Abstract
PURPOSE
In the phase III HIMALAYA study (ClinicalTrials.gov identifier: NCT03298451) in unresectable hepatocellular carcinoma (uHCC), the Single Tremelimumab Regular Interval Durvalumab (STRIDE) regimen significantly improved overall survival versus sorafenib, and durvalumab monotherapy was noninferior to sorafenib. Patient-reported outcomes (PROs), a secondary outcome from HIMALAYA, are reported here.
METHODS
Participants were randomly assigned to receive STRIDE, durvalumab, or sorafenib. PROs were assessed (preplanned secondary outcome) using the European Organization for Research and Treatment of Cancer 30-item Quality of Life Questionnaire and the 18-item HCC module. Time to deterioration (TTD), change from baseline and improvement rate in global health status/quality of life (GHS/QoL), functioning, and disease-related symptoms were analyzed.
RESULTS
In total, 1,171 participants were randomly assigned to STRIDE (n = 393), durvalumab (n = 389), or sorafenib (n = 389) and were evaluable for PRO assessments. Across treatment arms, compliance rates for PROs were >77% at baseline and >70% overall. Baseline scores were comparable across treatment arms. TTD in GHS/QoL, physical functioning, fatigue, appetite loss, and abdominal pain was numerically longer for both STRIDE and durvalumab versus sorafenib. Clinically meaningful deterioration in PROs was not observed in any treatment arm. However, TTD in nausea and abdominal swelling was numerically longer for STRIDE versus sorafenib, and the likelihood of clinically meaningful improvement in GHS/QoL, role, emotional and social functioning, and disease-related symptoms was greater with STRIDE and durvalumab versus sorafenib. PROs with STRIDE and durvalumab were generally similar.
CONCLUSION
Compared with sorafenib, STRIDE and durvalumab were associated with clinically meaningful, patient-centered GHS/QoL, functioning, and symptom benefits in people with uHCC. These findings support the benefits of the STRIDE regimen compared with sorafenib for a diverse population reflective of the global uHCC population.
INTRODUCTION
Liver cancer is the sixth most common cancer and the third leading cause of cancer-related death worldwide.1,2 Most people with hepatocellular carcinoma (HCC), the most common type of liver cancer, present with advanced stage disease at diagnosis and also have cirrhosis.3-5 Therapeutic options are often limited in these individuals because of poor overall health status, impaired liver function, and comorbidities.5,6 Systemic therapies are the mainstay treatment options for people with unresectable HCC (uHCC), and, until recently, these were limited to the tyrosine kinase inhibitors, sorafenib and lenvatinib.7,8
CONTEXT
Key Objective
Are patient-reported outcomes (PROs) improved with Single Tremelimumab Regular Interval Durvalumab (STRIDE) versus sorafenib treatment for unresectable hepatocellular carcinoma (uHCC)?
Knowledge Generated
Time to deterioration in global health status/quality of life (QoL), functioning, and disease-related symptoms was longer with STRIDE versus sorafenib, and the likelihood of clinically meaningful improvement was greater with STRIDE versus sorafenib. In general, STRIDE and durvalumab regimens were associated with similar times to deterioration and similar improvements for PROs.
Relevance (A.H. Ko)
-
These data lend further support to STRIDE as an option for the frontline treatment of advanced HCC, with the clinical benefits of this regimen extending to QoL and symptomatic control.*
*Relevance section written by JCO Associate Editor Andrew H. Ko, MD, FASCO.
The phase III HIMALAYA study (ClinicalTrials.gov identifier: NCT03298451) evaluated a single dose of tremelimumab, an anticytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) antibody, plus durvalumab, an anti–PD-L1 antibody, in the Single Tremelimumab Regular Interval Durvalumab (STRIDE) regimen, and durvalumab monotherapy versus sorafenib in participants with uHCC with no prior systemic therapy.9 STRIDE significantly improved overall survival (OS) versus sorafenib, with an OS hazard ratio (HR) of 0.78 (96.02% CI, 0.65 to 0.93; P = .0035).9 STRIDE also demonstrated a long-term survival benefit, with a 36-month OS rate of 30.7% versus 20.2% with sorafenib.9 OS with durvalumab monotherapy was noninferior to sorafenib (HR, 0.86 [95.67% CI, 0.73 to 1.03]; noninferiority margin, 1.08).9 The incidence, frequency, and severity of the adverse events with STRIDE and durvalumab were consistent with the known safety profiles of each agent, and no new safety signals were identified.9 STRIDE recently received approval from the US Food and Drug Administration,10,11 Japanese Ministry of Health, Labor, and Welfare,12 and the European Medicines Agency13,14 for the treatment of adults with uHCC.
Symptoms and toxicity from systemic therapies can be a substantial burden for people with uHCC and can significantly impair their health-related quality of life (HRQoL).15,16 The most prevalent and disturbing impacts identified by people with HCC include fatigue, emotional impacts, and impacts on social life.16 Sorafenib is associated with deteriorations in HRQoL and functioning for many people with uHCC.17 While immunotherapy-containing regimens have been shown to extend OS versus sorafenib,9,18 it is important to understand their impact on HRQoL through patient-reported outcomes (PROs). Evidence supporting improvement or preservation of HRQoL is needed to facilitate therapeutic decision making and demonstrate clinically meaningful benefits for people with uHCC.15,19
Here, we report the preplanned, secondary PRO analyses from the HIMALAYA study in terms of HRQoL and disease-related symptoms in participants treated with STRIDE, durvalumab monotherapy, or sorafenib.
METHODS
Study Design and Participants
HIMALAYA was a randomized, open-label, phase III study.9 Participants age ≥18 years with uHCC were randomly assigned to STRIDE (tremelimumab 300 mg for one dose plus durvalumab 1,500 mg once every 4 weeks), durvalumab monotherapy (1,500 mg once every 4 weeks), or sorafenib (400 mg twice daily). Study methodology has been described previously.9
HIMALAYA was conducted in accordance with the Declaration of Helsinki, International Conference on Harmonization and Good Clinical Practice guidelines, and applicable regulatory requirements. Informed consent was obtained before participation.
PRO Assessments
Assessment of PROs was a secondary objective of HIMALAYA. PROs were assessed using the European Organization for Research and Treatment of Cancer (EORTC) 30-item Quality of Life Questionnaire (QLQ-C30),20 validated to assess HRQoL in cancer clinical trials, and its HCC-specific module, the EORTC 18-item HCC Health-Related Quality of Life Questionnaire (QLQ-HCC18).21,22 Both have been validated across multiple language and cultural groups to capture the relevant signs/symptoms that are most meaningful to people with HCC.16,20,22
Questionnaires captured global health status/quality of life (GHS/QoL), functioning, and symptoms on scales scored from 0 to 100. A high GHS/QoL or functioning score represents a high level of global HRQoL or functioning. A high score for a symptom scale/item represents a high level of symptom burden.
Questionnaires were administered via an electronic tablet PRO device and were completed by participants at the study site before any other procedures or meetings with the study nurse or physician to discuss cancer-related issues or health status. Questionnaires were completed on day 1 of treatment and then every 8 weeks (±7 days relative to the first dose of treatment) for the first 48 weeks and then every 12 weeks ± 7 days thereafter, until treatment discontinuation. Participants who discontinued treatment also completed the questionnaires as described above until disease progression and up to 3 months after treatment discontinuation, if participants had disease progression at treatment discontinuation.
Further details are provided in Appendix 1 (online only).
Data Analyses
PRO analyses were conducted in participants in the full analysis set (FAS; all randomly assigned participants) with an evaluable baseline assessment and ≥one evaluable postbaseline assessment. For subscales where <50% of the subscale items were missing, a prorated score was calculated. At each postbaseline assessment, the change in score from baseline was categorized as improvement, no change, or deterioration. A clinically meaningful change (deterioration or improvement) was defined as an absolute change ≥10 points from baseline.
The time to deterioration (TTD) was analyzed in participants in the FAS with baseline scores ≥10 for GHS/QoL and functioning domains or ≤90 for symptoms. TTD was defined as time from random assignment until first clinically meaningful deterioration that was confirmed at a subsequent visit (unless observed at last available assessment) or death (any cause) in the absence of clinically meaningful deterioration. Further details on censoring events are in Appendix 1. Prespecified secondary endpoints included TTD in GHS/QoL, physical functioning, role functioning, fatigue, appetite loss, nausea, and diarrhea, per EORTC QLQ-C30, and TTD in shoulder pain, abdominal pain, abdominal swelling, and jaundice, per EORTC QLQ-HCC18.
TTD was analyzed using a stratified log-rank test. HRs and 95% CIs were calculated for STRIDE versus sorafenib and durvalumab versus sorafenib using a Cox proportional hazards model adjusted for treatment, etiology, Eastern Cooperative Oncology Group (ECOG) performance status (PS), and macrovascular invasion.
Adjusted mean change from baseline in PROs was assessed in participants in the FAS with a baseline and ≥one postbaseline assessment using a mixed-effect model repeated measures (MMRM) analysis. The MMRM included treatment, visit, and treatment-by-visit interaction as explanatory variables, and the baseline score and baseline score-by-visit interaction as covariates.
Improvement rate in PROs was a secondary end point. A best overall response of improved was defined as two consecutive visit responses of improvement ≥21 days apart or one visit response of improvement, no further assessments, and no death within two visits. The improvement rate was defined as the percentage of participants with a best overall response of improved and was assessed using logistic regression adjusted for etiology, ECOG PS, and macrovascular invasion. Odds ratios (ORs) with 95% CIs were reported.
