Abstract
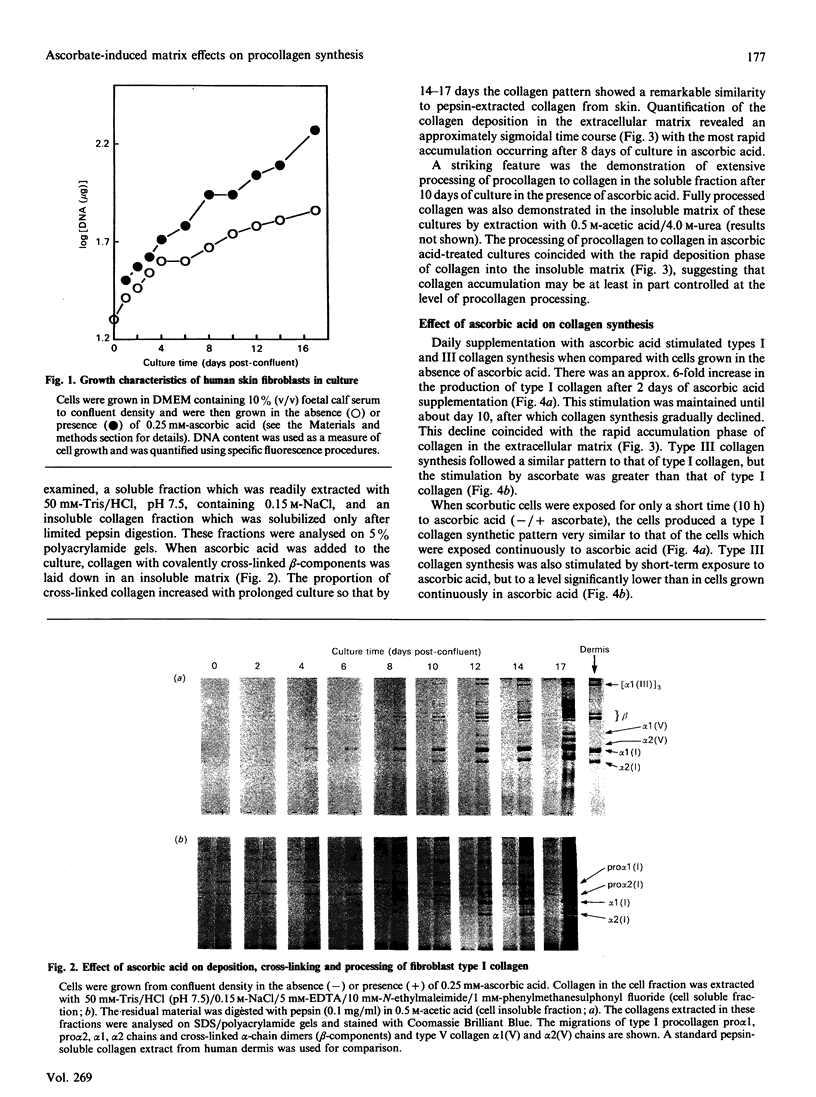
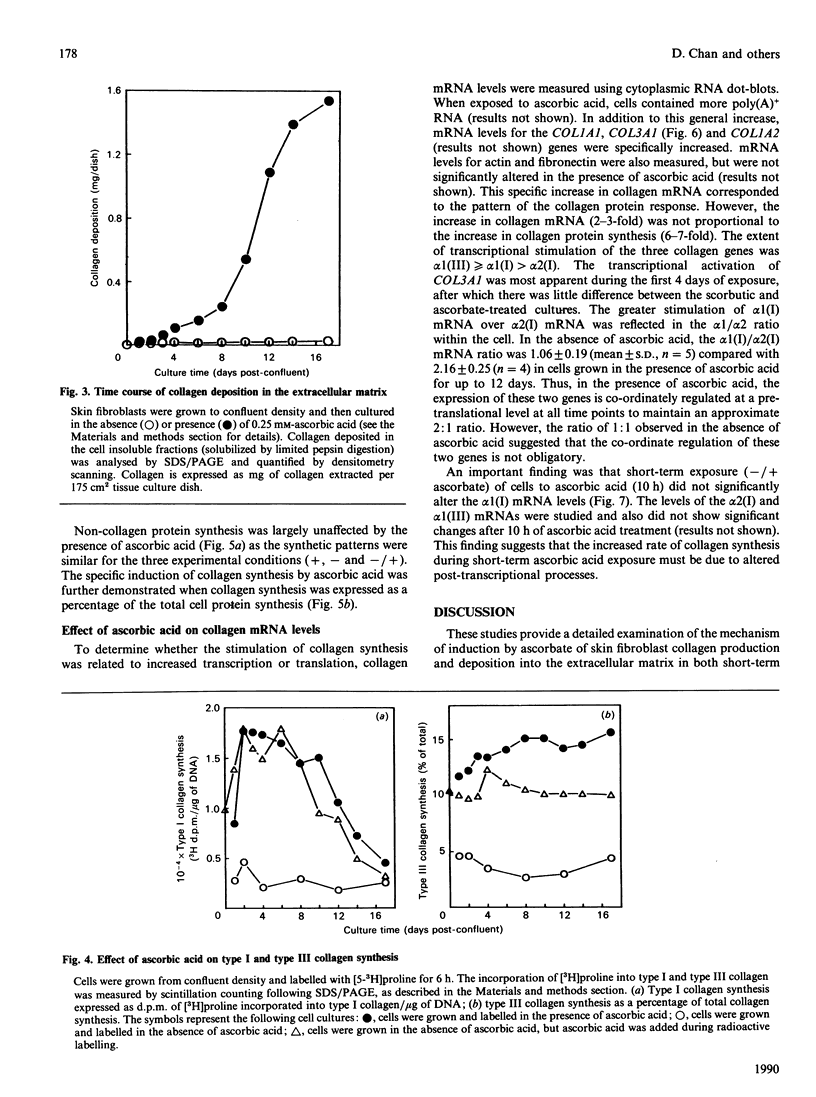
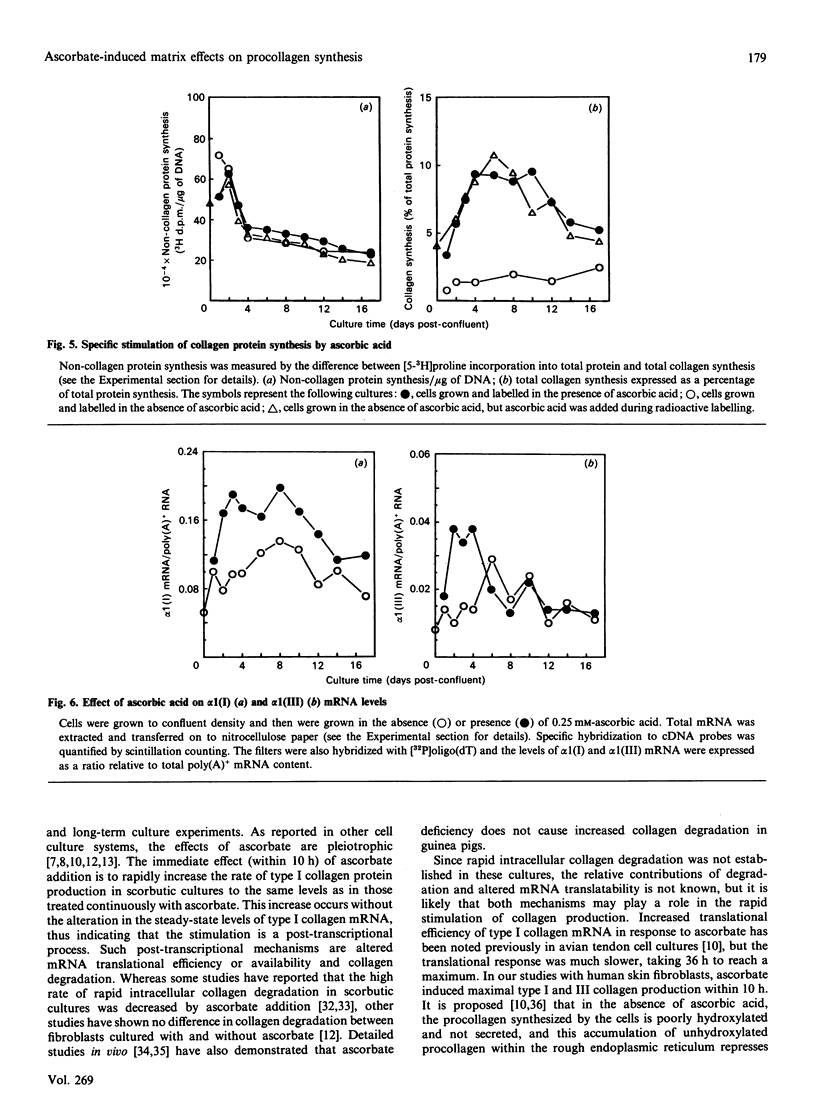
Procollagen biosynthesis and matrix deposition were studied in long-term human skin fibroblast cultures exposed to ascorbic acid. Ascorbic acid specifically stimulated types I and III collagen synthesis, reaching a maximum at day 2 and maintaining a specific high rate of production until day 10 of ascorbate exposure, after which collagen production declined. The increased level of collagen synthesis after different exposure times could also be achieved by only brief treatment (10 h) of parallel scorbutic (ascorbic-acid-deficient) cultures with ascorbic acid. This brief exposure did not result in increased collagen mRNA, thus demonstrating that the ascorbate-induced increase in collagen synthesis at all stages of ascorbic acid exposure was due to post-transcriptional mechanisms, most likely a rapid increase in type 1 collagen mRNA translational efficiency. This mechanism, rather than the transcriptional activation, was the primary response and is adequate to explain the ascorbate-induced increase in collagen synthesis. These data also demonstrate that the presence of a collagenous extracellular matrix was not involved in this collagen biosynthetic regulation. During long-term exposure (18 days) to ascorbic acid, a substantial cross-linked collagenous matrix formed, following an approximately sigmoidal time course. The most rapid matrix deposition occurred during the later days of exposure when the rate of collagen synthesis was decreasing, suggesting that the presence of a pre-existing matrix is important for further collagen accumulation. Procollagen was also efficiently processed to collagen during this phase, demonstrating that efficient procollagen processing is an important regulatory event in collagen matrix deposition.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alonso S., Minty A., Bourlet Y., Buckingham M. Comparison of three actin-coding sequences in the mouse; evolutionary relationships between the actin genes of warm-blooded vertebrates. J Mol Evol. 1986;23(1):11–22. doi: 10.1007/BF02100994. [DOI] [PubMed] [Google Scholar]
