Abstract
A single fructose-1,6-bisphosphate (FBP) aldolase has been detected in extracts from carrot storage roots (Daucus carota L.). The enzyme was purified 850-fold to electrophoretic homogeneity and a final specific activity of 26.3 mumols of FBP utilized/min per mg of protein. SDS/PAGE of the final preparation revealed a single protein-staining band of 40 kDa. The native molecular mass was determined by analytical gel filtration to be 159 kDa, indicating that the enzyme is a homotetramer. Denaturing isoelectric focusing revealed two predominant protein-staining bands, with pI values of 5.6 and 5.7. The enzyme is a class I aldolase, since EDTA or metal ions had no effect on its activity. The enzyme was relatively heat-stable, had an activation energy (Ea) of 68.3 kJ.mol-1, and had an absorption coefficient of 8.08 x 10(4) M-1.cm-1 at 280 nm. Km values for FBP and sedoheptulose 1,7-bisphosphate (SBP) were both determined to be 6 microM (pH optima 7.4). The specificity constant with FBP was 2.6 times that obtained with SBP. Ribose 5-phosphate, 6-phosphogluconate, MgAMP, glucose 1-phosphate and phosphoenolpyruvate (PEP) were inhibitors. PEP was a mixed-type inhibitor with respect to FBP (Ki = 3.2 mM, K'i = 5.1 mM). No activators were found. Rabbit anti-(carrot aldolase) polyclonal antibodies immunoprecipitated the activity of both carrot root aldolase and spinach leaf cytosolic aldolase, but not that of spinach leaf plastid aldolase. Western-blot analysis also revealed cross-reactivity with cytosolic, but not plastid, spinach leaf aldolase, indicating that the single carrot root aldolase is cytosolic.
Full text
PDF

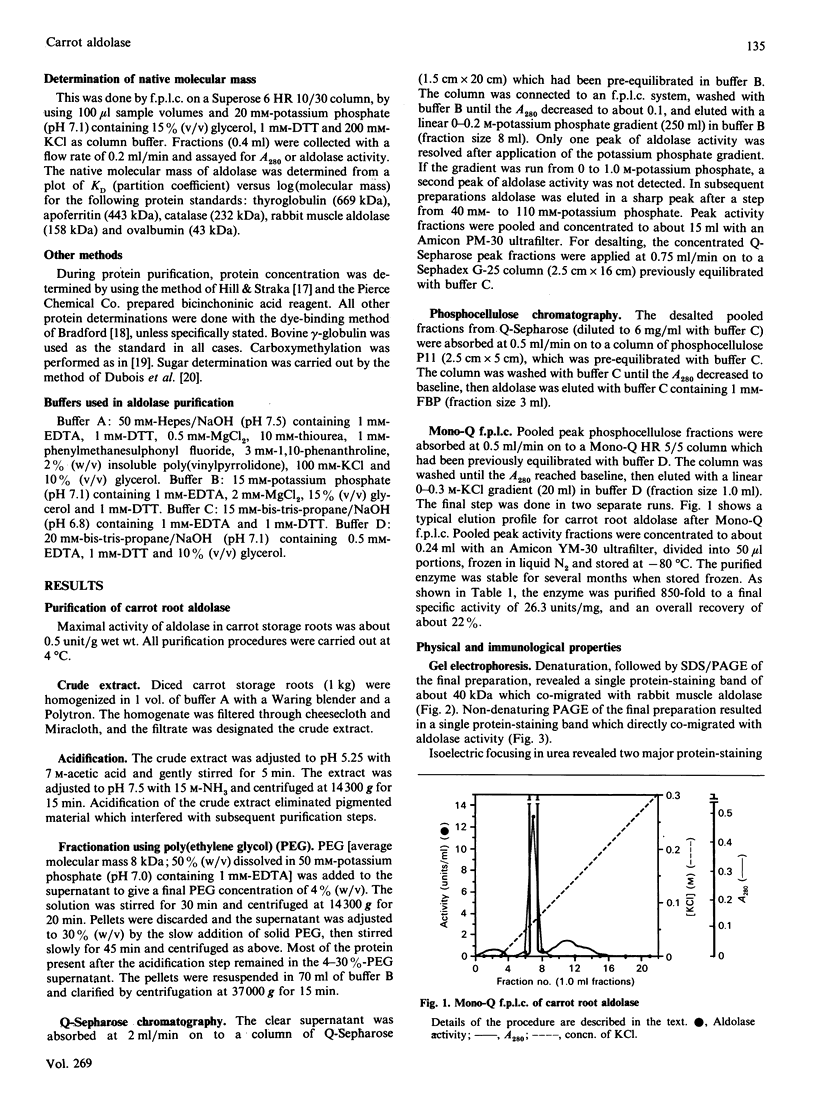




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babul J., Fraenkel D. G. Phosphate modification of fructose-1,6-bisphosphate aldolase in Escherichia coli. Biochem Biophys Res Commun. 1988 Mar 30;151(3):1033–1038. doi: 10.1016/s0006-291x(88)80469-8. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Doucet J. P., Trifaró J. M. A discontinuous and highly porous sodium dodecyl sulfate-polyacrylamide slab gel system of high resolution. Anal Biochem. 1988 Feb 1;168(2):265–271. doi: 10.1016/0003-2697(88)90317-x. [DOI] [PubMed] [Google Scholar]
- Heil J. A., Lebherz H. G. "Hybridization" between aldolase subunits derived from mammalian and plant origin. J Biol Chem. 1978 Sep 25;253(18):6599–6605. [PubMed] [Google Scholar]
- Hill H. D., Straka J. G. Protein determination using bicinchoninic acid in the presence of sulfhydryl reagents. Anal Biochem. 1988 Apr;170(1):203–208. doi: 10.1016/0003-2697(88)90109-1. [DOI] [PubMed] [Google Scholar]