These preplanned, secondary PRO analyses were not powered for statistical significance. HIMALAYA was designed to evaluate STRIDE and durvalumab monotherapy versus sorafenib. The study was not designed to support any formal comparisons between the STRIDE and durvalumab monotherapy arms.
RESULTS
Study Population
In HIMALAYA, 1,171 participants were randomly assigned to receive STRIDE (n = 393), durvalumab (n = 389), or sorafenib (n = 389) and were evaluable for PRO analyses (Fig 1). As previously reported, baseline demographics and disease characteristics were generally balanced across treatment arms.9
FIG 1.
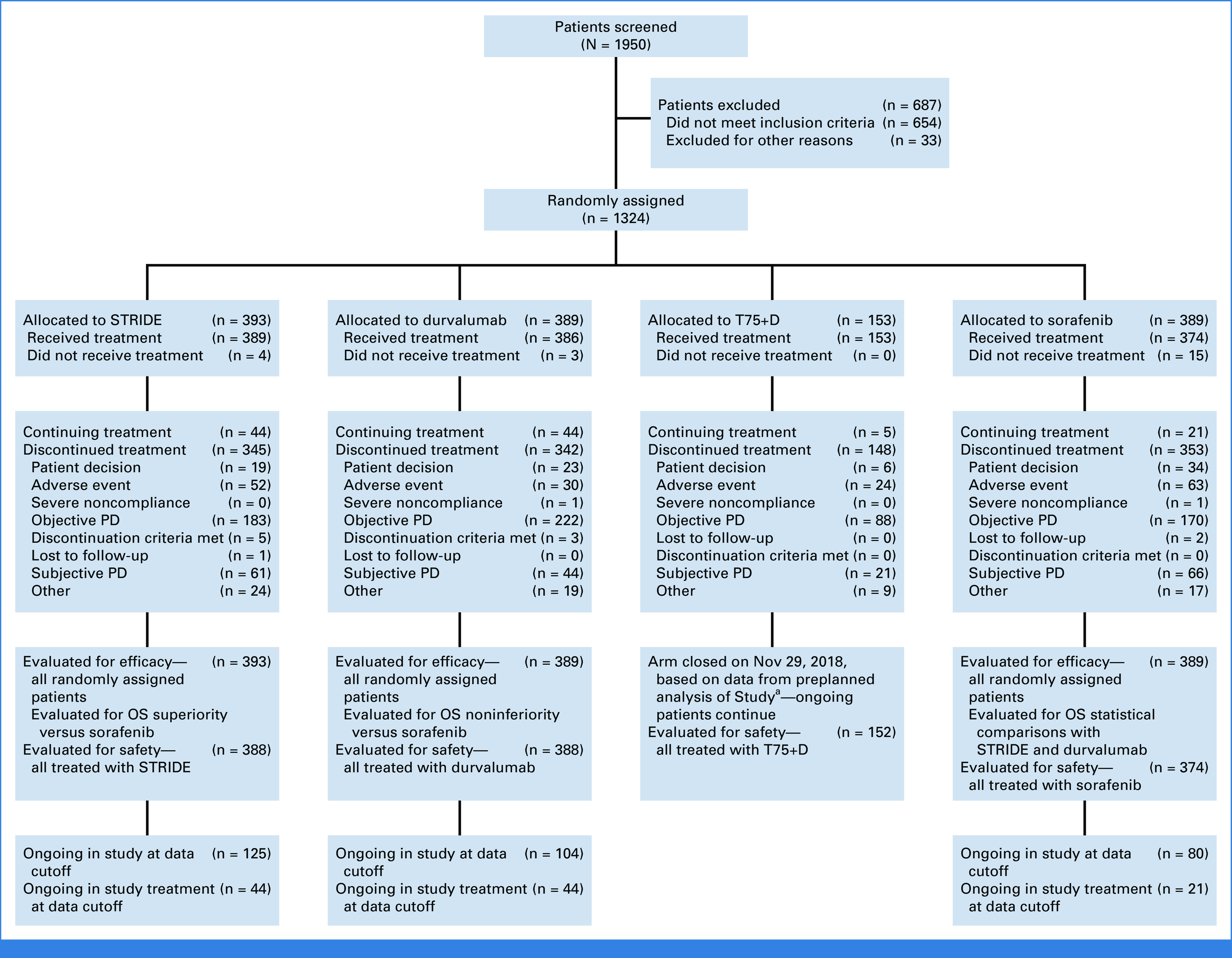
CONSORT diagram. aA preplanned analysis of Study 22 demonstrated that T75+D did not meaningfully differentiate from durvalumab monotherapy in terms of efficacy, and toxicity was slightly increased. Thus, enrollment to T75+D in HIMALAYA was closed on Nov 29, 2018. OS, overall survival; PD, progressive disease; STRIDE, Single Tremelimumab Regular Interval Durvalumab; T75+D, tremelimumab 75 mg once every 4 weeks for four doses plus durvalumab 1,500 mg once every 4 weeks.
Questionnaire Compliance Rates
Across treatment arms, compliance rates for completion of EORTC QLQ-C30 and QLQ-HCC18 PRO questionnaires were high (>80% and >77%, respectively, at baseline and >76% and >72%, respectively, overall during PRO follow-up; Appendix Tables A1 and A2). Compliance for EORTC QLQ-C30 was >65% for STRIDE and durvalumab through week 72 (majority of time points, >70%) and >60% for sorafenib through week 72 (except week 60 [51%]). Compliance for EORTC QLQ-HCC18 was >63% for STRIDE and durvalumab through week 72 (majority of time points, >70%) and for sorafenib through week 72 (except weeks 60 [51%] and 72 [61%]).
Baseline Scores
Baseline EORTC QLQ-C30 and QLQ-HCC18 scores were comparable (<7% difference) in the STRIDE, durvalumab, or sorafenib arms (Table 1; Appendix Table A3) and were generally high for physical functioning (approximately 83/100) but reduced for GHS/QoL (approximately 69/100). Participants in the STRIDE and durvalumab arms showed generally similar baseline symptom scores. There appeared to be a trend toward more severe baseline symptoms, indicated by higher scores, with sorafenib than STRIDE and durvalumab for fatigue, appetite loss, abdominal pain, and shoulder pain. Across all treatment groups, the mean scores were <10 at baseline for nausea and diarrhea and <20 for appetite loss, shoulder pain, abdominal pain, abdominal swelling, and jaundice.
TABLE 1.
EORTC QLQ-C30 and EORTC QLQ-HCC18 PRO Scores at Baseline
| Scale/Item | STRIDE (n = 393), Mean (SD) | Durvalumab (n = 389), Mean (SD) | Sorafenib (n = 389), Mean (SD) |
|---|---|---|---|
| EORTC QLQ-C30 | |||
| GHS/QoL | 70.5 (18.89) | 69.9 (18.87) | 67.4 (19.94) |
| Functional—physical functioning | 84.6 (16.54) | 84.4 (16.32) | 81.0 (20.52) |
| Functional—role | 84.7 (21.69) | 84.9 (21.92) | 80.5 (26.91) |
| Multiple symptoms—fatigue | 26.2 (20.73) | 24.9 (20.49) | 31.3 (25.65) |
| Single item—appetite loss | 12.2 (21.02) | 14.3 (25.06) | 17.8 (25.61) |
| Single item—nausea | 7.5 (17.84) | 7.4 (18.21) | 9.8 (21.24) |
| Single item—diarrhea | 6.0 (15.91) | 5.4 (13.40) | 7.6 (16.07) |
| EORTC QLQ-HCC18 | |||
| Single item—shoulder pain | 14.1 (22.37) | 15.0 (23.32) | 18.0 (26.70) |
| Single item—abdominal pain | 15.5 (21.45) | 13.0 (19.62) | 17.7 (24.70) |
| Single item—abdominal swelling | 11.9 (21.49) | 12.3 (21.39) | 12.0 (21.07) |
| Multiple symptoms—jaundice | 8.5 (14.18) | 8.6 (13.38) | 10.9 (16.14) |
Abbreviations: EORTC, European Organization for Research and Treatment of Cancer; GHS, global health status; HCC, hepatocellular carcinoma; PRO, patient-reported outcome; QLQ-C30, 30-item Quality of Life Questionnaire; QLQ-HCC18, 18-item HCC Health-Related Quality of Life Questionnaire; QoL, quality of life; SD, standard deviation; STRIDE, Single Tremelimumab Regular Interval Durvalumab.
TTD in PROs
Median TTD was numerically longer with STRIDE versus sorafenib for GHS/QoL (7.5 [95% CI, 5.8 to 10.8] v 5.7 [95% CI, 4.8 to 7.4] months), physical functioning (12.9 [95% CI, 9.2 to 16.8] v 7.4 [95% CI, 5.7 to 10.2] months), role functioning (9.3 [95% CI, 7.4 to 13.9] v 7.1 [95% CI, 5.6 to 9.2] months; Fig 2) and for cognitive, emotional, and social functioning (Appendix Fig A1). Similar trends for longer median TTD were observed with durvalumab versus sorafenib for GHS/QoL (7.4 [95% CI, 5.7 to 9.3] months), physical functioning (14.1 [95% CI, 9.2 to 18.5] months), role functioning (9.1 [95% CI, 6.3 to 11.3] months; Fig 2), and other functioning scales (Appendix Fig A1). Although not statistically tested, median TTD was generally numerically similar with STRIDE and durvalumab for GHS/QoL and across functioning categories (Fig 2; Appendix Fig A1). The probability of remaining deterioration-free in GHS/QoL and functioning was consistently higher over the follow-up period with STRIDE and durvalumab versus sorafenib (Fig 2; Appendix Fig A1).
FIG 2.