- Bateman J. F., Chan D., Mascara T., Rogers J. G., Cole W. G. Collagen defects in lethal perinatal osteogenesis imperfecta. Biochem J. 1986 Dec 15;240(3):699–708. doi: 10.1042/bj2400699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman J. F., Harley V., Chan D., Cole W. G. Comprehensive analysis of collagen metabolism in vitro using [4(3H)]/[14C]proline dual-labeling and polyacrylamide gel electrophoresis. Anal Biochem. 1988 Jan;168(1):171–175. doi: 10.1016/0003-2697(88)90025-5. [DOI] [PubMed] [Google Scholar]
- Bateman J. F., Lamande S., Chan D., Cole W. G. Peptide analysis of collagen produced from cDNA by transcription and translation in vitro. Biochem J. 1987 Jul 15;245(2):393–398. doi: 10.1042/bj2450393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman J. F., Mascara T., Chan D., Cole W. G. Abnormal type I collagen metabolism by cultured fibroblasts in lethal perinatal osteogenesis imperfecta. Biochem J. 1984 Jan 1;217(1):103–115. doi: 10.1042/bj2170103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg R. A., Steinmann B., Rennard S. I., Crystal R. G. Ascorbate deficiency results in decreased collagen production: under-hydroxylation of proline leads to increased intracellular degradation. Arch Biochem Biophys. 1983 Oct 15;226(2):681–686. doi: 10.1016/0003-9861(83)90338-7. [DOI] [PubMed] [Google Scholar]
- Bernard M. P., Kolbe M., Weil D., Chu M. L. Human cellular fibronectin: comparison of the carboxyl-terminal portion with rat identifies primary structural domains separated by hypervariable regions. Biochemistry. 1985 May 21;24(11):2698–2704. doi: 10.1021/bi00332a016. [DOI] [PubMed] [Google Scholar]
- Bissell M. J., Barcellos-Hoff M. H. The influence of extracellular matrix on gene expression: is structure the message? J Cell Sci Suppl. 1987;8:327–343. doi: 10.1242/jcs.1987.supplement_8.18. [DOI] [PubMed] [Google Scholar]
- Cesarone C. F., Bolognesi C., Santi L. Improved microfluorometric DNA determination in biological material using 33258 Hoechst. Anal Biochem. 1979 Nov 15;100(1):188–197. doi: 10.1016/0003-2697(79)90131-3. [DOI] [PubMed] [Google Scholar]