- Jacobshagen S., Schnarrenberger C. Two Class I Aldolases in the Green Alga Chara foetida (Charophyceae). Plant Physiol. 1988 May;87(1):78–82. doi: 10.1104/pp.87.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Job D., Cochet C., Dhien A., Chambaz E. M. A rapid method for screening inhibitor effects: determination of I50 and its standard deviation. Anal Biochem. 1978 Jan;84(1):68–77. doi: 10.1016/0003-2697(78)90484-0. [DOI] [PubMed] [Google Scholar]
- Kelley P. M., Tolan D. R. The complete amino Acid sequence for the anaerobically induced aldolase from maize derived from cDNA clones. Plant Physiol. 1986 Dec;82(4):1076–1080. doi: 10.1104/pp.82.4.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüger I., Schnarrenberger C. Purification, subunit structure and immunological comparison of fructose-bisphosphate aldolases from spinach and corn leaves. Eur J Biochem. 1983 Oct 17;136(1):101–106. doi: 10.1111/j.1432-1033.1983.tb07711.x. [DOI] [PubMed] [Google Scholar]
- Lebherz H. G., Leadbetter M. M., Bradshaw R. A. Isolation and characterization of the cytosolic and chloroplast forms of spinach leaf fructose diphosphate aldolase. J Biol Chem. 1984 Jan 25;259(2):1011–1017. [PubMed] [Google Scholar]
- Masters C. J., Reid S., Don M. Glycolysis--new concepts in an old pathway. Mol Cell Biochem. 1987 Jul;76(1):3–14. doi: 10.1007/BF00219393. [DOI] [PubMed] [Google Scholar]
- Moorhead G. B., Plaxton W. C. Binding of glycolytic enzymes to a particulate fraction in carrot and sugar beet storage roots : dependence on metabolic state. Plant Physiol. 1988 Feb;86(2):348–351. doi: 10.1104/pp.86.2.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhoff V., Arold N., Taube D., Ehrhardt W. Improved staining of proteins in polyacrylamide gels including isoelectric focusing gels with clear background at nanogram sensitivity using Coomassie Brilliant Blue G-250 and R-250. Electrophoresis. 1988 Jun;9(6):255–262. doi: 10.1002/elps.1150090603. [DOI] [PubMed] [Google Scholar]
- Plaxton W. C. Molecular and immunological characterization of plastid and cytosolic pyruvate kinase isozymes from castor-oil-plant endosperm and leaf. Eur J Biochem. 1989 May 1;181(2):443–451. doi: 10.1111/j.1432-1033.1989.tb14745.x. [DOI] [PubMed] [Google Scholar]
- Porter M. A., Stringer C. D., Hartman F. C. Characterization of the regulatory thioredoxin site of phosphoribulokinase. J Biol Chem. 1988 Jan 5;263(1):123–129. [PubMed] [Google Scholar]
- Robertson E. F., Dannelly H. K., Malloy P. J., Reeves H. C. Rapid isoelectric focusing in a vertical polyacrylamide minigel system. Anal Biochem. 1987 Dec;167(2):290–294. doi: 10.1016/0003-2697(87)90166-7. [DOI] [PubMed] [Google Scholar]
- Schnarrenberger C., Krüger I. Distinction between Cytosol and Chloroplast Fructose-Bisphosphate Aldolases from Pea, Wheat, and Corn Leaves. Plant Physiol. 1986 Feb;80(2):301–304. doi: 10.1104/pp.80.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]