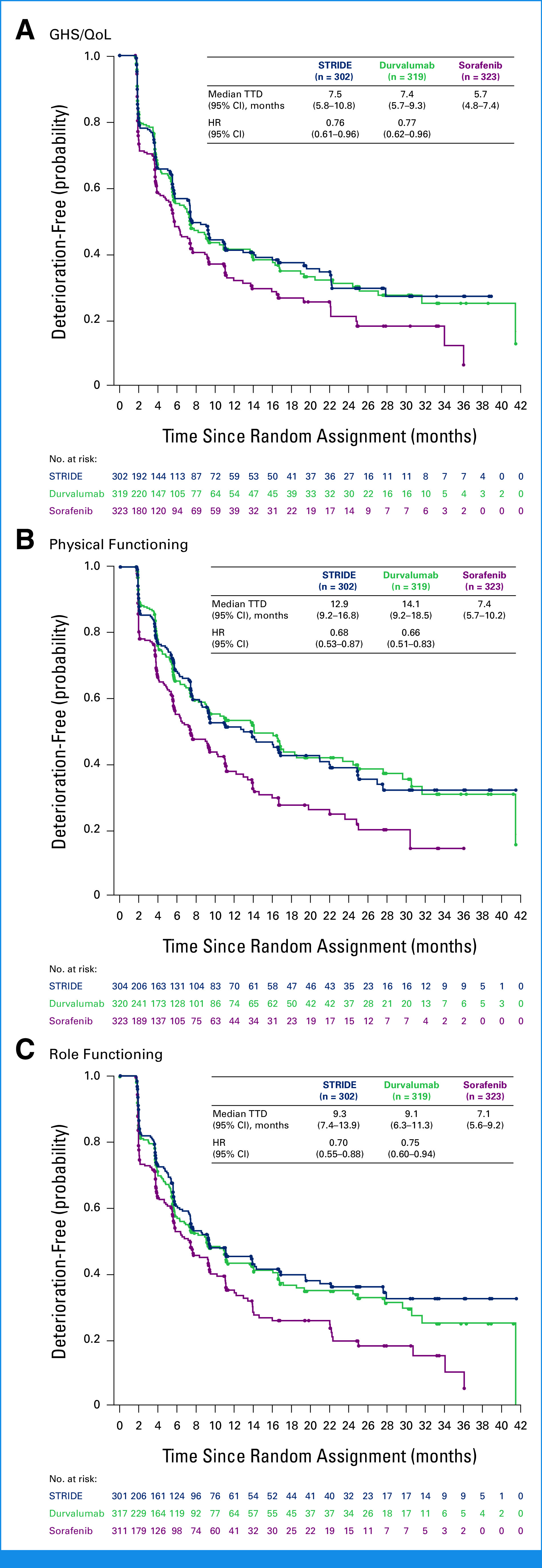
TTD in (A) GHS/QoL, (B) physical functioning, and (C) role functioning. HRs were calculated versus sorafenib. GHS, global health status; HR, hazard ratio; QoL, quality of life; STRIDE, Single Tremelimumab Regular Interval Durvalumab; TTD, time to deterioration.
Median TTD was numerically longer with STRIDE versus sorafenib for fatigue (7.4 [95% CI, 5.6 to 9.4] v 5.4 [95% CI, 3.8 to 6.3] months), appetite loss (12.6 [95% CI, 9.3 to 20.9] v 6.9 [95% CI, 5.6 to 7.6] months), abdominal pain (16.8 [95% CI, 11.2 to not reached, NR] v 8.9 [95% CI, 7.2 to 11.1] months), diarrhea (19.6 [95% CI, 12.7 to NR] v 5.6 [95% CI, 3.9 to 6.4] months), nausea (25.0 [95% CI, 16.0 to NR] v 11.0 [95% CI, 9.2 to 13.7] months), and abdominal swelling (20.9 [95% CI, 12.9 to 36.0] v 11.1 [95% CI, 9.3 to 13.7] months; Fig 3A). Numerically longer median TTD was observed with durvalumab versus sorafenib for symptoms of fatigue (6.9 [95% CI, 5.6 to 7.5] months), appetite loss (11.1 [95% CI, 8.9 to 16.8] months), abdominal pain (14.1 [95% CI, 9.5 to 24.4] months), and diarrhea (16.0 [95% CI, 9.1 to 24.4] months; Fig 3B).
FIG 3.
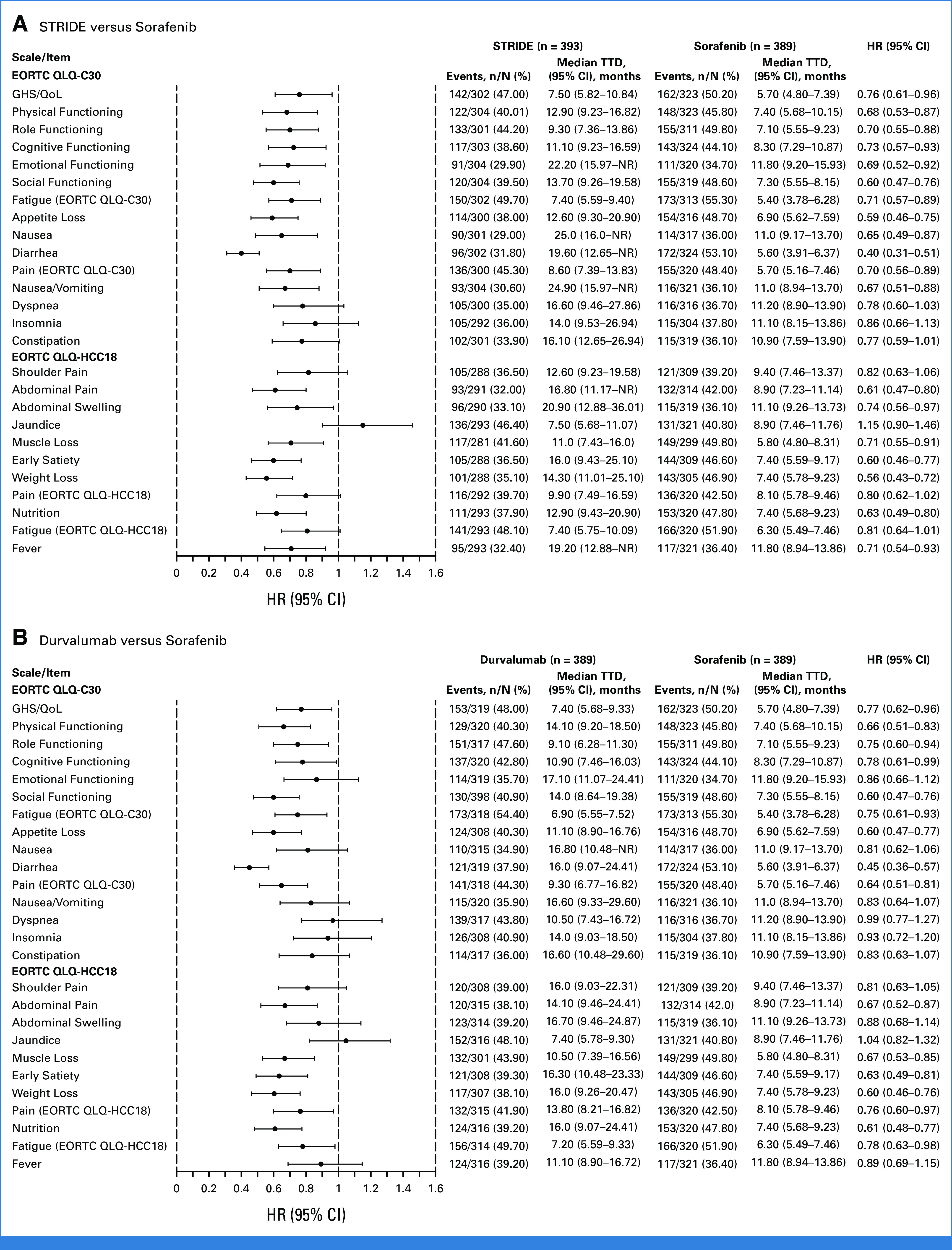
TTD in PROs with (A) STRIDE versus sorafenib and (B) durvalumab versus sorafenib. EORTC, European Organization for Research and Treatment of Cancer; GHS, global health status; HCC, hepatocellular carcinoma; HR, hazard ratio; NR, not reached; PRO, patient-reported outcome; QLQ-C30, 30-item Quality of Life Questionnaire; QLQ-HCC18, 18-item HCC Health-Related Quality of Life Questionnaire; QoL, quality of life; STRIDE, Single Tremelimumab Regular Interval Durvalumab; TTD, time to deterioration.