- Chojkier M., Spanheimer R., Peterkofsky B. Specifically decreased collagen biosynthesis in scurvy dissociated from an effect on proline hydroxylation and correlated with body weight loss. In vitro studies in guinea pig calvarial bones. J Clin Invest. 1983 Sep;72(3):826–835. doi: 10.1172/JCI111053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu M. L., Myers J. C., Bernard M. P., Ding J. F., Ramirez F. Cloning and characterization of five overlapping cDNAs specific for the human pro alpha 1(I) collagen chain. Nucleic Acids Res. 1982 Oct 11;10(19):5925–5934. doi: 10.1093/nar/10.19.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geesin J. C., Darr D., Kaufman R., Murad S., Pinnell S. R. Ascorbic acid specifically increases type I and type III procollagen messenger RNA levels in human skin fibroblast. J Invest Dermatol. 1988 Apr;90(4):420–424. doi: 10.1111/1523-1747.ep12460849. [DOI] [PubMed] [Google Scholar]
- Gerstenfeld L. C., Chipman S. D., Kelly C. M., Hodgens K. J., Lee D. D., Landis W. J. Collagen expression, ultrastructural assembly, and mineralization in cultures of chicken embryo osteoblasts. J Cell Biol. 1988 Mar;106(3):979–989. doi: 10.1083/jcb.106.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell F., Fukamizu H., Pawelek P., Nakagawa S. Collagen processing, crosslinking, and fibril bundle assembly in matrix produced by fibroblasts in long-term cultures supplemented with ascorbic acid. Exp Cell Res. 1989 Apr;181(2):483–491. doi: 10.1016/0014-4827(89)90105-5. [DOI] [PubMed] [Google Scholar]
- Harley C. B. Hybridization of oligo(dT) to RNA on nitrocellulose. Gene Anal Tech. 1987 Mar-Apr;4(2):17–22. doi: 10.1016/0735-0651(87)90013-6. [DOI] [PubMed] [Google Scholar]
- Limeback H. F., Sodek J. Procollagen synthesis and processing in periodontal ligament in vivo and in vitro. A comparative study using slab-gel fluorography. Eur J Biochem. 1979 Oct 15;100(2):541–550. doi: 10.1111/j.1432-1033.1979.tb04200.x. [DOI] [PubMed] [Google Scholar]
