar]
- 3.Grommes C, Oxnard GR, Kris MG, et al. : “Pulsatile” high-dose weekly erlotinib for CNS metastases from EGFR mutant non-small cell lung cancer. Neuro Oncol 13:1364-1369, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colclough N, Chen K, Johnström P, et al. : Preclinical comparison of the blood-brain barrier permeability of osimertinib with other EGFR TKIs. Clin Cancer Res 27:189-201, 2021 [DOI] [PubMed] [Google Scholar]
- 5.Ballard P, Yates JW, Yang Z, et al. : Preclinical comparison of osimertinib with other EGFR-TKIs in EGFR-mutant NSCLC brain metastases models, and early evidence of clinical brain metastases activity. Clin Cancer Res 22:5130-5140, 2016 [DOI] [PubMed] [Google Scholar]
- 6.Varrone A, Varnäs K, Jucaite A, et al. : A PET study in healthy subjects of brain exposure of (11)C-labelled osimertinib—A drug intended for treatment of brain metastases in non-small cell lung cancer. J Cereb Blood Flow Metab 40:799-807, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herbst RS, Wu YL, John T, et al. : Adjuvant osimertinib for resected EGFR-mutated stage IB-IIIA non-small-cell lung cancer: Updated results from the phase III randomized ADAURA trial. J Clin Oncol 41:1830-1840, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reungwetwattana T, Nakagawa K, Cho BC, et al. : CNS response to osimertinib versus standard epidermal growth factor receptor tyrosine kinase inhibitors in patients with untreated EGFR-mutated advanced non-small-cell lung cancer. J Clin Oncol 36:3290-3297, 2018 [DOI] [PubMed] [Google Scholar]
- 9.Yang JCH, Kim SW, Kim DW, et al. : Osimertinib in patients with epidermal growth factor receptor mutation-positive non-small-cell lung cancer and leptomeningeal metastases: The BLOOM study. J Clin Oncol 38:538-547, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park S, Lee MH, Seong M, et al. : A phase II, multicenter, two cohort study of 160 mg osimertinib in EGFR T790M-positive non-small-cell lung cancer patients with brain metastases or leptomeningeal disease who progressed on prior EGFR TKI therapy. Ann Oncol 31:1397-1404, 2020 [DOI] [PubMed] [Google Scholar]
- 11.Lee J, Choi Y, Han J, et al. : Osimertinib improves overall survival in patients with EGFR-mutated NSCLC with leptomeningeal metastases regardless of T790M mutational status. J Thorac Oncol 15:1758-1766, 2020 [DOI] [PubMed] [Google Scholar]
- 12.Yang J, Ahn M, Kim D, et al. : Osimertinib in pretreated T790M-positive advanced non-small-cell lung cancer: AURA study phase II extension component. J Clin Oncol 35:1288-1296, 2017 [DOI] [PubMed] [Google Scholar]
- 13.Goss G, Tsai CM, Shepherd FA, et al. : Osimertinib for pretreated EGFR Thr790Met-positive advanced non-small-cell lung cancer (AURA2): A multicentre, open-label, single-arm, phase 2 study. Lancet Oncol 17:1643-1652, 2016 [DOI] [PubMed] [Google Scholar]
- 14.Zhou C, Wang M, Cheng Y, et al. : AURA17 study of osimertinib in Asia-Pacific patients (pts) with EGFR T790M-positive advanced non-small cell lung cancer (NSCLC): Updated phase II results including overall survival (OS). Ann Oncol 29:ix157, 2018 [Google Scholar]
- 15.Mok TS, Wu Y-L, Ahn M-J, et al. : Osimertinib or platinum–pemetrexed in EGFR T790M–positive lung cancer. N Engl J Med 376:629-640, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahn MJ, Chiu CH, Cheng Y, et al. : Osimertinib for patients with leptomeningeal metastases associated with EGFR T790M-positive advanced NSCLC: The AURA leptomeningeal metastases analysis. J Thorac Oncol 15:637-648, 2020 [DOI] [PubMed] [Google Scholar]
- 17.Chamberlain M, Junck L, Brandsma D, et al. : Leptomeningeal metastases: A RANO proposal for response criteria. Neuro Oncol 19:484-492, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eisenhauer EA, Therasse P, Bogaerts J, et al. : New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 45:228-247, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Ramalingam SS, Vansteenkiste J, Planchard D, et al. : Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med 382:41-50, 2020 [DOI] [PubMed] [Google Scholar]
- 20.Planchard D, Jänne PA, Cheng Y, et al. : Osimertinib with or without chemotherapy in EGFR-mutated advanced NSCLC. N Engl J Med 389:1935-1948, 2023 [DOI] [PubMed] [Google Scholar]
- 21.Cho B, Felip E, Spira A, et al. : LBA14 Amivantamab plus lazertinib vs osimertinib as first-line treatment in patients with EGFR-mutated, advanced non-small cell lung cancer (NSCLC): Primary results from MARIPOSA, a phase III, global, randomized, controlled trial. Ann Oncol 34:S1306, 2023 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
A data sharing statement provided by the authors is available with this article at DOI https://doi.org/10.1200/JCO.24.00708.