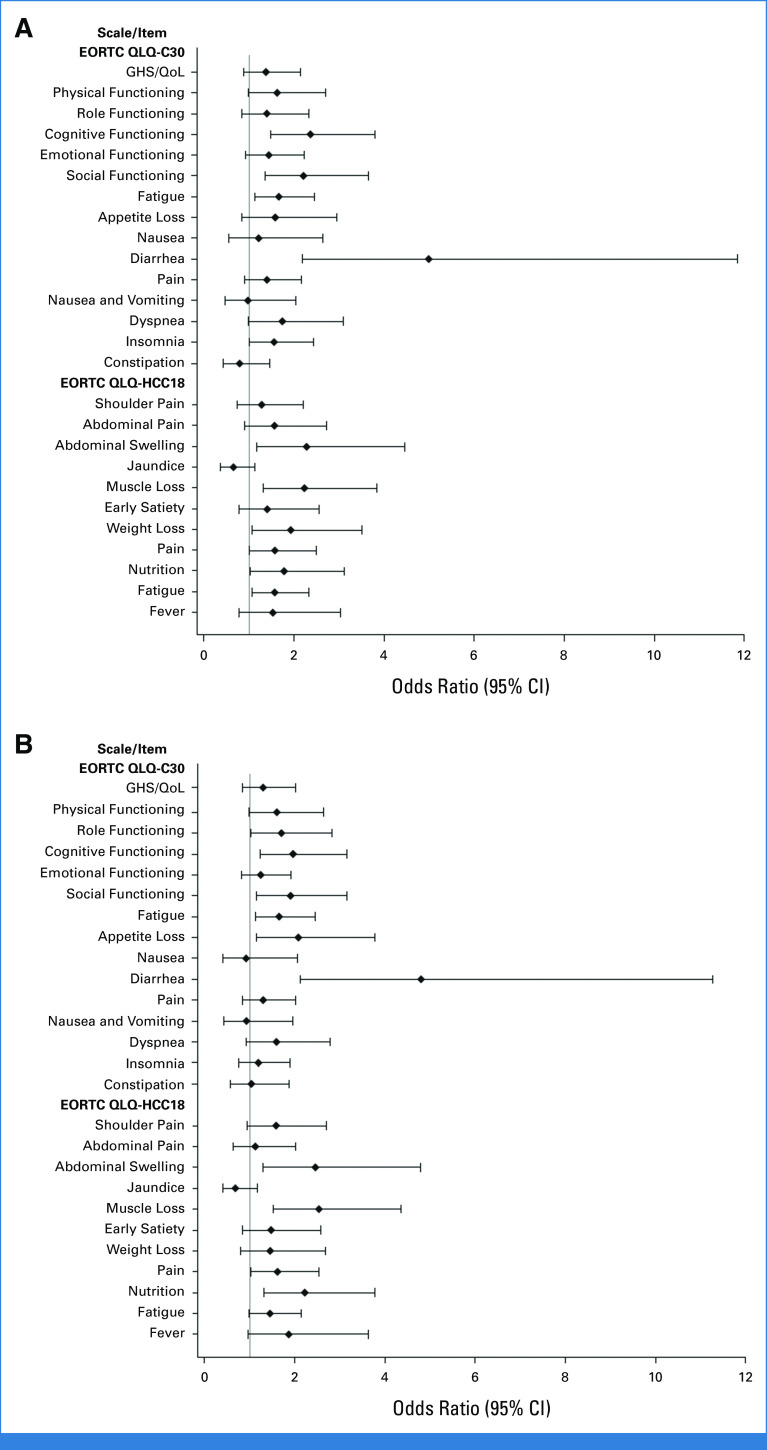
Trends for longer median TTD were also observed with STRIDE or durvalumab versus sorafenib in pain, muscle loss, early satiety, weight loss, and nutrition (Fig 3). There were no substantial differences in shoulder pain or jaundice with STRIDE versus sorafenib or for nausea, shoulder pain, abdominal swelling, or jaundice with durvalumab versus sorafenib (Fig 3). Although not statistically tested, median TTD was generally numerically similar with STRIDE and durvalumab across most symptoms (Fig 3).
Change From Baseline in PROs
MMRM analysis of adjusted mean change from baseline for GHS/QoL, physical functioning, and role functioning indicated no clinically meaningful deterioration over 24 weeks in any treatment arm (Fig 4A). No clinically meaningful deterioration in cognitive, emotional, or social functioning was observed over 24 weeks in any treatment arm (Appendix Fig A2). The adjusted mean change from baseline over 24 weeks was numerically less with durvalumab than with STRIDE for GHS/QoL, physical, role, cognitive, and social functioning (Fig 4A; Appendix Fig A2).
FIG 4.
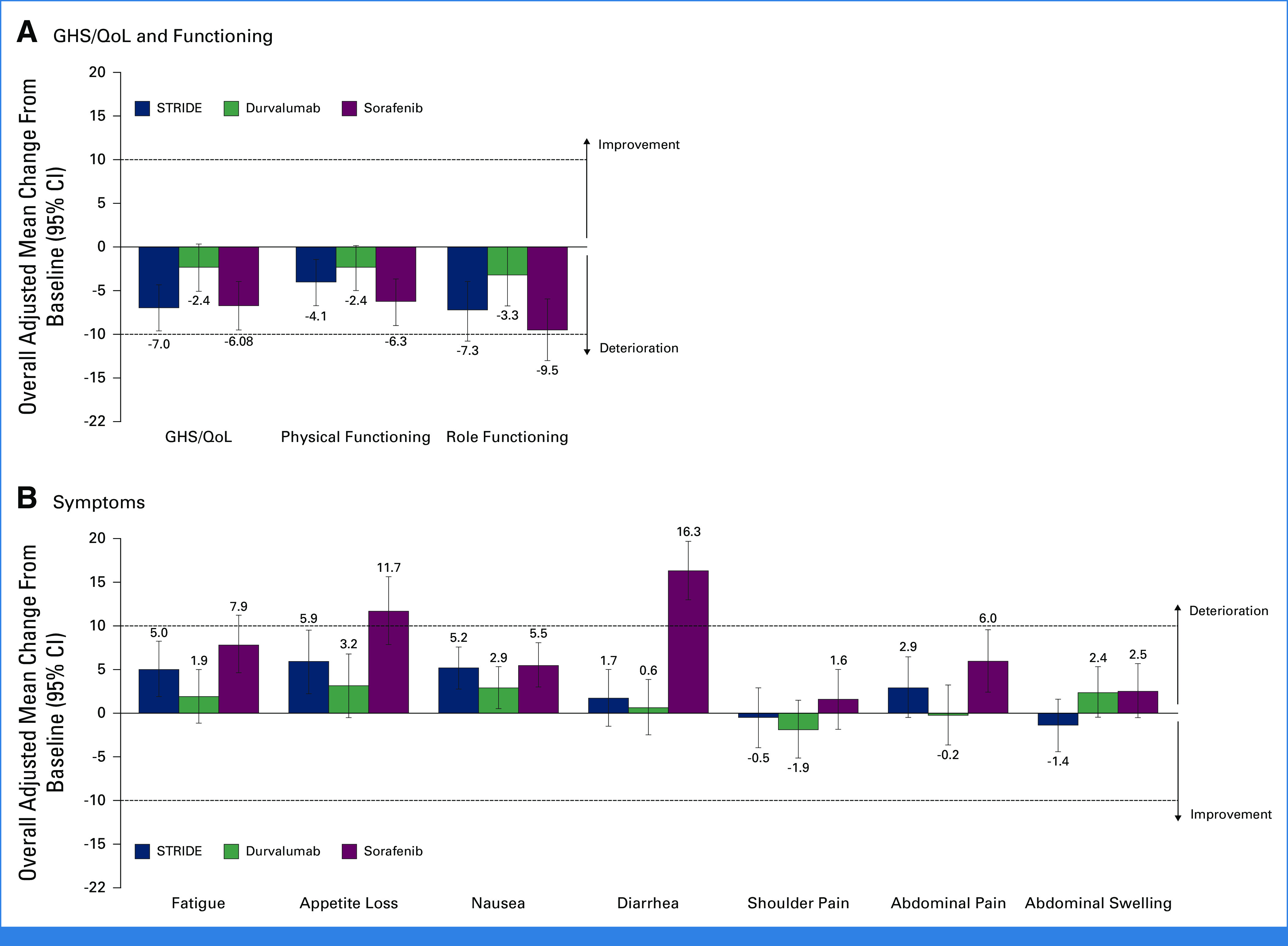
Adjusted mean change from baseline over 24 weeks in (A) GHS/QoL and functioning and (B) symptoms. Adjusted mean change from baseline was calculated using an MMRM analysis, including treatment, visit, and treatment-by-visit interaction as explanatory variables and the baseline score as a covariate. Data reported are the adjusted mean change from baseline averaged over 24 weeks. Error bars represent the 95% CIs. Dotted lines represent the threshold for clinically meaningful change: an absolute change in the score of ≥10 points from the baseline score. Change from baseline in jaundice was not calculated. GHS, global health status; MMRM, mixed-effect model repeated measures; QoL, quality of life; STRIDE, Single Tremelimumab Regular Interval Durvalumab.
No clinically meaningful deterioration in participants' symptom scores was observed over 24 weeks with STRIDE or durvalumab (Fig 4B). Clinically meaningful deterioration in appetite loss and diarrhea was observed with sorafenib (Fig 4B). Increases in fatigue, nausea, shoulder pain, and abdominal pain scores were numerically the highest with sorafenib (Fig 4B).
Across multiple visits (weeks 8, 16, 24, and/or 32), deterioration in PROs (two-sided P < .05) was nominally significantly less with STRIDE versus sorafenib at one or more visits for physical, emotional, cognitive, and social functioning and for fatigue, appetite loss, abdominal pain, and swelling (data not shown). Deterioration in PROs was nominally significantly less with durvalumab versus sorafenib at one or more visits for GHS/QoL, physical, emotional, cognitive, role, and social functioning and for fatigue, appetite loss, diarrhea, and abdominal pain (data not shown). Although the above results are nominally significant, none reached the 10-point absolute change from baseline threshold for clinical relevance.