- Lyons B. L., Schwarz R. I. Ascorbate stimulation of PAT cells causes an increase in transcription rates and a decrease in degradation rates of procollagen mRNA. Nucleic Acids Res. 1984 Mar 12;12(5):2569–2579. doi: 10.1093/nar/12.5.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinkoth J., Wahl G. Hybridization of nucleic acids immobilized on solid supports. Anal Biochem. 1984 May 1;138(2):267–284. doi: 10.1016/0003-2697(84)90808-x. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miskulin M., Dalgleish R., Kluve-Beckerman B., Rennard S. I., Tolstoshev P., Brantly M., Crystal R. G. Human type III collagen gene expression is coordinately modulated with the type I collagen genes during fibroblast growth. Biochemistry. 1986 Mar 25;25(6):1408–1413. doi: 10.1021/bi00354a033. [DOI] [PubMed] [Google Scholar]
- Miyahara M., Njieha F. K., Prockop D. J. Formation of collagen fibrils in vitro by cleavage of procollagen with procollagen proteinases. J Biol Chem. 1982 Jul 25;257(14):8442–8448. [PubMed] [Google Scholar]
- Murad S., Grove D., Lindberg K. A., Reynolds G., Sivarajah A., Pinnell S. R. Regulation of collagen synthesis by ascorbic acid. Proc Natl Acad Sci U S A. 1981 May;78(5):2879–2882. doi: 10.1073/pnas.78.5.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers J. C., Chu M. L., Faro S. H., Clark W. J., Prockop D. J., Ramirez F. Cloning a cDNA for the pro-alpha 2 chain of human type I collagen. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3516–3520. doi: 10.1073/pnas.78.6.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltonen L., Halila R., Ryhänen L. Enzymes converting procollagens to collagens. J Cell Biochem. 1985;28(1):15–21. doi: 10.1002/jcb.240280104. [DOI] [PubMed] [Google Scholar]
- Peterkofsky B. Regulation of collagen secretion by ascorbic acid in 3T3 and chick embryo fibroblasts. Biochem Biophys Res Commun. 1972 Dec 4;49(5):1343–1350. doi: 10.1016/0006-291x(72)90614-6. [DOI] [PubMed] [Google Scholar]
- Pinnel S. R., Murad S., Darr D. Induction of collagen synthesis by ascorbic acid. A possible mechanism. Arch Dermatol. 1987 Dec;123(12):1684–1686. doi: 10.1001/archderm.123.12.1684. [DOI] [PubMed] [Google Scholar]
- Prockop D. J., Kivirikko K. I. Heritable diseases of collagen. N Engl J Med. 1984 Aug 9;311(6):376–386. doi: 10.1056/NEJM198408093110606. [DOI] [PubMed] [Google Scholar]