Improvement Rate
Participants in the STRIDE and durvalumab arms, compared with the sorafenib arm, had an increased likelihood (OR >1) of reporting a clinically meaningful improvement in GHS/QoL, physical, role, and emotional functioning, and across most disease-related symptoms (Appendix Fig A3). Nominally significant differences (lower bound of 95% CI >1) for STRIDE versus sorafenib were observed for cognitive functioning, social functioning, fatigue (EORTC QLQ-C30), diarrhea, insomnia, abdominal swelling, muscle loss, weight loss, pain (EORTC QLQ-HCC18), nutrition, and fatigue (EORTC QLQ-HCC18; Appendix Fig A3A). Nominally significant differences for durvalumab versus sorafenib were observed for role functioning, cognitive functioning, social functioning, fatigue (EORTC QLQ-C30), appetite loss, diarrhea, abdominal swelling, muscle loss, pain (EORTC QLQ-HCC18), and nutrition (Appendix Fig A3B).
DISCUSSION
In the primary HIMALAYA analysis, STRIDE was found to significantly improve OS versus sorafenib.9 Even when treatment improves survival, this benefit can be counterbalanced by the burden of treatment and/or its potential effect on HRQoL.15 Assessing the impact of treatment on HRQoL, functioning, and disease-related symptoms of people with HCC is important to understand how clinical benefit relates to well-being and to inform comprehensive benefit-risk assessments.15 Hence, we investigated PROs associated with STRIDE and durvalumab treatment regimens.
Marked and consistent benefits from the participants' perspectives were observed with STRIDE and durvalumab monotherapy compared with sorafenib. The STRIDE and durvalumab monotherapy regimens were both associated with delayed worsening of GHS/QoL, functioning, and disease-related symptoms, compared with sorafenib; an increased OR of clinically meaningful improvement with STRIDE and durvalumab versus sorafenib was observed for these scores. Thus, findings from analyses of PROs from HIMALAYA support the clinical utility and positive benefit-risk profile of STRIDE compared with sorafenib for the treatment of people with uHCC.
Compliance rates for completion of the EORTC QLQ-C30 and EORTC QLQ-HCC18 questionnaires were high and remained steady throughout the HIMALAYA study and were consistent with findings in HCC and other advanced tumors.17,23,24 In addition, data for PRO assessments were collected at many time points over an extended duration. Therefore, the HIMALAYA analyses of PROs were conducted on a representative and thorough sample of participants. In HIMALAYA, participants with uHCC generally had poor overall QoL (GHS/QoL) before treatment, suggesting a high burden of the disease on participants general HRQoL; overall functioning domains were on the higher end of the scale before treatment initiation. PRO scores at baseline in HIMALAYA appear similar to those reported for the IMbrave150 study (ClinicalTrials.gov identifier: NCT03434379) in uHCC, further supporting that the HIMALAYA analyses of PROs were conducted on a representative sample of people with uHCC.17
Overall, treatment with the STRIDE and durvalumab was associated with less deterioration in GHS/QoL and functioning and reduced symptom burden from baseline, compared with sorafenib, suggesting that the STRIDE and durvalumab regimens are favorable from the participants' perspectives. In general, STRIDE and durvalumab were associated with a similar delay in worsening and a similar improvement for GHS/QoL, functioning, and symptoms. Neither STRIDE nor durvalumab was associated in any clinically meaningful changes from baseline in PROs. Thus, the addition of a single dose of tremelimumab to durvalumab monotherapy, in the STRIDE regimen, was associated with comparable PROs.
The clinically meaningful, favorable, and consistent benefits in PROs for STRIDE compared with sorafenib support the novel dose selection of the tremelimumab (anti–CTLA-4) and durvalumab (anti–PD-L1) combination regimen for the treatment of uHCC. Other anti–CTLA-4 therapies have been previously reported to maintain or improve PROs; for example, ipilimumab maintained PROs in advanced melanoma,25 nivolumab plus ipilimumab maintained PROs in metastatic colorectal cancer,26 and nivolumab plus ipilimumab improved PROs in advanced HCC,27 advanced non–small cell lung cancer,28 and advanced renal cell carcinoma.29
In addition, while more treatment-emergent adverse events (TEAEs) leading to discontinuation and dose delay were observed with sorafenib, STRIDE and durvalumab were associated with more immune-mediated TEAEs that were generally low grade and low frequency.9 The delayed worsening and clinically meaningful improvement in PROs observed with STRIDE and durvalumab monotherapy versus sorafenib are consistent with their generally favorable safety profiles. Thus, the analyses of PROs reported here further support the overall tolerability of STRIDE in people with uHCC.
The HIMALAYA PRO analyses had several strengths. As PRO assessments were prespecified secondary objectives of the HIMALAYA study, they provide qualitative insight into the experiences of people with HCC, in line with the patient-focused drug development guidelines.16 Additionally, the EORTC QLQ-C30 and EORTC QLQ-HCC18 questionnaires are well established, widely used in uHCC clinical trials, and have been validated.15,20-22
Potential limitations of the HIMALAYA PRO analyses included the open-label study design, which could have led to reporting bias because of lack of blinding to treatment. However, benefits were observed in PROs directly affected by disease or treatment, such as physical functioning, which are less prone to open-label bias.30 Furthermore, consistent benefits in TTD for all PROs, along with significant improvement in OS for STRIDE, and favorable benefit-risk profiles for STRIDE and durvalumab monotherapy, compared with sorafenib,9 may indicate that open-label bias did not affect these findings. Another potential limitation of the HIMALAYA study is that PRO measurements were a secondary objective, and, as such, the study was not powered to establish significant treatment differences for these PRO analyses. Baseline compliance rates may have been impacted by delays in delivery and technical difficulties with PRO devices. In this study, an absolute change ≥10 points from baseline was defined as clinically meaningful. Although the threshold for a clinically meaningful change in PROs has not been formally established in HCC, this definition is consistent with cutoffs suggested in QoL interpretation guidelines31,32 and with the IMbrave150 PROs analysis for uHCC.17
In conclusion, both STRIDE and durvalumab were associated with clinically meaningful, patient-centered benefits, compared with sorafenib, in participants with uHCC. Thus, the addition of a single dose of tremelimumab to durvalumab monotherapy, in the STRIDE regimen, did not appear to be associated with concerns from the participants' perspectives. Along with the superior OS and favorable safety profile,9 these findings support the positive benefit-risk profile of the novel STRIDE regimen compared with sorafenib, with STRIDE offering a well-tolerated and effective treatment option for people with uHCC.
ACKNOWLEDGMENT
The authors would like to thank the participants in the HIMALAYA study, their families, and the investigators and study site personnel. We would like to thank Mindy Ye for their work on study participant safety. Medical writing support, under the guidance of the authors, was provided by Claire Tinderholm, PhD, of CMC Connect, a division of IPG Health Medical Communications and was funded by AstraZeneca, in accordance with Good Publication Practice (GPP 2022) guidelines.34
APPENDIX 1. SUPPLEMENTARY METHODS
European Organization for Research and Treatment of Cancer 30-Item Quality of Life Questionnaire and European Organization for Research and Treatment of Cancer 18-Item Hepatocellular Carcinoma Health-Related Quality of Life Questionnaire Questionnaires
The European Organization for Research and Treatment of Cancer (EORTC) 30-item Quality of Life Questionnaire (QLQ-C30)20 was developed to assess health-related quality of life (QoL) in cancer clinical trials. It has undergone extensive testing and validation, including cross-cultural testing and validation,20 and has been used extensively in hepatocellular carcinoma (HCC) studies.15 The EORTC 18-item HCC Health-Related Quality of Life Questionnaire (QLQ-HCC18)21,22 is an 18-item self-administered questionnaire developed and validated specifically for HCC.
The EORTC QLQ-C30 includes multi-item scales scored from 0 to 100, namely a global health status (GHS)/QoL scale, five functional scales (physical, role, cognitive, emotional, and social), three symptom scales (fatigue, pain, and nausea/vomiting), and five single-item symptom scales (dyspnea, loss of appetite, insomnia, constipation, and diarrhea).20,22 The EORTC QLQ-HCC18 includes six multi-item scales (fatigue, jaundice, nutrition, pain, fever, and body image) scored from 0 to 100 and two single-item symptom scales (abdominal swelling and sexual interest).21,22
All items assessed by EORTC QLQ-C30 and EORTC QLQ-HCC18 were scored according to the published scoring guidelines of the EORTC QLQ-C30 Scoring Manual.33 All patient-reported outcome (PRO) items ranged in score from 0 to 100. Approved translations of all PROs underwent cultural and linguistic validation for the countries involved in the study before use.
Time to Deterioration Censoring
For the analysis of time to deterioration, participants whose GHS/QoL, function, or symptoms did not show clinically meaningful deterioration and who were alive at the time of the analysis were censored at the time of their last PRO assessment where GHS/QoL, function, or symptoms could be evaluated. Participants with no postbaseline assessment were censored at the date of random assignment. In the case that GHS/QoL, function, or symptoms deteriorated, the participant was censored at the time of the last PRO assessment where GHS/QoL, function, or symptoms could be evaluated. Death was not a censoring event. If a participant died without deterioration within two PRO assessment visits from the last available PRO assessment, it was considered as a deterioration event; however, if they did not have a deterioration and died after two or more missed PRO assessment visits or after the last PRO assessment, the participant was censored as alive at the time of the last PRO assessment where GHS/QoL, function, or symptoms could be evaluated before the two missed visits.
TABLE A1.
EORTC QLQ-C30 Compliance Rates
| Week | Compliance Rate, % | ||
|---|---|---|---|
| STRIDE (n = 393) | Durvalumab (n = 389) | Sorafenib (n = 389) | |
| Baseline | 80.0 | 84.7 | 87.8 |
| Overall | 76.1 | 83.4 | 80.9 |
| 8 | 85.2 | 89.9 | 83.8 |
| 16 | 74.3 | 78.4 | 68.7 |
| 24 | 73.1 | 70.7 | 69.5 |
| 32 | 74.4 | 71.3 | 75.8 |
| 40 | 69.0 | 67.8 | 64.0 |
| 48 | 70.3 | 76.6 | 70.8 |
| 60 | 65.5 | 80.2 | 51.3 |
| 72 | 74.5 | 71.3 | 60.7 |
| 84 | 67.0 | 73.6 | 58.5 |
| 96 | 70.7 | 69.5 | 56.8 |
| 108 | 66.7 | 68.6 | 63.9 |
| 120 | 70.0 | 70.7 | 59.1 |
| 132 | 75.0 | 71.4 | 71.4 |
| 144 | 65.4 | 66.7 | 63.6 |
| 156 | 100.0 | 63.6 | 75.0 |
| 168 | 120.0 | 83.3 | 0.0 |
| 180 | 66.7 | 50.0 | 0.0 |
| 192 | 0.0 | 0.0 | 0.0 |
NOTE. Baseline compliance rate was defined as the proportion of total participants with an evaluable baseline questionnaire. Overall compliance rate was defined as the proportion of total participants with an evaluable baseline questionnaire and at least one evaluable follow-up questionnaire. Compliance rate at each time point was defined as the proportion of participants still under PRO follow-up with an evaluable questionnaire.
Abbreviations: EORTC, European Organization for Research and Treatment of Cancer; PRO, patient-reported outcome; QLQ-C30, 30-item Quality of Life Questionnaire; STRIDE, Single Tremelimumab Regular Interval Durvalumab.
TABLE A2.
EORTC QLQ-HCC18 Compliance Rates
| Week | Compliance Rate, % | ||
|---|---|---|---|
| STRIDE (n = 393) | Durvalumab (n = 389) | Sorafenib (n = 389) | |
| Baseline | 77.1 | 83.6 | 87.5 |
| Overall | 72.7 | 82.8 | 80.8 |
| 8 | 86.1 | 89.1 | 82.7 |
| 16 | 73.3 | 77.4 | 68.6 |
| 24 | 71.8 | 69.4 | 68.6 |
| 32 | 73.3 | 71.3 | 75.2 |
| 40 | 67.9 | 67.8 | 64.0 |
| 48 | 69.7 | 76.6 | 70.8 |
| 60 | 65.5 | 80.2 | 51.3 |
| 72 | 74.5 | 71.3 | 60.7 |
| 84 | 67.0 | 73.6 | 73.6 |
| 96 | 70.7 | 69.5 | 56.8 |
| 108 | 66.7 | 68.6 | 63.9 |
| 120 | 70.0 | 70.7 | 59.1 |
| 132 | 75.0 | 71.4 | 71.4 |
| 144 | 65.4 | 66.7 | 63.6 |
| 156 | 100.0 | 63.6 | 75.0 |
| 168 | 120.0 | 83.3 | 0.0 |
| 180 | 66.7 | 50.0 | 0.0 |
| 192 | 0.0 | 0.0 | 0.0 |
NOTE. Baseline compliance rate was defined as the proportion of total participants with an evaluable baseline questionnaire. Overall compliance rate was defined as the proportion of total participants with an evaluable baseline questionnaire and at least one evaluable follow-up questionnaire. Compliance rate at each time point was defined as the proportion of participants still under PRO follow-up with an evaluable questionnaire.
Abbreviations: EORTC, European Organization for Research and Treatment of Cancer; HCC, hepatocellular carcinoma; PRO, patient-reported outcome; QLQ-HCC18, 18-item HCC Health-Related Quality of Life Questionnaire; STRIDE, Single Tremelimumab Regular Interval Durvalumab.
TABLE A3.
EORTC QLQ-C30 and EORTC QLQ-HCC18 PRO Scores at Baseline for Additional Functioning and Symptom Items
| Scale/Item | STRIDE (n = 393), Mean (SD) | Durvalumab (n = 389), Mean (SD) | Sorafenib (n = 389), Mean (SD) |
|---|---|---|---|
| EORTC QLQ-C30 | |||
| Functional—cognitive functioning | 87.3 (15.41) | 87.6 (15.18) | 87.1 (16.30) |
| Functional—emotional functioning | 80.9 (18.06) | 80.3 (18.94) | 77.3 (20.48) |
| Functional—social functioning | 85.1 (19.64) | 85.2 (20.04) | 84.0 (22.74) |
| Multiple symptoms—pain | 18.5 (22.20) | 16.4 (20.12) | 22.3 (25.37) |
| Multiple symptoms—nausea/vomiting | 4.7 (11.15) | 4.9 (12.18) | 7.1 (16.62) |
| Single item—dyspnea | 13.2 (21.21) | 14.7 (20.86) | 18.6 (24.74) |
| Single item—insomnia | 24.1 (27.67) | 22.1 (27.27) | 27.3 (29.46) |
| Single item—constipation | 11.8 (21.12) | 10.8 (19.05) | 13.1 (22.35) |
| EORTC QLQ-HCC18 | |||
| Single item—muscle loss | 20.7 (27.79) | 22.0 (28.53) | 23.9 (30.30) |
| Single item—early satiety | 14.8 (24.39) | 17.2 (25.12) | 18.3 (27.32) |
| Single item—weight loss | 14.8 (23.27) | 15.2 (25.50) | 16.5 (28.00) |
| Multiple symptoms—pain | 14.8 (17.54) | 14.0 (17.28) | 17.9 (21.28) |
| Multiple symptoms—nutrition | 14.2 (15.80) | 14.7 (16.22) | 16.1 (17.77) |
| Multiple symptoms—fatigue | 21.4 (18.92) | 21.0 (20.29) | 24.6 (23.16) |
| Multiple symptoms—fever | 5.1 (11.05) | 5.4 (11.32) | 7.1 (14.13) |
Abbreviations: EORTC, European Organization for Research and Treatment of Cancer; HCC, hepatocellular carcinoma; PRO, patient-reported outcome; QLQ-C30, 30-item Quality of Life Questionnaire; QLQ-HCC18, 18-item HCC Health-Related Quality of Life Questionnaire; SD, standard deviation; STRIDE, Single Tremelimumab Regular Interval Durvalumab.
FIG A1.

TTD in (A) cognitive functioning, (B) emotional functioning, and (C) social functioning. HRs were calculated versus sorafenib. HR, hazard ratio; NR, not reached; STRIDE, Single Tremelimumab Regular Interval Durvalumab; TTD, time to deterioration.
FIG A2.
Adjusted mean change from baseline over 24 weeks in EORTC QLQ-C30 functioning domains. Adjusted mean change from baseline was calculated using an MMRM analysis including treatment, visit, and treatment-by-visit interaction as explanatory variables and the baseline score as a covariate. Data reported are the adjusted mean change from baseline averaged over 24 weeks. Error bars represent the 95% CIs. Dotted lines represent the threshold for clinically meaningful change (an absolute change ≥10 points from baseline). EORTC, European Organization for Research and Treatment of Cancer; MMRM, mixed-effect model repeated measures; QLQ-C30, 30-item Quality of Life Questionnaire; STRIDE, Single Tremelimumab Regular Interval Durvalumab.
FIG A3.
Improvement rate (best overall response of improveda) in PROs assessed with (A) STRIDE versus sorafenib and (B) durvalumab versus sorafenib. The analysis was performed using a logistic regression model adjusted for treatment with factors for etiology of liver disease, ECOG, and macrovascular invasion. An odds ratio >1 favors the STRIDE regimen or durvalumab versus sorafenib. For the GHS/QoL and function improvement rate, the set for the analysis includes a subset of the FAS who have a baseline GHS/QoL or function score ≤90. For the symptom improvement rate, the set for the analysis includes a subset of the FAS who have baseline symptom scores of ≥10. aTwo consecutive visit responses of improvement ≥21 days apart or one visit response of improvement and no further assessments and no death within two visits. ECOG, Eastern Cooperative Oncology Group; EORTC, European Organization for Research and Treatment of Cancer; FAS, full analysis set; GHS, global health status; HCC, hepatocellular carcinoma; PRO, patient-reported outcome; QLQ-C30, 30-item Quality of Life Questionnaire; QLQ-HCC18, 18-item HCC Health-Related Quality of Life Questionnaire; QoL, quality of life; STRIDE, Single Tremelimumab Regular Interval Durvalumab.