- Rowe L. B., Schwarz R. I. Role of procollagen mRNA levels in controlling the rate of procollagen synthesis. Mol Cell Biol. 1983 Feb;3(2):241–249. doi: 10.1128/mcb.3.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz E., Bienkowski R. S., Coltoff-Schiller B., Goldfischer S., Blumenfeld O. O. Changes in the components of extracellular matrix and in growth properties of cultured aortic smooth muscle cells upon ascorbate feeding. J Cell Biol. 1982 Feb;92(2):462–470. doi: 10.1083/jcb.92.2.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz R. I., Bissell M. J. Dependence of the differentiated state on the cellular environment: modulation of collagen synthesis in tendon cells. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4453–4457. doi: 10.1073/pnas.74.10.4453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz R. I., Mandell R. B., Bissell M. J. Ascorbate induction of collagen synthesis as a means for elucidating a mechanism of quantitative control of tissue-specific function. Mol Cell Biol. 1981 Sep;1(9):843–853. doi: 10.1128/mcb.1.9.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz R. I. Procollagen secretion meets the minimum requirements for the rate-controlling step in the ascorbate induction of procollagen synthesis. J Biol Chem. 1985 Mar 10;260(5):3045–3049. [PubMed] [Google Scholar]
- Spanheimer R. G., Peterkofsky B. A specific decrease in collagen synthesis in acutely fasted, vitamin C-supplemented, guinea pigs. J Biol Chem. 1985 Apr 10;260(7):3955–3962. [PubMed] [Google Scholar]
- Steinmann B., Rao V. H., Gitzelmann R. Intracellular degradation of newly synthesized collagen is conformation-dependent. FEBS Lett. 1981 Oct 12;133(1):142–144. doi: 10.1016/0014-5793(81)80491-7. [DOI] [PubMed] [Google Scholar]
- Tajima S., Pinnell S. R. Regulation of collagen synthesis by ascorbic acid. Ascorbic acid increases type I procollagen mRNA. Biochem Biophys Res Commun. 1982 May 31;106(2):632–637. doi: 10.1016/0006-291x(82)91157-3. [DOI] [PubMed] [Google Scholar]
- Zern M. A., Schwartz E., Giambrone M. A., Blumenfeld O. O. Ascorbate-generated endogenous extracellular matrix affects cell protein synthesis in calf aortic smooth muscle cells. Exp Cell Res. 1985 Oct;160(2):307–318. doi: 10.1016/0014-4827(85)90178-8. [DOI] [PubMed] [Google Scholar]