Bruno Sangro
Consulting or Advisory Role: Bristol Myers Squibb, AstraZeneca, Bayer, Sirtex Medical, Roche/Genentech, Eisai, Incyte, Boston Scientific, Sanofi Pasteur
Speakers' Bureau: AstraZeneca, Eisai, Incyte, Roche
Research Funding: Bristol Myers Squibb (Inst), Roche (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Bristol Myers Squibb, Sirtex Medical, Eisai, Roche
Peter R. Galle
Honoraria: Bristol-Myers Squibb, Bayer Schering Pharma, Sirtex Medical, Roche/Genentech, Ipsen, Adaptimmune, MSD, AstraZeneca/MedImmune
Consulting or Advisory Role: Bayer Schering Pharma, Sirtex Medical, Lilly, Bristol-Myers Squibb, MSD, Roche/Genentech, Adaptimmune, Boston Scientific
Speakers' Bureau: Bayer Schering Pharma, Lilly, Roche, Ipsen
Research Funding: Roche/Genentech
Travel, Accommodations, Expenses: Bayer Schering Pharma, Lilly, Sirtex Medical, AstraZeneca
Robin Kate Kelley
Consulting or Advisory Role: Agios (Inst), AstraZeneca (Inst), Merck (Inst), Kinnate Biopharma, Exelixis/Ipsen (Inst), Regeneron, Tyra Biosciences, Compass Therapeutics, Elevar Therapeutics, J-Pharma, Moderna Therapeutics
Research Funding: Lilly (Inst), Exelixis (Inst), Novartis (Inst), Bristol-Myers Squibb (Inst), MedImmune (Inst), Merck Sharp & Dohme (Inst), Agios (Inst), AstraZeneca (Inst), Adaptimmune (Inst), Taiho Pharmaceutical (Inst), Bayer (Inst), QED Therapeutics (Inst), EMD Serono (Inst), Partner Therapeutics (Inst), Genentech/Roche (Inst), Surface Oncology (Inst), Relay Therapeutics (Inst), Loxo/Lilly (Inst), Servier (Inst), Compass Therapeutics (Inst), Tyra Biosciences (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Merck
Chaiyut Charoentum
Research Funding: AstraZeneca (Inst), Roche/Genentech (Inst), Novartis (Inst), MSD Oncology (Inst)
Enrico N. De Toni
Employment: Boehringer Ingelheim
Honoraria: AstraZeneca, Bayer, Bristol-Myers Squibb, Eisai, Lilly, Pfizer, Ipsen, Terumo, Roche
Consulting or Advisory Role: AstraZeneca, Bayer, Bristol-Myers Squibb, Eisai, Lilly, Pfizer, Ipsen, Terumo, Roche
Research Funding: AstraZeneca, Bristol-Myer Squibb, Bayer, Lilly, Ipsen, Roche
Travel, Accommodations, Expenses: AstraZeneca, Bristol-Myers Squibb, Bayer, Celsion, Roche, Ipsen
Jeong Heo
Consulting or Advisory Role: AstraZeneca/MedImmune
Speakers' Bureau: Roche, AstraZeneca/MedImmune
Ann-Lii Cheng
Honoraria: Bayer Yakuhin, AstraZeneca, Eisai, Genentech/Roche
Consulting or Advisory Role: Bristol-Myers Squibb, Bayer Schering Pharma, Eisai, Ono Pharmaceutical, AstraZeneca, Genentech/Roche, MSD, BeiGene, IQVIA, Ipsen, Roche, Omega Therapeutics, Inc
Andrea Wilson Woods
Employment: Blue Faery: The Adrienne Wilson Liver Cancer Association
Honoraria: HCC TAG, American Institute Continuing Medical Education
Consulting or Advisory Role: Eisai (Inst), Humanise Health, AstraZeneca
Charu Gupta
Employment: AstraZeneca/MedImmune
Stock and Other Ownership Interests: AstraZeneca/MedImmune
Jayne Abraham
Employment: UCB
Stock and Other Ownership Interests: UCB
Carrie L. McCoy
Employment: AstraZeneca/MedImmune
Stock and Other Ownership Interests: AstraZeneca/MedImmune
Nikunj Patel
Employment: AstraZeneca
Stock and Other Ownership Interests: AstraZeneca
Alejandra Negro
Employment: AstraZeneca
Stock and Other Ownership Interests: AstraZeneca
Arndt Vogel
Honoraria: Roche, Amgen, Lilly, Bristol-Myers Squibb, MSD, Pierre Fabre, Ipsen, Janssen, Incyte, AstraZeneca/MedImmune, Sirtex Medical, Daichi-Sankyo, Terumo, Taiho Oncology, Eisai, BeiGene, Boehringer Pharma GmbH, GlaxoSmithKline, Boston Scientific, Servier
Consulting or Advisory Role: Novartis, Lilly, Roche, Amgen, Baxalta, AstraZeneca, Eisai, BTG, Ipsen, Pierre Fabre, Terumo, Daichi-Sankyo, Sirtex Medical, Boehringer Pharma GmbH, Incyte, Taiho Oncology
Research Funding: Novartis
Travel, Accommodations, Expenses: Roche, Ipsen, AstraZeneca, MSD, Lilly
Ghassan K. Abou-Alfa
Consulting or Advisory Role: Eisai, Ipsen, Merck Serono, AstraZeneca, Yiviva, Roche/Genentech, Autem Medical, Exelixis, QED Therapeutics, Boehringer Ingelheim, Novartis, Berry Genomics, BioNtech, Bristol-Myers Squibb/Medarex, Merus NV, Neogene Therapeutics, Tempus, Vector Health, Servier, J-Pharma, AbbVie
Research Funding: AstraZeneca (Inst), Bristol-Myers Squibb (Inst), Puma Biotechnology (Inst), QED Therapeutics (Inst), BioNtech (Inst), Genentech/Roche (Inst), Helsinn Healthcare (Inst), Yiviva (Inst), Elicio Therapeutics (Inst), Agenus (Inst), Parker Institute for Cancer Immunotherapy (Inst), Pertzye (Inst), Arcus Ventures (Inst)
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented at the 2022 ASCO Annual Meeting, Chicago, IL, June 3-7, 2022.
SUPPORT
Supported by AstraZeneca.
CLINICAL TRIAL INFORMATION
NCT03298451 (HIMALAYA)
AUTHOR CONTRIBUTIONS
Conception and design: Nikunj Patel, Alejandra Negro, Ghassan K. Abou-Alfa
Financial support: Nikunj Patel
Administrative support: Bruno Sangro, Chaiyut Charoentum, Yurii Ostapenko, Ghassan K. Abou-Alfa
Provision of study materials or patients: Bruno Sangro, Robin Kate Kelley, Chaiyut Charoentum, Yurii Ostapenko, Jeong Heo, Ann-Lii Cheng, Nikunj Patel, Arndt Vogel, Ghassan K. Abou-Alfa
Collection and assembly of data: Robin Kate Kelley, Chaiyut Charoentum, Enrico N. De Toni, Yurii Ostapenko, Jeong Heo, Nikunj Patel, Alejandra Negro, Ghassan K. Abou-Alfa
Data analysis and interpretation: Bruno Sangro, Peter R. Galle, Robin Kate Kelley, Chaiyut Charoentum, Yurii Ostapenko, Jeong Heo, Ann-Lii Cheng, Andrea Wilson Woods, Charu Gupta, Jayne Abraham, Carrie L. McCoy, Nikunj Patel, Alejandra Negro, Arndt Vogel, Ghassan K. Abou-Alfa
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Patient-Reported Outcomes From the Phase III HIMALAYA Study of Tremelimumab Plus Durvalumab in Unresectable Hepatocellular Carcinoma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Bruno Sangro
Consulting or Advisory Role: Bristol Myers Squibb, AstraZeneca, Bayer, Sirtex Medical, Roche/Genentech, Eisai, Incyte, Boston Scientific, Sanofi Pasteur
Speakers' Bureau: AstraZeneca, Eisai, Incyte, Roche
Research Funding: Bristol Myers Squibb (Inst), Roche (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Bristol Myers Squibb, Sirtex Medical, Eisai, Roche
Peter R. Galle
Honoraria: Bristol-Myers Squibb, Bayer Schering Pharma, Sirtex Medical, Roche/Genentech, Ipsen, Adaptimmune, MSD, AstraZeneca/MedImmune
Consulting or Advisory Role: Bayer Schering Pharma, Sirtex Medical, Lilly, Bristol-Myers Squibb, MSD, Roche/Genentech, Adaptimmune, Boston Scientific
Speakers' Bureau: Bayer Schering Pharma, Lilly, Roche, Ipsen
Research Funding: Roche/Genentech
Travel, Accommodations, Expenses: Bayer Schering Pharma, Lilly, Sirtex Medical, AstraZeneca
Robin Kate Kelley
Consulting or Advisory Role: Agios (Inst), AstraZeneca (Inst), Merck (Inst), Kinnate Biopharma, Exelixis/Ipsen (Inst), Regeneron, Tyra Biosciences, Compass Therapeutics, Elevar Therapeutics, J-Pharma, Moderna Therapeutics
Research Funding: Lilly (Inst), Exelixis (Inst), Novartis (Inst), Bristol-Myers Squibb (Inst), MedImmune (Inst), Merck Sharp & Dohme (Inst), Agios (Inst), AstraZeneca (Inst), Adaptimmune (Inst), Taiho Pharmaceutical (Inst), Bayer (Inst), QED Therapeutics (Inst), EMD Serono (Inst), Partner Therapeutics (Inst), Genentech/Roche (Inst), Surface Oncology (Inst), Relay Therapeutics (Inst), Loxo/Lilly (Inst), Servier (Inst), Compass Therapeutics (Inst), Tyra Biosciences (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Merck
Chaiyut Charoentum
Research Funding: AstraZeneca (Inst), Roche/Genentech (Inst), Novartis (Inst), MSD Oncology (Inst)
Enrico N. De Toni
Employment: Boehringer Ingelheim
Honoraria: AstraZeneca, Bayer, Bristol-Myers Squibb, Eisai, Lilly, Pfizer, Ipsen, Terumo, Roche
Consulting or Advisory Role: AstraZeneca, Bayer, Bristol-Myers Squibb, Eisai, Lilly, Pfizer, Ipsen, Terumo, Roche
Research Funding: AstraZeneca, Bristol-Myer Squibb, Bayer, Lilly, Ipsen, Roche
Travel, Accommodations, Expenses: AstraZeneca, Bristol-Myers Squibb, Bayer, Celsion, Roche, Ipsen
Jeong Heo
Consulting or Advisory Role: AstraZeneca/MedImmune
Speakers' Bureau: Roche, AstraZeneca/MedImmune
Ann-Lii Cheng
Honoraria: Bayer Yakuhin, AstraZeneca, Eisai, Genentech/Roche
Consulting or Advisory Role: Bristol-Myers Squibb, Bayer Schering Pharma, Eisai, Ono Pharmaceutical, AstraZeneca, Genentech/Roche, MSD, BeiGene, IQVIA, Ipsen, Roche, Omega Therapeutics, Inc
Andrea Wilson Woods
Employment: Blue Faery: The Adrienne Wilson Liver Cancer Association
Honoraria: HCC TAG, American Institute Continuing Medical Education
Consulting or Advisory Role: Eisai (Inst), Humanise Health, AstraZeneca
Charu Gupta
Employment: AstraZeneca/MedImmune
Stock and Other Ownership Interests: AstraZeneca/MedImmune
Jayne Abraham
Employment: UCB
Stock and Other Ownership Interests: UCB
Carrie L. McCoy
Employment: AstraZeneca/MedImmune
Stock and Other Ownership Interests: AstraZeneca/MedImmune
Nikunj Patel
Employment: AstraZeneca
Stock and Other Ownership Interests: AstraZeneca
Alejandra Negro
Employment: AstraZeneca
Stock and Other Ownership Interests: AstraZeneca
Arndt Vogel
Honoraria: Roche, Amgen, Lilly, Bristol-Myers Squibb, MSD, Pierre Fabre, Ipsen, Janssen, Incyte, AstraZeneca/MedImmune, Sirtex Medical, Daichi-Sankyo, Terumo, Taiho Oncology, Eisai, BeiGene, Boehringer Pharma GmbH, GlaxoSmithKline, Boston Scientific, Servier
Consulting or Advisory Role: Novartis, Lilly, Roche, Amgen, Baxalta, AstraZeneca, Eisai, BTG, Ipsen, Pierre Fabre, Terumo, Daichi-Sankyo, Sirtex Medical, Boehringer Pharma GmbH, Incyte, Taiho Oncology
Research Funding: Novartis
Travel, Accommodations, Expenses: Roche, Ipsen, AstraZeneca, MSD, Lilly
Ghassan K. Abou-Alfa
Consulting or Advisory Role: Eisai, Ipsen, Merck Serono, AstraZeneca, Yiviva, Roche/Genentech, Autem Medical, Exelixis, QED Therapeutics, Boehringer Ingelheim, Novartis, Berry Genomics, BioNtech, Bristol-Myers Squibb/Medarex, Merus NV, Neogene Therapeutics, Tempus, Vector Health, Servier, J-Pharma, AbbVie
Research Funding: AstraZeneca (Inst), Bristol-Myers Squibb (Inst), Puma Biotechnology (Inst), QED Therapeutics (Inst), BioNtech (Inst), Genentech/Roche (Inst), Helsinn Healthcare (Inst), Yiviva (Inst), Elicio Therapeutics (Inst), Agenus (Inst), Parker Institute for Cancer Immunotherapy (Inst), Pertzye (Inst), Arcus Ventures (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Sung H, Ferlay J, Siegel RL, et al. : Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209-249, 2021 [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Ervik M, Lam F, et al. : Global Cancer Observatory: Cancer Today: Liver Factsheet. Lyon, France, International Agency for Research on Cancer, 2020 [Google Scholar]
- 3.Park J-W, Chen M, Colombo M, et al. : Global patterns of hepatocellular carcinoma management from diagnosis to death: The BRIDGE study. Liver Int 35:2155-2166, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bray F, Ferlay J, Soerjomataram I, et al. : Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394-424, 2018 [DOI] [PubMed] [Google Scholar]
- 5.Llovet JM, Kelley RK, Villanueva A, et al. : Hepatocellular carcinoma. Nat Rev Dis Primers 7:6, 2021 [DOI] [PubMed] [Google Scholar]
- 6.Rimassa L, Personeni N, Czauderna C, et al. : Systemic treatment of HCC in special populations. J Hepatol 74:931-943, 2021 [DOI] [PubMed] [Google Scholar]
- 7.European Association for the Study of the Liver : EASL clinical practice guidelines: Management of hepatocellular carcinoma. J Hepatol 69:182-236, 2018 [DOI] [PubMed] [Google Scholar]
- 8.Zhang H, Zhang W, Jiang L, et al. : Recent advances in systemic therapy for hepatocellular carcinoma. Biomark Res 10:3, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abou-Alfa GK, Lau G, Kudo M, et al. : Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. NEJM Evid 1:EVIDoa2100070, 2022 [DOI] [PubMed] [Google Scholar]
- 10.US Food and Drug Administration : Imfinzi (durvalumab): Highlights of prescribing information, 2023
- 11.US Food and Drug Administration : Imjudo (tremelimumab-actl): Highlights of prescribing information, 2023
- 12.AstraZeneca : Imfinzi plus Imjudo approved in Japan for advanced liver and non-small cell lung cancers, and Imfinzi approved for unresectable biliary tract and liver cancers, 2022
- 13.European Medicines Agency : Imfinzi (durvalumab): Summary of product characteristics, 2023
- 14.European Medicines Agency : Imjudo (tremelimumab): Summary of product characteristics, 2023
- 15.Gandhi S, Khubchandani S, Iyer R: Quality of life and hepatocellular carcinoma. J Gastrointest Oncol 5:296-317, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel N, Maher J, Lie X, et al. : Understanding the patient experience in hepatocellular carcinoma: A qualitative patient interview study. Qual Life Res 31:473-485, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galle PR, Finn RS, Qin S, et al. : Patient-reported outcomes with atezolizumab plus bevacizumab versus sorafenib in patients with unresectable hepatocellular carcinoma (IMbrave150): An open-label, randomised, phase 3 trial. Lancet Oncol 22:991-1001, 2021 [DOI] [PubMed] [Google Scholar]
- 18.Cheng A-L, Qin S, Ikeda M, et al. : Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol 76:862-873, 2022 [DOI] [PubMed] [Google Scholar]
- 19.Li L, Mo FKF, Chan SL, et al. : Prognostic values of EORTC QLQ-C30 and QLQ-HCC18 index-scores in patients with hepatocellular carcinoma—Clinical application of health-related quality-of-life data. BMC Cancer 17:8, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aaronson NK, Ahmedzai S, Bergman B, et al. : The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 85:365-376, 1993 [DOI] [PubMed] [Google Scholar]
- 21.Blazeby JM, Currie E, Zee BC, et al. : Development of a questionnaire module to supplement the EORTC QLQ-C30 to assess quality of life in patients with hepatocellular carcinoma, the EORTC QLQ-HCC18. Eur J Cancer 40:2439-2444, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Chie WC, Blazeby JM, Hsiao CF, et al. : International cross-cultural field validation of an European Organization for Research and Treatment of Cancer questionnaire module for patients with primary liver cancer, the European Organization for Research and Treatment of Cancer quality-of-life questionnaire HCC18. Hepatology 55:1122-1129, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Leighl NB, Karaseva N, Nakagawa K, et al. : Patient-reported outcomes from FLAURA: Osimertinib versus erlotinib or gefitinib in patients with EGFR-mutated advanced non-small-cell lung cancer. Eur J Cancer 125:49-57, 2020 [DOI] [PubMed] [Google Scholar]
- 24.Goldman JW, Garassino MC, Chen Y, et al. : Patient-reported outcomes with first-line durvalumab plus platinum-etoposide versus platinum-etoposide in extensive-stage small-cell lung cancer (CASPIAN): A randomized, controlled, open-label, phase III study. Lung Cancer 149:46-52, 2020 [DOI] [PubMed] [Google Scholar]
- 25.Dalle S, Mortier L, Corrie P, et al. : Long-term real-world experience with ipilimumab and non-ipilimumab therapies in advanced melanoma: The IMAGE study. BMC Cancer 21:642, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lenz H-J, Van Cutsem E, Luisa Limon M, et al. : First-line nivolumab plus low-dose ipilimumab for microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: The phase II CheckMate 142 study. J Clin Oncol 40:161-170, 2022 [DOI] [PubMed] [Google Scholar]
- 27.Yau T, Kang YK, Kim TY, et al. : Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: The CheckMate 040 randomized clinical trial. JAMA Oncol 6:e204564, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reck M, Schenker M, Lee KH, et al. : Nivolumab plus ipilimumab versus chemotherapy as first-line treatment in advanced non–small-cell lung cancer with high tumour mutational burden: Patient-reported outcomes results from the randomised, open-label, phase III CheckMate 227 trial. Eur J Cancer 116:137-147, 2019 [DOI] [PubMed] [Google Scholar]
- 29.Cella D, Grünwald V, Escudier B, et al. : Patient-reported outcomes of patients with advanced renal cell carcinoma treated with nivolumab plus ipilimumab versus sunitinib (CheckMate 214): A randomised, phase 3 trial. Lancet Oncol 20:297-310, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roydhouse JK, Mishra-Kalyani PS, Bhatnagar V, et al. : Does knowledge of treatment assignment affect patient report of symptoms, function, and health status? An evaluation using multiple myeloma trials. Value Health 24:822-829, 2021 [DOI] [PubMed] [Google Scholar]
- 31.Osoba D, Rodrigues G, Myles J, et al. : Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol 16:139-144, 1998 [DOI] [PubMed] [Google Scholar]
- 32.Cocks K, King MT, Velikova G, et al. : Evidence-based guidelines for interpreting change scores for the European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire Core 30. Eur J Cancer 48:1713-1721, 2012 [DOI] [PubMed] [Google Scholar]
- 33.Fayers PM, Aaronson NK, Bjordal K, et al. : EORTC QLQ-C30 Scoring Manual (ed 3). Brussels, Belgium, European Organisation for Research and Treatment of Cancer, 2001 [Google Scholar]
- 34.DeTora LM, Toroser D, Sykes A, et al. : Good Publication Practice (GPP) guidelines for company-sponsored biomedical research: 2022 update. Ann Intern Med 175:1298-1304, 2022 [DOI] [PubMed] [Google Scholar]